Trans-Coronary Sinus Intra-Septal Radiofrequency Ablation (TIRA) for Hypertrophic Obstructive Cardiomyopathy: First-in-Human Results
Abstract
1. Introduction
2. Methods
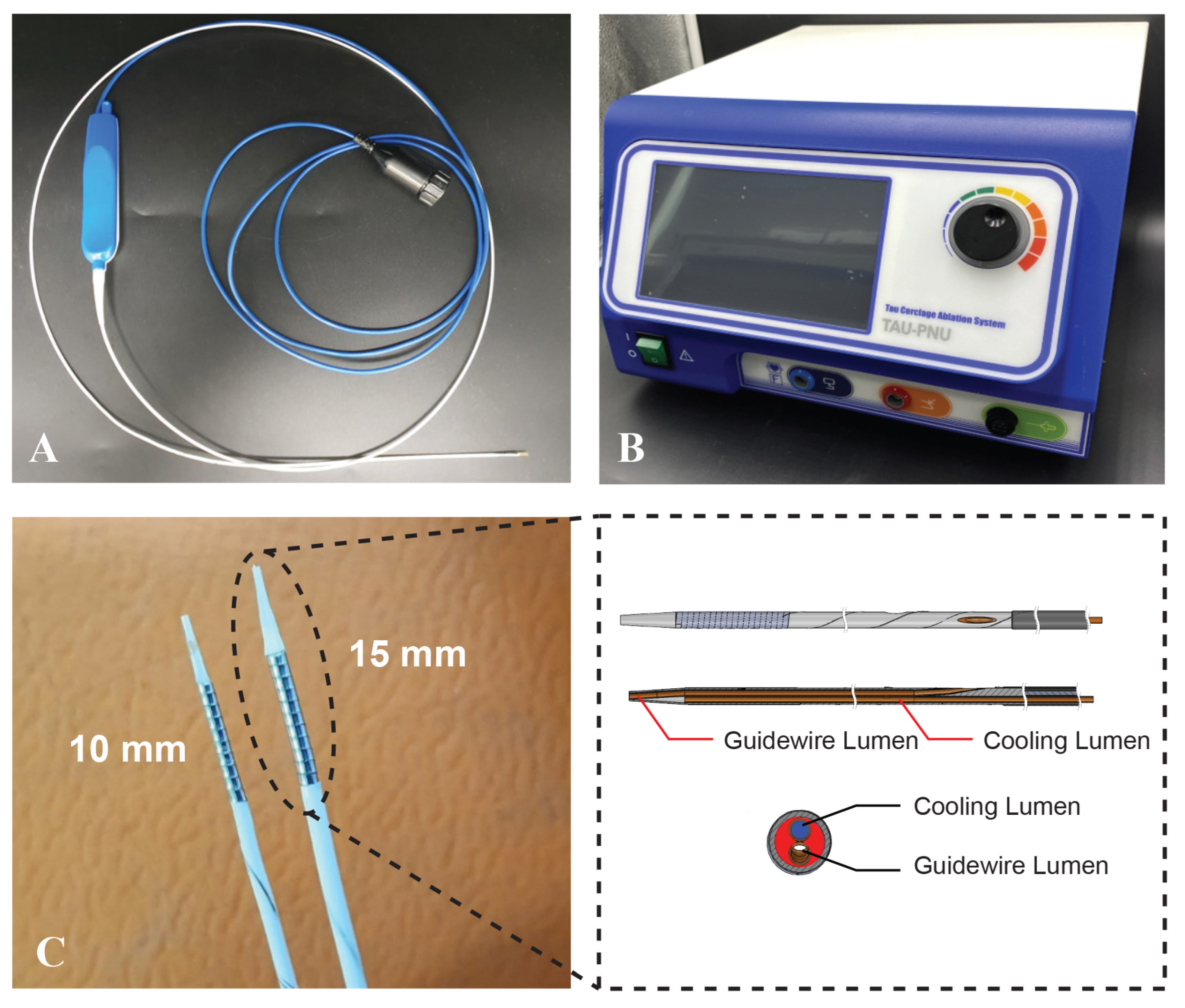
2.1. TIRA-HOCM Device
2.2. Patients and Study Design
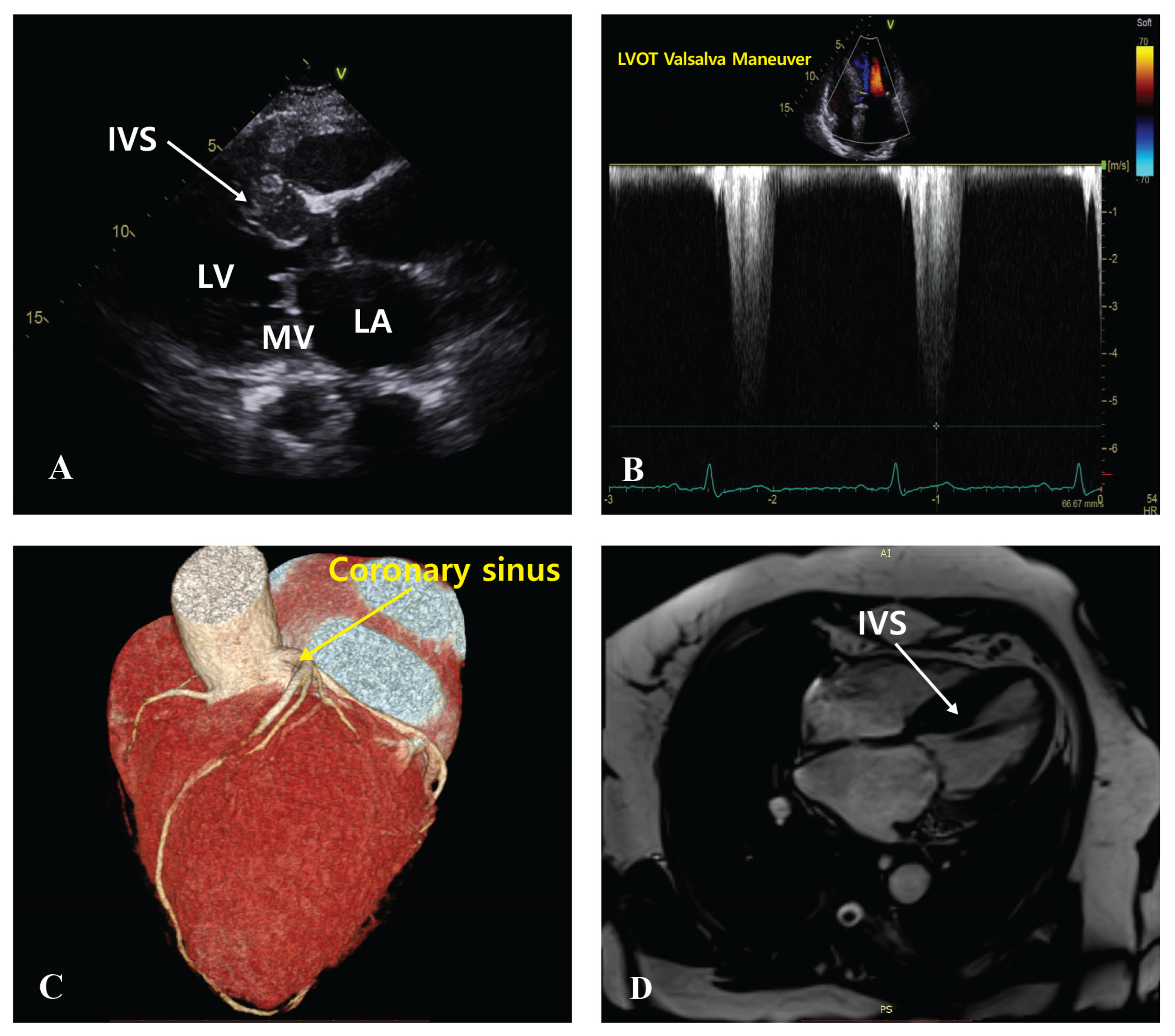
2.3. Procedure
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maron, B.J.; Gardin, J.M.; Flack, J.M.; Gidding, S.S.; Kurosaki, T.T.; Bild, D.E. Prevalence of hypertrophic cardiomyopathy in a general population of young adults: Echocardiographic analysis of 4111 subjects in the CARDIA study. Circulation 1995, 92, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Semsarian, C.; Ingles, J.; Maron, M.S.; Maron, B.J. New perspectives on the prevalence of hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2015, 65, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.R.; Ho, C.Y.; Elliott, P.M. Genetics of hypertrophic cardiomyopathy: Established and emerging implications for clinical practice. Eur. Heart J. 2024, 45, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Maisch, B.; Mahrholdt, H. The 2014 ESC guidelines on the diagnosis and management of hypertrophic cardiomyopathy: What is new? Herz 2014, 39, 919–930. [Google Scholar] [CrossRef]
- Niimura, H.; Bachinski, L.L.; Sangwatanaroj, S.; Watkins, H.; Chudley, A.E.; McDonough, B.; Wakimoto, H.; Setoguchi, M.; Yubeta, T.; Nakada, K.; et al. Sarcomere protein gene mutations in hypertrophic cardiomyopathy of the elderly. Circulation 2002, 105, 446–451. [Google Scholar] [CrossRef]
- Sherrid, M.V.; Shetty, A.; Winson, G.; Palacios, I.; McKenna, W.J.; Elliott, P.M.; Rahman, S.; Wheeler, M.; Dinkes, R.; Treat, J.; et al. Treatment of obstructive hypertrophic cardiomyopathy symptoms and gradient resistant to first-line therapy with β-blockade or verapamil. Circ. Heart Fail. 2013, 6, 694–702. [Google Scholar] [CrossRef]
- Maekawa, Y.; Akita, K.; Takanashi, S. Contemporary septal reduction therapy in drug-refractory hypertrophic obstructive cardiomyopathy. Circ. J. 2018, 82, 1977–1984. [Google Scholar] [CrossRef]
- Tower-Rader, A.; Ramchand, J.; Nissen, S.E.; Desai, M.Y. Mavacamten: A novel small molecule modulator of β-cardiac myosin for treatment of hypertrophic cardiomyopathy. Expert Opin. Investig. Drugs 2020, 29, 1171–1178. [Google Scholar] [CrossRef]
- Savage, P.; Mahfoud, F.; Freedman, B.; O’Donoghue, M.; Clarke, J.; Dunne, L.; Schulman, S.P.; Selvanayagam, J.B.; Andrews, J.; Moses, J.W.; et al. Advances in clinical cardiology 2021: A summary of key clinical trials. Adv. Ther. 2022, 39, 2398–2437. [Google Scholar] [CrossRef]
- Brown, M.L.; Schaff, H.V. Surgical management of obstructive hypertrophic cardiomyopathy: The gold standard. Expert Rev. Cardiovasc. Ther. 2008, 6, 715–722. [Google Scholar] [CrossRef]
- Maron, B.J.; Nishimura, R.A. Surgical septal myectomy versus alcohol septal ablation: Assessing the status of the controversy in 2014. Circulation 2014, 130, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Masry, H.E.; Breall, J.A. Alcohol septal ablation for hypertrophic obstructive cardiomyopathy. Curr. Cardiol. Rev. 2008, 4, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Califf, R.M.; Abdelmeguid, A.E.; Kuntz, R.E.; Popma, J.J.; Davidson, C.J.; Cohen, E.A.; Kleiman, N.S.; Mahaffey, K.W.; Topol, E.J.; Ohman, E.M.; et al. Myonecrosis after revascularization procedures. J. Am. Coll. Cardiol. 1998, 31, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, Y.; Chen, R.; Luo, J.; Zhou, X.; Chen, Z.; Zhao, H.; Jiang, L.; Zhuang, W.; Li, J.; et al. Percutaneous intramyocardial septal radiofrequency ablation for hypertrophic obstructive cardiomyopathy. J. Am. Coll. Cardiol. 2018, 72, 1898–1909. [Google Scholar] [CrossRef]
- Wolfram, D.; Tzankov, A.; Pülzl, P.; Piza-Katzer, H. Hypertrophic scars and keloids—A review of their pathophysiology, risk factors, and therapeutic management. Dermatol. Surg. 2009, 35, 171–181. [Google Scholar] [CrossRef]
- Shin, E.-S.; Nair, P.B.; Kim, J.-S.; Choe, Y.H.; Baek, S.H.; Jeong, J.O.; Park, J.-C.; Kang, S.-H.; Yoon, Y.E.; Yang, D.-H.; et al. Septal reduction using transvenous intramyocardial cerclage radiofrequency ablation: Preclinical feasibility. Basic Transl. Sci. 2020, 5, 988–998. [Google Scholar]
- Park, Y.-H.; Sun, B.J.; Lee, P.H.; Jung, S.H.; Kim, D.H.; Song, J.K.; Lee, J.W.; Kim, J.-J.; Na, C.Y.; Lim, S.-H.; et al. Mitral loop cerclage annuloplasty for secondary mitral regurgitation: First human results. JACC Cardiovasc. Interv. 2017, 10, 597–610. [Google Scholar] [CrossRef]
- Bennett, J.A.; Riegel, B.; Bittner, V.; Nichols, J. Validity and reliability of the NYHA classes for measuring research outcomes in patients with cardiac disease. Heart Lung 2002, 31, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Barkagan, M.; Riahi, S.; Choi, S.H.; Lazzara, R.; Liem, L.B.; Deyell, M.; Kelen, G.; Gokce, A.; Trachtenberg, B.; Coelho, D.; et al. Histopathological characterization of radiofrequency ablation in ventricular scar tissue. JACC Clin. Electrophysiol. 2019, 5, 920–931. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Tzikas, S.; Kozerke, S.; Hess, M.; Bremerich, J.; Nagel, E.; Gebker, R.; Uecker, M.; Schuster, A.; Rees, C.; et al. Left ventricular chamber dimensions and wall thickness by cardiovascular magnetic resonance: Comparison with transthoracic echocardiography. Eur. Heart J.–Cardiovasc. Imaging 2013, 14, 240–246. [Google Scholar] [CrossRef]
- Oktay, V.; Arslan, S.; Gecit, M.H.; Bulat, Z.; Gokce, M.E. Short-and Mid-Term Outcomes of Early Alcohol Septal Ablation Therapy for Patients with Mildly Symptomatic Hypertrophic Obstructive Cardiomyopathy: A Tertiary Center Experience. J. Clin. Med. 2024, 13, 1444. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.Y.; Zain, A.; Bhatia, M.; Smedira, N.; Patel, P.; Sideris, E.; Kwan, J.; Mahajan, N.; Kellett, M.; Ray, I.; et al. Myosin inhibition in patients with obstructive hypertrophic cardiomyopathy referred for septal reduction therapy. J. Am. Coll. Cardiol. 2022, 80, 95–108. [Google Scholar] [CrossRef] [PubMed]

| Case | Sex/Age (yrs) | NYHA | Medical History | |||||
|---|---|---|---|---|---|---|---|---|
| Hypertension | Dyslipidemia | A. Fib | Angina Pectoris | Pacemaker | Imaging Guidance | |||
| 1 | F/79 | 2 | − | + | + | − | + | CT TTE |
| 2 | F/66 | 2 | + | + | − | − | − | CT MRI TTE |
| 3 | M/52 | 2 | + | + | − | − | − | CT MRI TTE |
| 4 | F/81 | 2 | + | + | − | + | − | CT MRI TTE |
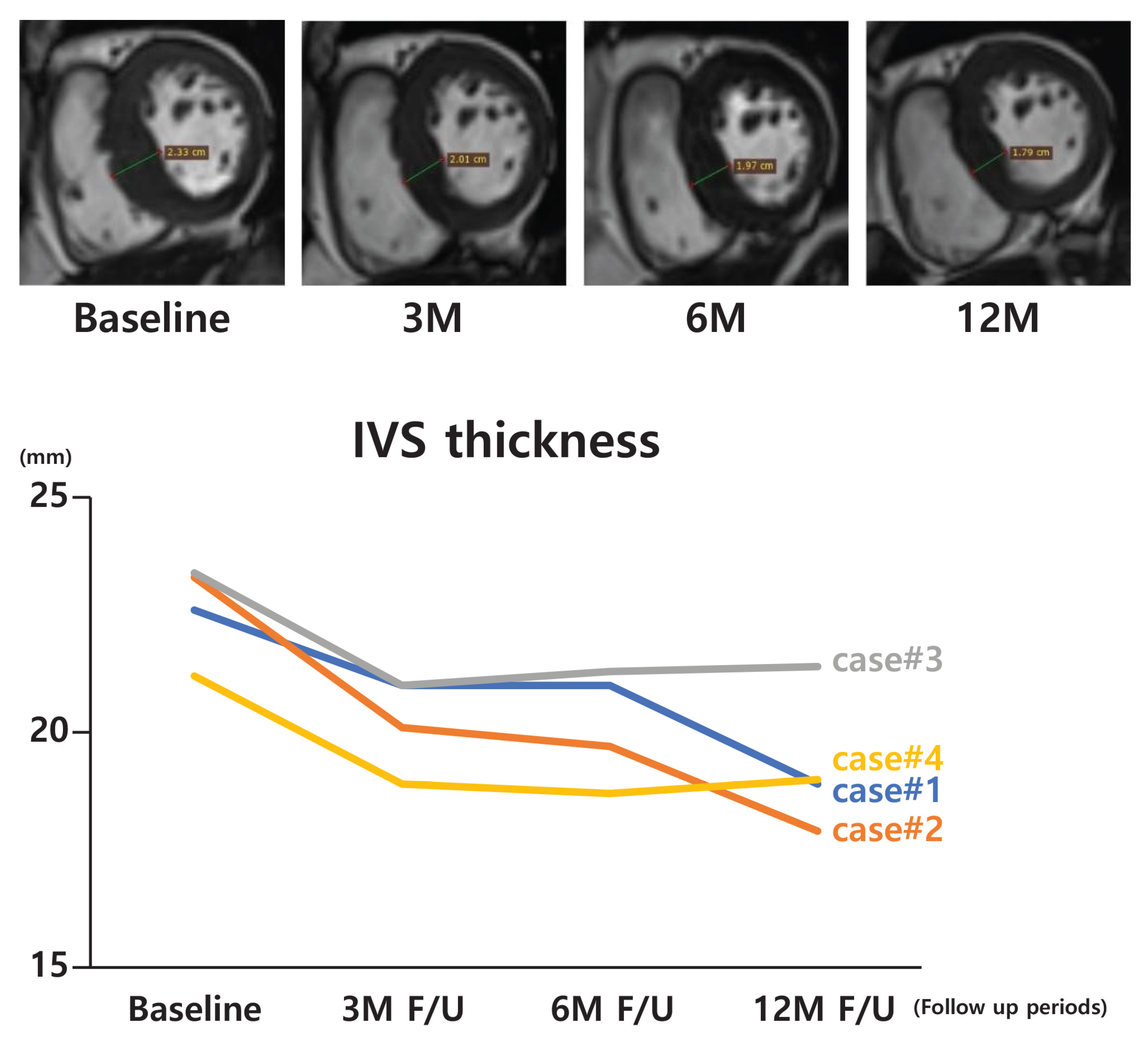
| Case | Computed Tomography or MRI Measurement Data (Interventricular Septal Thickness) | |||
|---|---|---|---|---|
| Baseline | 3 M | 6 M | 12 M | |
| 1 * | 22.6 | 21.0 | - | 18.9 |
| 2 | 23.3 | 20.1 | 19.7 | 17.9 |
| 3 | 23.4 | 21.0 | 21.3 | 21.4 |
| 4 | 21.2 | 18.9 | 18.7 | 19.0 |
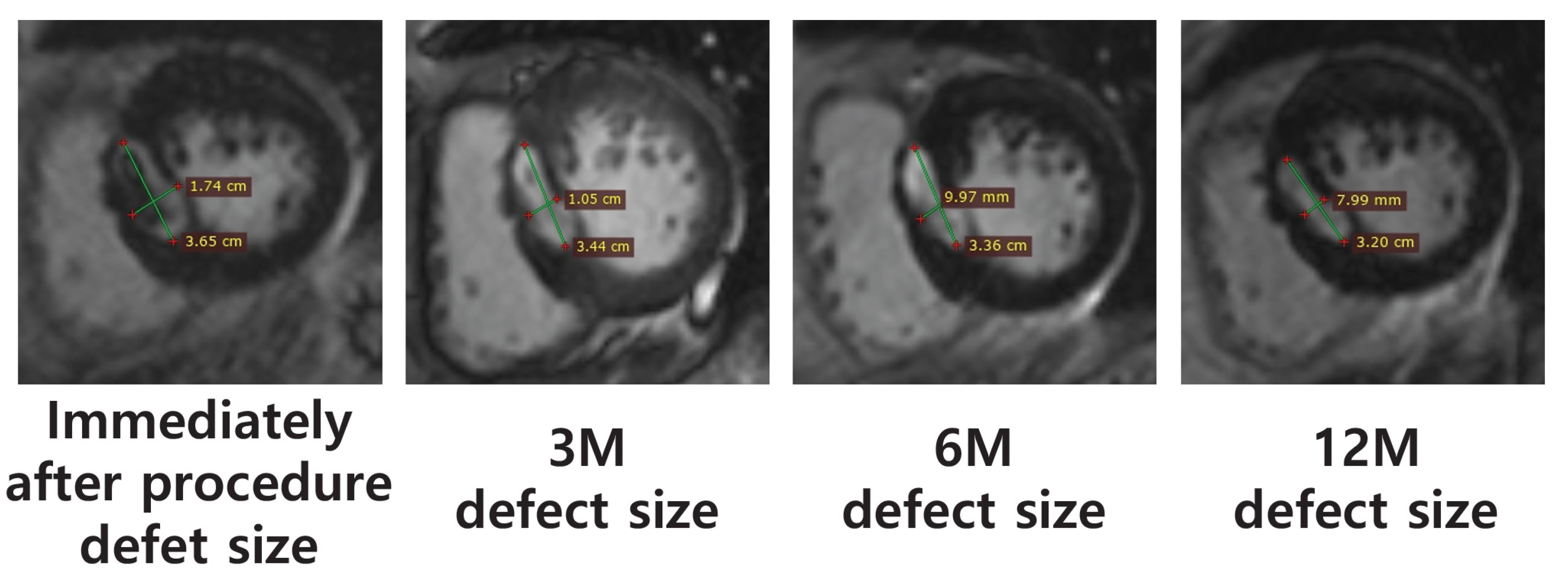
| Case | Ablation Defect Size (MRI, mm) | |||
|---|---|---|---|---|
| Immediately Following Procedure | 3 M | 6 M | 12 M | |
| 1 | - | - | - | - |
| 2 | 36.5 × 17.4 | 34.4 × 10.5 | 33.6 × 10.0 | 32.0 × 8.0 |
| 3 | 25.7 × 18.9 | 16.6 × 11.4 | 14.4 × 7.8 | 13.0 × 7.7 |
| 4 | 12.6 × 12.3 | 11.3 × 10.5 | 9.6 × 10.1 | 9.7 × 8.7 |
| Case | Echocardiogram Data | |||
|---|---|---|---|---|
| IVS Thickness (mm, Diastolic) | ||||
| Baseline | 3 M | 6 M | 12 M | |
| 1 | 17.2 | 18.4 | 18.1 | 17.8 |
| 2 | 23.2 | 21.2 | 21.7 | 20.6 |
| 3 | 23.5 | 23.4 | 23.1 | 23.0 |
| 4 | 21.4 | 22.5 | 22.5 | 22.6 |
| LVOT Gradient (mmHg): Resting Maneuver | ||||
| 1 | 84.64 | 92,16 | 125.44 | 43.56 |
| 2 | 100.00 | 54.76 | 46.24 | 38.44 |
| 3 | 40.96 | 36.00 | 25.00 | 25.00 |
| 4 | 112.36 | 116.64 | 92.16 | 100.00 |
| LVOT Gradient (mmHg): Valsalva Maneuver | ||||
| 1 | 129.96 | 134.56 | 219.04 | 108.16 |
| 2 | 129.96 | 116.64 | 54.76 | 43.56 |
| 3 | 70.56 | 70.56 | 38.44 | 33.64 |
| 4 | 184.96 | 148.84 | 121.00 | 148.84 |
| LVOT Diameter (mm) | ||||
| 1 | 15.2 | 15.6 | 16.1 | 15.6 |
| 2 | 20.5 | 16.5 | 17.0 | 17.2 |
| 3 | 17.5 | 18.3 | 17.9 | 18.0 |
| 4 | 21.9 | 21.9 | 21.8 | 22.1 |
| Case | Laboratory Data | |||
|---|---|---|---|---|
| 6MWD (m) | ||||
| Baseline | 3 M | 6 M | 12 M | |
| 1 | 315 | - | 340 | 303 |
| 2 | 300 | 405 | 410 | 375 |
| 3 | 375 | 385 | 410 | 405 |
| 4 | 316 | 303 | 320 | 320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.-S.; Seo, J.-Y.; Lee, C.-M.; Jung, S.-J.; Kim, J.-H.; Chon, M.-K. Trans-Coronary Sinus Intra-Septal Radiofrequency Ablation (TIRA) for Hypertrophic Obstructive Cardiomyopathy: First-in-Human Results. Biomedicines 2024, 12, 2762. https://doi.org/10.3390/biomedicines12122762
Oh J-S, Seo J-Y, Lee C-M, Jung S-J, Kim J-H, Chon M-K. Trans-Coronary Sinus Intra-Septal Radiofrequency Ablation (TIRA) for Hypertrophic Obstructive Cardiomyopathy: First-in-Human Results. Biomedicines. 2024; 12(12):2762. https://doi.org/10.3390/biomedicines12122762
Chicago/Turabian StyleOh, Ji-Soo, Jae-Young Seo, Cheol-Min Lee, Su-Jin Jung, June-Hong Kim, and Min-Ku Chon. 2024. "Trans-Coronary Sinus Intra-Septal Radiofrequency Ablation (TIRA) for Hypertrophic Obstructive Cardiomyopathy: First-in-Human Results" Biomedicines 12, no. 12: 2762. https://doi.org/10.3390/biomedicines12122762
APA StyleOh, J.-S., Seo, J.-Y., Lee, C.-M., Jung, S.-J., Kim, J.-H., & Chon, M.-K. (2024). Trans-Coronary Sinus Intra-Septal Radiofrequency Ablation (TIRA) for Hypertrophic Obstructive Cardiomyopathy: First-in-Human Results. Biomedicines, 12(12), 2762. https://doi.org/10.3390/biomedicines12122762






