Hyperinflammatory Immune Response in COVID-19: Host Genetic Factors in Pyrin Inflammasome and Immunity to Virus in a Spanish Population from Majorca Island
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject Enrollment
2.2. Next Generation Sequencing (NGS)
2.3. Inborn Errors Test Study
2.4. Statistical Analysis to Identify Potential Genetic Markers
3. Results
3.1. Patients Cohort: Clinical and Laboratory Data
3.2. Identifying Monogenic Inborn Errors of Immunity
3.3. Identifying Genetic Biomarkers
3.4. Distribution of Risk Variants According to Sex
3.5. Combined Analysis of Our Candidate Genetic Markers
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barbany, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Henderson, L.A.; Canna, S.W.; Schulert, G.S.; Volpi, S.; Lee, P.Y.; Kernan, K.F.; Caricchio, R.; Mahmud, S.; Hazen, M.M.; Halyabar, O.; et al. On the Alert for Cytokine Storm: Immunopathology in COVID-19. Arthritis Rheumatol. 2020, 72, 1059–1063. [Google Scholar] [CrossRef]
- Salzer, E.; Daschkey, S.; Choo, S.; Gombert, M.; Santos-Valente, E.; Ginzel, S.; Schewendinger, M.; Haas, O.A.; Fritsch, G.; Pickl, W.F.; et al. Combined immunodeficiency with life-threatening EBV-associated lymphoproliferative disorder in patients lacking functional CD27. Haematologica 2013, 98, 473–478. [Google Scholar] [CrossRef]
- Liang, Y.; Cucchetti, M.; Roncagalli, R.; Yokosuka, T.; Malzac, A.; Bertosio, E.; Imbert, J.; Nijman, I.J.; Suchanek, M.; Saito, T.; et al. The lymphoid lineage-specific actin-uncapping protein Rltpr is essential for costimulation via CD28 and the development of regulatory T cells. Nat. Immunol. 2013, 14, 858–866. [Google Scholar] [CrossRef]
- Molleran Lee, S.; Villanueva, J.; Sumegi, J.; Zhang, K.; Kogawa, K.; Davis, J.; Filipovich, A.H. Characterisation of diverse PRF1 mutations leading to decreased natural killer cell activity in North American families with haemophagocytic lymphohistiocytosis. J. Med. Genet. 2004, 41, 137–144. [Google Scholar] [CrossRef]
- Crayne, C.B.; Albeituni, S.; Nichols, K.E.; Cron, R.Q. The Immunology of Macrophage Activation Syndrome. Front. Immunol. 2019, 10, 119. [Google Scholar] [CrossRef]
- Giavridis, T.; van der Stegen, S.J.C.; Eyquem, J.; Hamieh, M.; Piersigilli, A.; Sadelain, M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 2018, 24, 731–738. [Google Scholar] [CrossRef]
- Schulert, G.S.; Cron, R.Q. The genetics of macrophage activation syndrome. Genes Immun. 2020, 21, 169–181. [Google Scholar] [CrossRef]
- Chinn, I.K.; Eckstein, O.S.; Peckham-Gregory, E.C.; Goldberg, B.R.; Forbes, L.R.; Nicholas, S.K.; Mace, E.M.; Vogel, T.P.; Abhyankar, H.A.; Diaz, M.I.; et al. Genetic and mechanistic diversity in pediatric hemophagocytic lymphohistiocytosis. Blood 2018, 132, 89–100. [Google Scholar] [CrossRef]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoniadou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe 2020, 27, 992–1000. [Google Scholar] [CrossRef]
- Kousathanas, A.; Pairo-Castineira, E.; Rawlik, K.; Stuckey, A.; Odhams, C.A.; Walker, S.; Russell, C.D.; Malinauskas, T.; Wu, Y.; Millar, J. Whole-genome sequencing reveals host factors underlying critical COVID-19. Nature 2022, 607, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Ellinghaus, D.; Degenhardt, F.; Bujanda, L.; Buti, M.; Albillos, A.; Invernizzi, P.; Fernández, J.; Prati, D.; Baselli, G.; Asselta, R. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N. Engl. J. Med. 2020, 383, 1522–1534. [Google Scholar] [PubMed]
- Pairo-Castineira, E.; Clohisey, S.; Klaric, L.; Bretherick, A.D.; Rawlik, K.; Pasko, D.; Walker, S.; Parkinson, N.; Fourman, M.H.; Russell, C.D. Genetic mechanisms of critical illness in COVID-19. Nature 2021, 591, 92–98. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J.; Ogishi, M.; Sabli, I.K.D.; Hodeib, S.; Korol, C. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef]
- Solanich, X.; Vargas-Parra, G.; Van der Made, C.I.; Simons, A.; Schuurs-Hoeijmakers, J.; Antolí, A.; Del Valle, J.; Rocamora-Blanch, G.; Setién, F.; Esteller, M. Genetic Screening for TLR7 Variants in Young and Previously Healthy Men with Severe COVID-19. Front. Immunol. 2021, 12, 719115. [Google Scholar] [CrossRef]
- Van der Made, C.I.; Simons, A.; Schuurs-Hoeijmakers, J.; Van den Heuvel, G.; Mantere, T.; Kersten, S.; Van Deuren, R.C.; Steehouwer, M.; van Reijmersdal, S.V.; Jaeger, M. Presence of Genetic Variants among Young Men with Severe COVID-19. JAMA 2020, 324, 663–673. [Google Scholar] [CrossRef]
- Zhang, Q.; Bastard, P.; COVID Human Genetic Effort; Cobat, A.; Casanova, J.L. Human genetic and immunological determinants of critical COVID-19 pneumonia. Nature 2022, 603, 587–598. [Google Scholar]
- Fardet, L.; Galicier, L.; Lambotte, O.; Marzac, C.; Aumont, C.; Chahwan, D.; Coppo, P.; Hejblum, G. Development and Validation of the HScore, a Score for the Diagnosis of Reactive Hemophagocytic Syndrome. Arthritis Rheumatol. 2014, 66, 2613–2620. [Google Scholar] [CrossRef]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C. Human Inborn Errors of Immunity: 2022 Update on the Classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Kitade, T.; Horiki, N.; Katsurahara, M.; Totoki, T.; Harada, T.; Tano, S.; Yamada, R.; Hamada, Y.; Inoue, H.; Tanaka, K. Usefulness of small intestinal endoscopy in a case of adult-onset Familial Mediterranean Fever associated with jejunoileitis. Intern. Med. 2015, 54, 1343–1347. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamagami, K.; Nakamura, T.; Nakamura, R.; Hanioka, Y.; Seki, K.; Chilba, H.; Kobayashi, K.; Agematsu, K. Familial Mediterranean fever with P369S/R408Q exon3 variant in pyrin presenting as symptoms of PFAPA. Mod. Rheumatol. 2017, 27, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Hannan, L.M.; Ward, J.; Ebringer, R.; Mcdonald, C.F. Late presentation of familial Mediterranean fever associated with P369S/R408Q variant in the MEFV gene. Intern. Med. 2012, 42, 952–954. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.G.; Masters, S.L.; Booty, M.G.; Habal, N.; Alexander, J.D.; Barham, B.K.; Remmers, E.F.; Barron, K.S.; Kastner, D.L.; Aksentijevich, I. Clinical features and functional significance of the P369S/R408Q variant in pyrin, the familial Mediterranean fever protein. Ann. Rheum. Dis. 2010, 69, 1383–1388. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. COVID-19 Weekly Surveillance Report 2022. Available online: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/weekly-surveillance-report/weekly-surveillance-report-alt (accessed on 2 September 2022).
- Xu, H.; Akinyemi, I.A.; Chitre, S.A.; Loeb, J.C.; Lednicky, J.A.; McIntosh, M.T.; Bhaduri-McIntosh, S. SARS-CoV-2 viroporin encoded by ORF3a triggers the NLRP3 inflammatory pathway. Virology 2022, 568, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Grandemange, S.; Soler, S.; Touitou, I. Expression of the familial Mediterranean fever gene is regulated by nonsense-mediated decay. Hum Mol Genet 2009, 18, 4746–4755. [Google Scholar] [CrossRef]
- Kirectepe, A.K.; Kasapcopur, O.; Arisoy, N.; Erdem, G.C.; Hatemi, G.; Ozdogan, H.; Turanli, E.T. Analysis of MEFV exon methylation and expression patterns in familial Mediterranean fever. BMC Med. Genet. 2011, 12, 105. [Google Scholar] [CrossRef]
- Papin, S.; Duquesnoy, P.; Cazeneuve, C.; Pantel, J.; Coppey-Moisan, M.; Dargemont, C.; Amselem, S. Alternative splicing at the MEFV locus involved in familial Mediterranean fever regulates translocation of the marenostrin/pyrin protein to the nucleus. Hum. Mol. Genet. 2000, 9, 3001–3009. [Google Scholar] [CrossRef]
- Erdem, G.C.; Erdemir, S.; Abaci, I.; Aydin, A.K.K.; Everest, E.; Turanli, E.T. Alternatively spliced MEFV transcript lacking exon 2 and its protein isoform pyrin-2d implies an epigenetic regulation of the gene in inflammatory cell culture models. Genet. Mol. Biol. 2017, 40, 688–697. [Google Scholar] [CrossRef]
- Smieszek, S.P.; Polymeropoulos, V.M.; Xiao, C.; Polymeropoulos, C.M.; Polymeropoulos, M.H. Loss-of-function mutations in IFNAR2 in COVID-19 severe infection susceptibility. J. Glob. Antimicrob. Resist. 2021, 26, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Ciancanelli, M.J.; Huang, S.X.; Luthra, P.; Garner, H.; Itan, Y.; Volpi, S.; Lafaille, F.G.; Trouillet, C.; Schmolke, M.; Albrecht, R.A. Life-threatening influenza and impaired interferon amplification in human IRF7 deficiency. Science 2015, 348, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q. Human genetics of life-threatening influenza pneumonitis. Hum. Genet. 2020, 139, 941–948. [Google Scholar] [CrossRef]
- Fricke-Galindo, I.; Martínez-Morales, A.; Chávez-Galán, L.; Ocaña-Guzmán, R.; Buendía-Roldán, I.; Pérez-Rubio, G.; Hernández-Zenteno, R.J.; Verónica-Aguilar, A.; Alarcón-Dionet, A.; Aguilar-Duran, H. IFNAR2 relevance in the clinical outcome of individuals with severe COVID-19. Front. Immunol. 2022, 13, 949413. [Google Scholar] [CrossRef] [PubMed]
- Nhung, V.P.; Ton, N.D.; Ngoc, T.T.B.; Thuong, M.T.H.; Hai, N.T.T.; Oanh, K.T.P.; Hien, L.T.T.; Thach, P.N.; Hai, N.V.; Ha, N.H. Host Genetic Risk Factors Associated with COVID-19 Susceptibility and Severity in Vietnamese. Genes 2022, 13, 1884. [Google Scholar] [CrossRef] [PubMed]
- Frodsham, A.J.; Zhang, L.; Dumpis, U.; Taib, N.A.M.; Best, S.; Durham, A.; Hennig, B.J.W.; Hellier, S.; Knapp, S.; Wright, M. Class II cytokine receptor gene cluster is a major locus for hepatitis B persistence. Proc. Natl. Acad. Sci. USA 2006, 103, 9148. [Google Scholar] [CrossRef]
- Roncagalli, R.; Cucchetti, M.; Jarmuzynski, N.; Grégorie, C.; Bergot, E.; Audebert, S.; Baudelet, E.; Menoita, M.G.; Joachim, A.; Durand, S. The scaffolding function of the RLTPR protein explains its essential role for CD28 co-stimulation in mouse and human T cells. J. Exp. Med. 2016, 213, 2437–2457. [Google Scholar] [CrossRef]
- Alazami, A.M.; Al-Helale, M.; Alhissi, S.; Al-Saud, B.; Alajlan, H.; Monies, D.; Shah, Z.; Abouelhoda, M.; Arnaout, R.; Al-Dhekr, H. Novel CARMIL2 mutations in patients with variable clinical dermatitis, infections, and combined immunodeficiency. Front. Immunol. 2018, 9, 203. [Google Scholar] [CrossRef]

| Patient | Gene | Genotype | ID dbSNP | MAF | ACMG Classification | Pathway |
|---|---|---|---|---|---|---|
| HP-38 | IRF7 | c.887-2A>C/WT | rs766015923 | 5.95 × 10−6 | Likely pathogenic | Predisposition to viral infection |
| HP-15 | MEFV | p.Arg653His/WT | rs104895085 | 3.54 × 10−5 | VUS | Autoinflammatory disorders |
| MEFV | p.Ala317Val/WT | ND | - | VUS | Autoinflammatory disorders | |
| HP-37 | MEFV | p.Lys695Arg/WT | rs104895094 | 5.83 × 10−3 | VUS | Autoinflammatory disorders |
| HP-44 | MEFV | p.Lys695Arg/WT | rs104895094 | 5.83 × 10−3 | VUS | Autoinflammatory disorders |
| HP-3 | MEFV | p.Arg408Gln/WT | rs11466024 | 1.34 × 10−2 | VUS | Autoinflammatory disorders |
| MEFV | p.Pro369Ser/WT | rs11466023 | 1.47 × 10−2 | VUS | Autoinflammatory disorders | |
| HP-18 | MEFV | p.Arg408Gln/WT | rs11466024 | 1.34 × 10−2 | VUS | Autoinflammatory disorders |
| MEFV | p.Pro369Ser/WT | rs11466023 | 1.47 × 10−2 | VUS | Autoinflammatory disorders |
| Patient Cohort | Hardy–Weinberg Disequilibrium Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Gene Genotype | H-P (%) | AM-Risk-P (%) | p | eMAF | HWE (%) | p (H-P) | p (AM-Risk-P) |
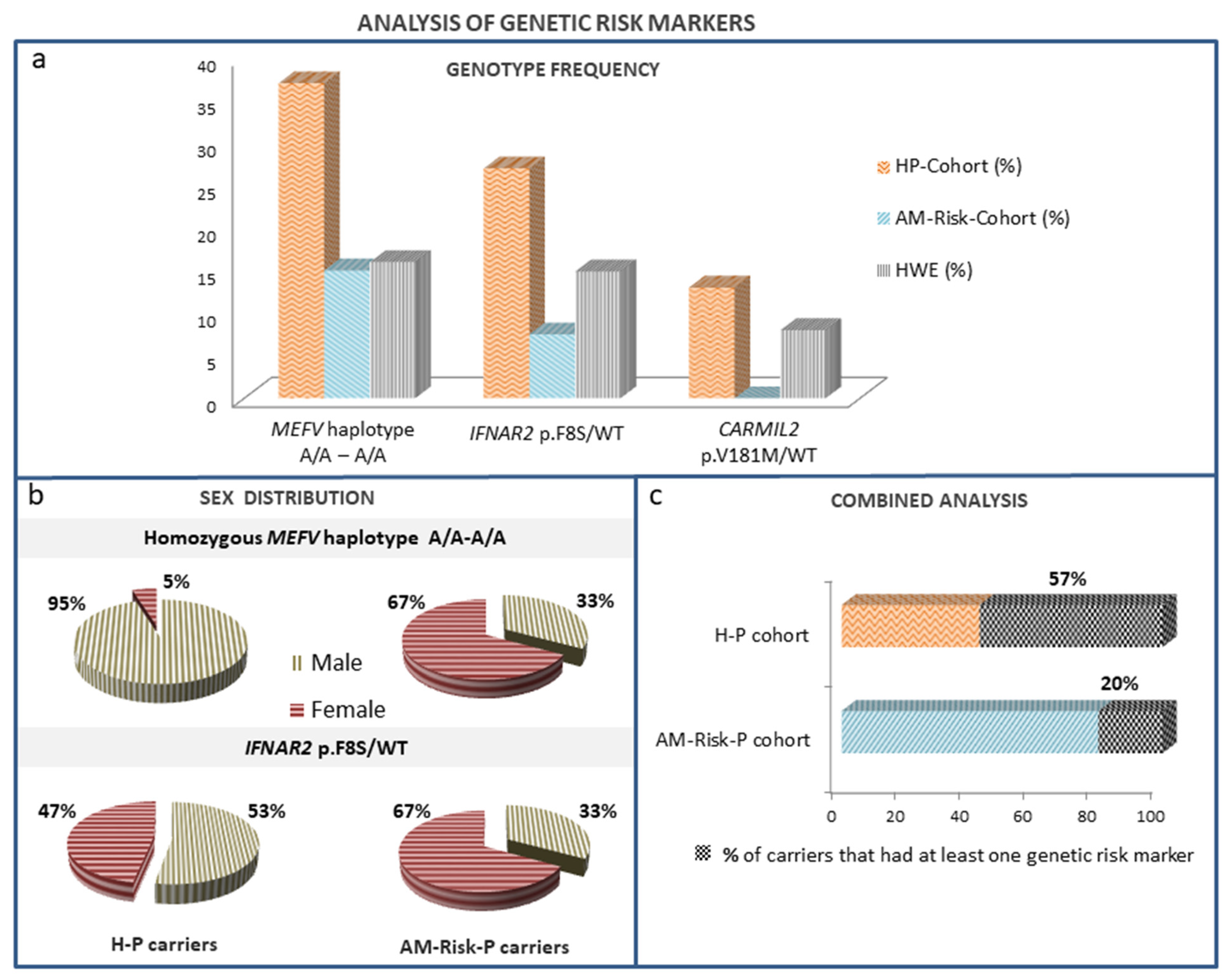
| MEFV Haplotype rs1231123A/A-rs1231122 A/A | 36.5 | 15 | <0.05 | 0.45 | 20.3 | <0.05 | NS |
| IFNAR2 p.Phe8Ser/WT | 28.8 | 7.5 | <0.05 | 0.08 | 14.7 | <0.05 | NS |
| CARMIL2 p.Val181Met/WT | 13 | 0 | <0.05 | 0.05 | 9.5 | NS | <0.01 |
| Risk Markers | OR | 95% CI |
|---|---|---|
| MEFV Haplotype rs1231123A/A-rs1231122 A/A | 3.143 | 1.123–8.793 |
| IFNAR2 Phe8Ser/WT | 4.500 | 1.204–16.826 |
| Combined analysis | 6.274 | 2.430–16.201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Pomar, N.; Cunill, V.; Segura-Guerrero, M.; Pol-Pol, E.; Escobar Oblitas, D.; Pons, J.; Ayestarán, I.; Pruneda, P.C.; Losada, I.; Toledo-Pons, N.; et al. Hyperinflammatory Immune Response in COVID-19: Host Genetic Factors in Pyrin Inflammasome and Immunity to Virus in a Spanish Population from Majorca Island. Biomedicines 2023, 11, 2548. https://doi.org/10.3390/biomedicines11092548
Martínez-Pomar N, Cunill V, Segura-Guerrero M, Pol-Pol E, Escobar Oblitas D, Pons J, Ayestarán I, Pruneda PC, Losada I, Toledo-Pons N, et al. Hyperinflammatory Immune Response in COVID-19: Host Genetic Factors in Pyrin Inflammasome and Immunity to Virus in a Spanish Population from Majorca Island. Biomedicines. 2023; 11(9):2548. https://doi.org/10.3390/biomedicines11092548
Chicago/Turabian StyleMartínez-Pomar, Natalia, Vanesa Cunill, Marina Segura-Guerrero, Elisabet Pol-Pol, Danilo Escobar Oblitas, Jaime Pons, Ignacio Ayestarán, Patricia C. Pruneda, Inés Losada, Nuria Toledo-Pons, and et al. 2023. "Hyperinflammatory Immune Response in COVID-19: Host Genetic Factors in Pyrin Inflammasome and Immunity to Virus in a Spanish Population from Majorca Island" Biomedicines 11, no. 9: 2548. https://doi.org/10.3390/biomedicines11092548
APA StyleMartínez-Pomar, N., Cunill, V., Segura-Guerrero, M., Pol-Pol, E., Escobar Oblitas, D., Pons, J., Ayestarán, I., Pruneda, P. C., Losada, I., Toledo-Pons, N., García Gasalla, M., & Ferrer Balaguer, J. M. (2023). Hyperinflammatory Immune Response in COVID-19: Host Genetic Factors in Pyrin Inflammasome and Immunity to Virus in a Spanish Population from Majorca Island. Biomedicines, 11(9), 2548. https://doi.org/10.3390/biomedicines11092548






