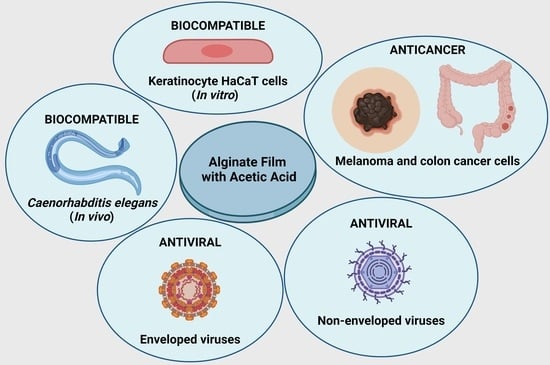
Biocompatible Alginate Hydrogel Film Containing Acetic Acid Manifests Broad-Spectrum Antiviral and Anticancer Activities
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Formulation of Hydrogel Film
2.3. Water Absorption Test
2.4. Toxicological Study
2.5. Proliferation Assay
2.6. Anticancer Study
2.7. In Vivo Toxicity Tests
2.8. Double-Stranded RNA Extraction and Quantification
2.9. Antiviral Test
2.9.1. Using Enveloped Bacteriophage Φ6
2.9.2. Using Non-Enveloped Bacteriophage MS2
3. Results and Discussion
3.1. Film Composition
3.2. Water Absorption
3.3. Toxicological Study
3.4. Proliferation Assay
3.5. Anticancer Study
3.6. In Vivo Toxicity Tests
3.7. Double-Stranded RNA Extraction and Quantification
3.8. Antiviral Test
3.8.1. Enveloped Bacteriophage Φ6
3.8.2. Non-Enveloped Bacteriophage MS2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cano-Vicen, A.; Hashimoto, R.; Takayama, K.; Serrano-Aroca, A. Biocompatible Films of Calcium Alginate Inactivate Enveloped Viruses Such as SARS-CoV-2. Polymers 2022, 14, 1483. [Google Scholar] [CrossRef]
- Cano-Vicent, A.; Martínez-Agut, A.; Tuñón-Molina, A.; Bakshi, H.; Serra, R.S.I.; Alfagih, I.M.; Tambuwala, M.M.; Serrano-Aroca, Á. In Vivo and In Vitro Biocompatible Alginate Film Crosslinked with Ca2+ and Co2+ Manifests Antiviral, Antibacterial and Anticancer Activity. Eur. Polym. J. 2023, 197, 112377. [Google Scholar] [CrossRef]
- Banerjee, E.R. (Ed.) Nanomaterials and Biomedicine: Therapeutic and Diagnostic Approach; Springer Nature: Singapore, 2020. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Lee, C.; Shin, J.; Lee, J.S.; Byun, E.; Ryu, J.H.; Um, S.H.; Kim, D.I.; Lee, H.; Cho, S.W. Bioinspired, calcium-free alginate hydrogels with tunable physical and mechanical properties and improved biocompatibility. Biomacromolecules 2013, 14, 2004–2013. [Google Scholar] [CrossRef]
- Nunamaker, E.A.; Purcell, E.K.; Kipke, D.R. In Vivo stability and biocompatibility of implanted calcium alginate disks. J. Biomed. Mater. Res. A 2007, 83A, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Serrano-Aroca, A. Alginate: Enhancement Strategies for Advanced Applications. Int. J. Mol. Sci. 2022, 23, 4486. [Google Scholar] [CrossRef]
- Anh, H.T.P.; Huang, C.M.; Huang, C.J. Intelligent Metal-Phenolic Metallogels as Dressings for Infected Wounds. Sci. Rep. 2019, 9, 11562. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.S. Acetic Acid. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 1–21. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/0471238961.0103052023010714.a01.pub3 (accessed on 8 November 2022).
- Pal, P.; Nayak, J. Acetic Acid Production and Purification: Critical Review Towards Process Intensification. Sep. Purif. Rev. 2017, 46, 44–61. [Google Scholar] [CrossRef]
- Gomes, R.J.; de Fatima Borges, M.; de Freitas Rosa, M.; Castro-Gómez, R.J.H.; Spinosa, W.A. Acetic Acid Bacteria in the Food Industry: Systematics, Characteristics and Applications. Food Technol. Biotechnol. 2018, 56, 139. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.Y.; Karabiyikli, S. Importance of acetic acid bacteria in food industry. Food Control 2011, 22, 647–656. [Google Scholar] [CrossRef]
- Seearunruangchai, A.; Tanasupawat, S.; Keeratipibul, S.; Thawai, C.; Itoh, T.; Yamada, Y. Identification of acetic acid bacteria isolated from fruits collected in Thailand. J. Gen. Appl. Microbiol. 2004, 50, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Solieri, L.; Giudici, P. Vinegars of the world. In Vinegars of the World; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–16. Available online: https://link.springer.com/chapter/10.1007/978-88-470-0866-3_1 (accessed on 10 November 2022).
- Samad, A.; Azlan, A.; Ismail, A. Therapeutic effects of vinegar: A review. Curr. Opin. Food Sci. 2016, 8, 56–61. [Google Scholar] [CrossRef]
- Johnston, C.S.; Gaas, C.A. Vinegar: Medicinal Uses and Antiglycemic Effect. Medscape Gen. Med. 2006, 8, 61. [Google Scholar]
- Zinn, M.K.; Bockmühl, D. Did granny know best? Evaluating the antibacterial, antifungal and antiviral efficacy of acetic acid for home care procedures. BMC Microbiol. 2020, 20, 265. [Google Scholar] [CrossRef] [PubMed]
- Halstead, F.D.; Rauf, M.; Moiemen, N.S.; Bamford, A.; Wearn, C.M.; Fraise, A.P.; Lund, P.A.; Oppenheim, B.A.; Webber, M.A. The Antibacterial Activity of Acetic Acid against Biofilm-Producing Pathogens of Relevance to Burns Patients. PLoS ONE 2015, 10, e0136190. [Google Scholar] [CrossRef]
- Sloss, J.M.; Cumberland, N.; Milner, S.M. Acetic acid used for the elimination of Pseudomonas aeruginosa from burn and soft tissue wounds. J. R. Army Med. Corps 1993, 139, 49–51. [Google Scholar] [CrossRef]
- Ryssel, H.; Kloeters, O.; Germann, G.; Schäfer, T.; Wiedemann, G.; Oehlbauer, M. The antimicrobial effect of acetic acid—An alternative to common local antiseptics? Burns 2009, 35, 695–700. [Google Scholar] [CrossRef]
- Cortesia, C.; Vilchèze, C.; Bernut, A.; Contreras, W.; Gómez, K.; De Waard, J.; Jacobs, W.R., Jr.; Kremer, L.; Takiff, H. Acetic Acid, the Active Component of Vinegar, Is an Effective Tuberculocidal Disinfectant. mBio 2014, 25, e00013-14. [Google Scholar] [CrossRef]
- Cerón, S.; North, B.J.; Taylor, S.A.; Leib, D.A. The STING agonist 5,6-dimethylxanthenone-4-acetic acid (DMXAA) stimulates an antiviral state and protects mice against herpes simplex virus-induced neurological disease. Virology 2019, 529, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Ostrovskii, V.A.; Danagulyan, G.G.; Nesterova, O.M.; Pavlyukova, Y.N.; Tolstyakov, V.V.; Zarubina, O.S.; Slepukhin, P.A.; Esaulkova, Y.L.; Muryleva, A.A.; Zarubaev, V.V.; et al. Synthesis and antiviral activity of nonannulated tetrazolylpyrimidines. Chem. Heterocycl. Compd. 2021, 57, 448–454. [Google Scholar] [CrossRef]
- Kuda, T.; Nishizawa, M.; Toshima, D.; Matsushima, K.; Yoshida, S.; Takahashi, H.; Kimura, B.; Yamagishi, T. Antioxidant and anti-norovirus properties of aqueous acetic acid macromolecular extracts of edible brown macroalgae. LWT 2021, 141, 110942. [Google Scholar] [CrossRef]
- Kim, D.S.; Kim, S.Y.; Jeong, Y.M.; Jeon, S.E.; Kim, M.K.; Kwon, S.B.; Na, J.I.; Park, K.C. Light-activated indole-3-acetic acid induces apoptosis in g361 human melanoma cells. Biol. Pharm. Bull. 2006, 29, 2404–2409. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Feng, X.; Kestell, P.; Paxton, J.W.; Baguley, B.C.; Chan, E. Transport of the investigational anti-cancer drug 5,6-dimethylxanthenone-4-acetic acid and its acyl glucuronide by human intestinal Caco-2 cells. Eur. J. Pharm. Sci. 2005, 24, 513–524. [Google Scholar] [CrossRef]
- Shirey, K.A.; Nhu, Q.M.; Yim, K.C.; Roberts, Z.J.; Teijaro, J.R.; Farber, D.L.; Blanco, J.C.; Vogel, S.N. The anti-tumor agent, 5,6-dimethylxanthenone-4-acetic acid (DMXAA), induces IFN-β-mediated antiviral activity in vitro and in vivo. J. Leukoc. Biol. 2011, 89, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Bibby, M.C.; Double, J.A.; Phillips, R.M.; Loadman, P.M. Factors involved in the anti-cancer activity of the investigational agents LM985 (flavone acetic acid ester) and LM975 (flavone acetic acid). Br. J. Cancer 1987, 55, 159–163. [Google Scholar] [CrossRef]
- Kaminskyy, D.; Zimenkovsky, B.; Lesyk, R. Synthesis and in vitro anticancer activity of 2,4-azolidinedione-acetic acids derivatives. Eur. J. Med. Chem. 2009, 44, 3627–3636. [Google Scholar] [CrossRef] [PubMed]
- Amruta, N.; Maness, N.J.; Gressett, T.E.; Tsuchiya, Y.; Kishi, M.; Bix, G. Effect of acetic acid inactivation of SARS-CoV-2. PLoS ONE 2023, 18, e0276578. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Briso, A.L.; Aparicio-Collado, J.L.; Serra, R.S.I.; Serrano-Aroca, Á. Graphene Oxide versus Carbon Nanofibers in Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Films: Degradation in Simulated Intestinal Environments. Polymers 2022, 14, 348. [Google Scholar] [CrossRef] [PubMed]
- Cano-Vicent, A.; Tuñón-Molina, A.; Bakshi, H.; Sabater i Serra, R.; Alfagih, I.M.; Tambuwala, M.M.; Serrano-Aroca, Á. Biocompatible Alginate Film Crosslinked with Ca2+ and Zn2+ Possesses Antibacterial, Antiviral, and Anticancer Activities. ACS Omega 2023, 8, 24396–24405. [Google Scholar] [CrossRef]
- Salesa, B.; Serra, R.S.I.; Serrano-Aroca, Á. Zinc chloride: Time-dependent cytotoxicity, proliferation and promotion of glycoprotein synthesis and antioxidant gene expression in human keratinocytes. Biology 2021, 10, 1072. [Google Scholar] [CrossRef]
- El Haidari, R.; Abbas, L.A.; Nerich, V.; Anota, A. Factors Associated with Health-Related Quality of Life in Women with Breast Cancer in the Middle East: A Systematic Review. Cancers 2020, 12, 696. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, H.A.; Quinn, G.A.; Nasef, M.M.; Mishra, V.; Aljabali, A.A.; El-Tanani, M.; Serrano-Aroca, Á.; Webba Da Silva, M.; McCarron, P.A.; Tambuwala, M.M. Crocin Inhibits Angiogenesis and Metastasis in Colon Cancer via TNF-α/NF-kB/VEGF Pathways. Cells 2022, 11, 1502. [Google Scholar] [CrossRef] [PubMed]
- Hakkim, F.L.; Bakshi, H.A.; Khan, S.; Nasef, M.; Farzand, R.; Sam, S.; Rashan, L.; Al-Baloshi, M.S.; Hasson, S.S.A.A.; Al Jabri, A.; et al. Frankincense essential oil suppresses melanoma cancer through down regulation of Bcl-2/Bax cascade signaling and ameliorates heptotoxicity via phase I and II drug metabolizing enzymes. Oncotarget 2019, 10, 3472–3490. [Google Scholar] [CrossRef]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. Methods Mol. Biol. 2009, 501, 69–76. [Google Scholar]
- Hismiogullari, S.; Hismiogullari, A.; Sahin, F.; Toksoy Öner, E.; Yenice, S.; Karasartova, D. Investigation of Antibacterial and Cytotoxic Effects of Organic Acids Including Ascorbic Acid, Lactic Acid and Acetic Acids on Mammalian Cells. J. Anim. Vet. Adv. 2008, 7, 343–354. [Google Scholar]
- Erencia, M.; Cano, F.; Tornero, J.A.; Fernandes, M.M.; Tzanov, T.; Macanás, J.; Carrillo, F. Electrospinning of gelatin fibers using solutions with low acetic acid concentration: Effect of solvent composition on both diameter of electrospun fibers and cytotoxicity. J. Appl. Polym. Sci. 2015, 132, 42115. [Google Scholar] [CrossRef]
- Gobbi, S.; Belluti, F.; Rampa, A.; Bisi, A. Flavonoid-Inspired Vascular Disrupting Agents: Exploring Flavone-8-Acetic Acid and Derivatives in the New Century. Molecules 2021, 26, 4228. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, P.E.; O’Neil, N. The use of functional genomics in C. elegans for studying human development and disease. J. Inherit. Metab. Dis. 2001, 24, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Sonnhammer, E.L.L.; Durbin, R. Analysis of Protein Domain Families in Caenorhabditis elegans. Genomics 1997, 46, 200–216. [Google Scholar] [CrossRef]
- Stutz, K.; Kaech, A.; Aebi, M.; Künzler, M.; Hengartner, M.O. Disruption of the C. elegans Intestinal Brush Border by the Fungal Lectin CCL2 Phenocopies Dietary Lectin Toxicity in Mammals. PLoS ONE 2015, 10, e0129381. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, X.; Ding, Y.; Wang, H.; Wu, X.; Liang, B. Comparative genomics and functional study of lipid metabolic genes in Caenorhabditis elegans. BMC Genom. 2013, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.M.; Orsi, G.; Brown, A.; Pritchard, D.I.; Aylott, J.W. Mapping the pharyngeal and intestinal pH of Caenorhabditis elegans and real-time luminal pH oscillations using extended dynamic range pH-sensitive nanosensors. ACS Nano 2013, 7, 5577–5587. [Google Scholar] [CrossRef]
- Hunt, P.R. The C. elegans model in toxicity testing. J. Appl. Toxicol. 2017, 37, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Fernández Romero, J.A.; Del Barrio Alonso, G.; Romeu Alvarez, B.; Gutierrez, Y.; Valdés, V.S.; Parra, F. In Vitro antiviral activity of Phyllanthus orbicularis extracts against herpes simplex virus type 1. Phytother. Res. 2003, 17, 980–982. [Google Scholar] [CrossRef] [PubMed]
- Pianta, L.; Vinciguerra, A.; Bertazzoni, G.; Morello, R.; Mangiatordi, F.; Lund, V.J.; Trimarchi, M. Acetic acid disinfection as a potential adjunctive therapy for non-severe COVID-19. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2921–2924. [Google Scholar] [CrossRef]
- Burton, P.D.; Tezak, M. Variable Chain Length Carboxylic Acids as Modifiers to Enhance the Antiviral Efficacy of Sodium Dodecyl Sulfate. 2020. Available online: https://www.osti.gov/servlets/purl/1634888/ (accessed on 20 April 2023).










| Sample Film | % Weight |
|---|---|
| Ac10 | 15.95 ± 1.45 |
| Ac0 | 0.00 ± 0.00 |
| Control | Ac0 | Ac10 | ||
|---|---|---|---|---|
| 5 min | PFU/mL | 3.37 × 106 ± 4.80 × 105 | 2.70 × 106 ± 3.34 × 105 | 1.34 × 106 ± 3.22 × 105 |
| log reduction | - | 0.10 | 0.41 | |
| % inactivation virus | - | ≈0 | 60.20 | |
| 30 min | PFU/mL | 3.89 × 106 ± 3.23 × 105 | 2.33 × 105 ± 1.94 × 105 | 1.33 × 103 ± 1.15 × 103 |
| log reduction | - | 1.32 | 4.39 | |
| % inactivation virus | - | 94.02 | 99.97 | |
| 5 h | PFU/mL | 3.04 × 106 ± 3.99 × 105 | 0.00 ± 0.00 | 2.00 × 103 ± 3.46 × 103 |
| log reduction | - | 6.48 | 5.22 | |
| % inactivation virus | - | 100.00 | 99.93 | |
| 24 h | PFU/mL | 3.89 × 106 ± 3.23 × 105 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| log reduction | - | 6.59 | 6.59 | |
| % inactivation virus | - | 100.00 | 100.00 |
| Control | Ac0 | Ac10 | ||
|---|---|---|---|---|
| 5 min | PFU/mL | 3.41 × 107 ± 6.93 × 105 | 3.43 × 107 ± 1.53 × 106 | 3.60 × 107 ± 1.00 × 106 |
| log reduction | - | 0 | 0 | |
| % inactivation virus | - | 0 | 0 | |
| 30 min | PFU/mL | 7.34 × 107 ± 4.40 × 106 | 6.88 × 107 ± 2.11 × 106 | 2.61 × 107 ± 3.34 × 107 |
| log reduction | - | 0.03 | 0.71 | |
| % inactivation virus | - | ≈0 | 64.50 | |
| 5 h | PFU/mL | 7.34 × 107 ± 4.40 × 106 | 6.08 × 107 ± 1.57 × 107 | 1.42 × 107 ± 1.64 × 107 |
| log reduction | - | 0.09 | 0.91 | |
| % inactivation virus | - | ≈0 | 80.59 | |
| 24 h | PFU/mL | 6.59 × 107 ± 2.72 × 106 | 5.04 × 107 ± 3.47 × 106 | 2.67 × 106 ± 2.30 × 105 |
| log reduction | - | 0.12 | 1.39 | |
| % inactivation virus | - | ≈0 | 95.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-Vicent, A.; Tuñón-Molina, A.; Bakshi, H.; Alfagih, I.M.; Tambuwala, M.M.; Serrano-Aroca, Á. Biocompatible Alginate Hydrogel Film Containing Acetic Acid Manifests Broad-Spectrum Antiviral and Anticancer Activities. Biomedicines 2023, 11, 2549. https://doi.org/10.3390/biomedicines11092549
Cano-Vicent A, Tuñón-Molina A, Bakshi H, Alfagih IM, Tambuwala MM, Serrano-Aroca Á. Biocompatible Alginate Hydrogel Film Containing Acetic Acid Manifests Broad-Spectrum Antiviral and Anticancer Activities. Biomedicines. 2023; 11(9):2549. https://doi.org/10.3390/biomedicines11092549
Chicago/Turabian StyleCano-Vicent, Alba, Alberto Tuñón-Molina, Hamid Bakshi, Iman M. Alfagih, Murtaza M. Tambuwala, and Ángel Serrano-Aroca. 2023. "Biocompatible Alginate Hydrogel Film Containing Acetic Acid Manifests Broad-Spectrum Antiviral and Anticancer Activities" Biomedicines 11, no. 9: 2549. https://doi.org/10.3390/biomedicines11092549
APA StyleCano-Vicent, A., Tuñón-Molina, A., Bakshi, H., Alfagih, I. M., Tambuwala, M. M., & Serrano-Aroca, Á. (2023). Biocompatible Alginate Hydrogel Film Containing Acetic Acid Manifests Broad-Spectrum Antiviral and Anticancer Activities. Biomedicines, 11(9), 2549. https://doi.org/10.3390/biomedicines11092549