Optimizing Dental Bond Strength: Insights from Comprehensive Literature Review and Future Implications for Clinical Practice
Abstract
1. Introduction
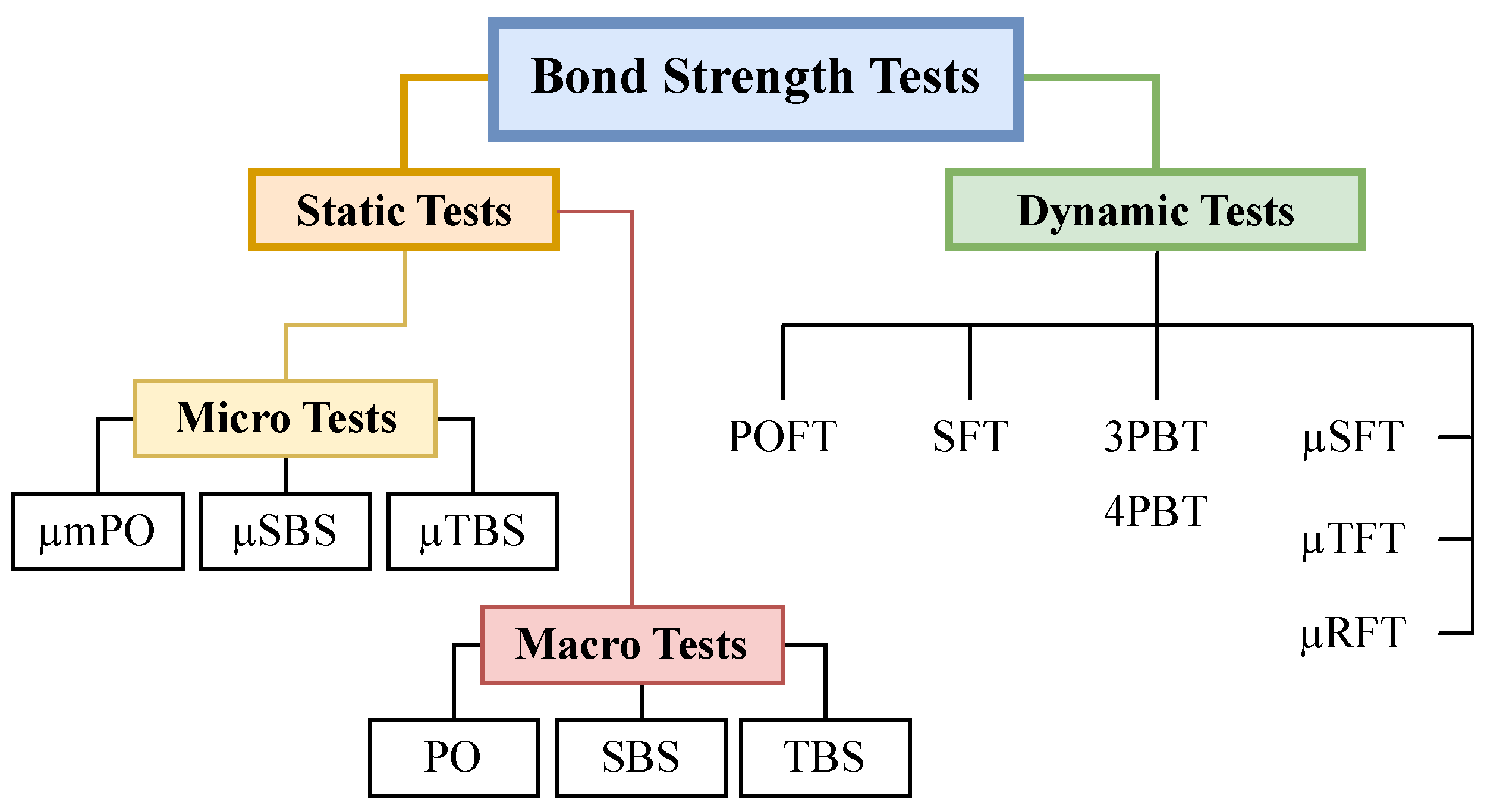
2. Bond Strength Testing Strategy
2.1. Dynamic Tests
2.2. Static Tests
3. Dentin-Resin Bonding
3.1. Smear Layer
3.2. Matrix Metalloproteinase (MMP)
3.3. Effects on MMP by Simplified Adhesive
3.4. MMP Inhibitors
- TIMPs
- Quaternary ammonium methacrylates (QAM)
- Protease inhibitors
- Tetracyclines and non-antimicrobial chemically modified tetracyclines (CMTs).
3.5. Hybrid Layer and Its Degradation
- The application of both etch-and-rinse and self-etch adhesive systems influences the bonding process.
- Elevated MMP levels and increased activity in adhesive-treated dentin can lead to a reduced inhibitory function of TIMPs, affecting the maintenance of bond strength.
- Saliva containing cholesterol esterase and pseudocholinesterase contributes to a decrease in bond strength. Furthermore, bacterial collagenases induce nanoleakage at the dentin-resin interface, and acids produced by cariogenic bacteria activate MMPs, resulting in a reduction in the durability of resin-dentin bonds.
3.6. Methods for Better Monomer Infiltration and Inhibition of Hybrid Layer Degradation
- Hydrophobic adhesives (application of a hydrophobic coating).
- Application of multiple layers.
- Extended polymerization time by lengthening the curing time.
- Increase solvent evaporation.
- Use of electric current that can enhance monomer infiltration in dentin.
- Adhesive with remineralization function.
- Antibacterial bonding system.
- MMP inhibitors.
4. Resin-Resin Bond
4.1. Impact of Contaminations on Resin-Resin Bond Strength
4.2. Blood
4.3. Saliva
5. Ceramic-Resin Bond
5.1. Nano Resin and Ceromer
5.2. Surface Pretreatment
5.3. Coloring Shades
6. Conclusions
- The micro test measurements are crucial for assessing bond strength in dental procedures, but they are underutilized due to their labor-intensive nature and sensitivity to technique.
- The condition of the smear layer on dentin significantly influences bond strength, emphasizing the importance of maintaining the proper phosphoric acid concentration during preparation and selecting appropriate bur types, such as superfine-grit or diamond burs.
- MMPs, particularly MMP-2 and MMP-9, pose challenges to dentin-resin bonding, with peak activity within the pH range of 2.3 to 4.5. The integration of MMP inhibitors into adhesive systems can effectively prevent hybrid layer degradation.
- Contaminants like blood and saliva can impact resin bonding, and effective remedies include thorough flushing with water and the adoption of wet bonding techniques involving ethanol and acetone.
- In the context of indirect restorations, ceromers are preferred for their superior wear resistance and bond strength compared to resin nanoceramics. Shade selection is also crucial, with A3 having minimal impact on bond strength, while shades B2 and C1 exhibit a linear decrease, and D4 contributes to an increase in bond strength.
Author Contributions
Funding
Conflicts of Interest
References
- Cramer, N.B.; Stansbury, J.W.; Bowman, C.N. Recent advances and developments in composite dental restorative materials. J. Dent. Res. 2011, 90, 402–416. [Google Scholar] [CrossRef] [PubMed]
- Pratap, B.; Gupta, R.K.; Bhardwaj, B.; Nag, M. Resin based restorative dental materials: Characteristics and future perspectives. Jpn. Dent. Sci. Rev. 2019, 55, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Mathur, S.; Sachdev, V.; Singh, D. Evaluation of compressive strength, shear bond strength, and microhardness values of glass-ionomer cement Type IX and Cention N. J. Conserv. Dent. 2020, 23, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Askar, H.; Krois, J.; Göstemeyer, G.; Bottenberg, P.; Zero, D.; Banerjee, A.; Schwendicke, F. Secondary caries: What is it, and how it can be controlled, detected, and managed? Clin. Oral Investig. 2020, 24, 1869–1876. [Google Scholar] [CrossRef]
- Dionysopoulos, D.; Gerasimidou, O. Wear of contemporary dental composite resin restorations: A literature review. Restor. Dent. Endod. 2021, 46, e18. [Google Scholar] [CrossRef]
- Cardoso, M.; de Almeida Neves, A.; Mine, A.; Coutinho, E.; Van Landuyt, K.; De Munck, J.; Van Meerbeek, B. Current aspects on bonding effectiveness and stability in adhesive dentistry. Aust. Dent. J. 2011, 56, 31–44. [Google Scholar] [CrossRef]
- El Mourad, A.M. Assessment of Bonding Effectiveness of Adhesive Materials to Tooth Structure using Bond Strength Test Methods: A Review of Literature. Open Dent. J. 2018, 12, 664–678. [Google Scholar] [CrossRef]
- Papia, E.; Larsson, C.; du Toit, M.; von Steyern, P.V. Bonding between oxide ceramics and adhesive cement systems: A systematic review. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 395–413. [Google Scholar] [CrossRef]
- Kudva, A.; Raghunath, A.; PM, S.N.; Shetty, H.K.; D’Costa, V.F.; Jayaprakash, K. Comparative evaluation of shear bond strength of a bioactive material to composite resin using three different universal bonding agents: An in vitro study. J. Conserv. Dent. 2022, 25, 54–57. [Google Scholar]
- Bahrololoomi, Z.; Mehravar, F. Comparison of different adhesive systems on bond strength of resin composite posts placed in primary teeth. Int. J. Dent. 2022, 2022, 1968781. [Google Scholar] [CrossRef]
- Ye, S.; Lin, J.-C.; Kang, L.-L.; Li, C.-L.; Hou, S.-S.; Lee, T.-L.; Chuang, S.-F. Investigations of silane-MDP interaction in universal adhesives: A ToF-SIMS analysis. Dent. Mater. 2022, 38, 183–193. [Google Scholar] [CrossRef]
- Peng, T.-Y.; Kang, C.-M.; Feng, S.-W.; Hung, C.-Y.; Iwaguro, S.; Lin, D.-J. Effects of glass-ceramic spray deposition manipulation on the surface characteristics of zirconia dental restorations. Ceram. Int. 2022, 48, 29873–29881. [Google Scholar] [CrossRef]
- Younis, M.; Unkovskiy, A.; Drexler, T.; Qian, J.; Wan, G.; Spintzyk, S. The impact of non-thermal plasma on the adhesion of polyetherketoneketone (PEKK) to a veneering composite system. J. Mech. Behav. Biomed. Mater. 2020, 112, 104065. [Google Scholar]
- Ye, S.; Chuang, S.F.; Hou, S.S.; Lin, J.C.; Kang, L.L.; Chen, Y.C. Interaction of silane with 10-MDP on affecting surface chemistry and resin bonding of zirconia. Dent. Mater. 2022, 38, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Mazumdara, P.; Singhb, S.; Das, D. Method for assessing the bond strength of dental restorative materials: An overview. J. Pierre Fauchard Acad. 2021, 35, 73–77. [Google Scholar] [CrossRef]
- Poitevin, A.; De Munck, J.; Cardoso, M.V.; Mine, A.; Peumans, M.; Lambrechts, P.; Van Meerbeek, B. Dynamic versus static bond-strength testing of adhesive interfaces. Dent. Mater. 2010, 26, 1068–1076. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; De Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; Van Landuyt, K.; Lambrechts, P.; Vanherle, G. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. Univ. Wash. 2003, 28, 215–235. [Google Scholar]
- Tezvergil-Mutluay, A.; Pashley, D.; Mutluay, M.M. Long-term durability of dental adhesives. Curr. Oral Health Rep. 2015, 2, 174–181. [Google Scholar] [CrossRef]
- Flury, S.; Lussi, A.; Peutzfeldt, A.; Zimmerli, B. Push-out bond strength of CAD/CAM-ceramic luted to dentin with self-adhesive resin cements. Dent. Mater. 2010, 26, 855–863. [Google Scholar] [CrossRef]
- Baldea, B.; Gabriel, F.; Antal, M.; Nagy, K.; Popescu, D.; Nica, L. Push-out bond strength and SEM analysis of two self-adhesive resin cements: An in vitro study. J. Dent. Sci. 2013, 8, 296–305. [Google Scholar] [CrossRef]
- Holderegger, C.; Sailer, I.; Schuhmacher, C.; Schläpfer, R.; Hämmerle, C.; Fischer, J. Shear bond strength of resin cements to human dentin. Dent. Mater. 2008, 24, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Sunnegårdh-Grönberg, K.; Peutzfeldt, A.; van Dijken, J.W.V. Flexural strength and modulus of a novel ceramic restorative cement intended for posterior restorations as determined by a three-point bending test. Acta Odontol. Scand. 2003, 61, 87–92. [Google Scholar] [CrossRef]
- Junior, S.A.R.; Ferracane, J.L.; Bona, Á.D. Flexural strength and Weibull analysis of a microhybrid and a nanofill composite evaluated by 3- and 4-point bending tests. Dent. Mater. 2008, 24, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Mirmohammadi, H.; Aboushelib, M.N.; Kleverlaan, C.J.; de Jager, N.; Feilzer, A.J. The influence of rotating fatigue on the bond strength of zirconia-composite interfaces. Dent. Mater. 2010, 26, 627–633. [Google Scholar] [CrossRef]
- Nalla, R.K.; Kinney, J.H.; Marshall, S.J.; Ritchie, R.O. On the in vitro fatigue behavior of human dentin: Effect of mean stress. J. Dent. Res. 2004, 83, 211–215. [Google Scholar] [CrossRef]
- Nalla, R.K.; Imbeni, V.; Kinney, J.H.; Staninec, M.; Marshall, S.J.; Ritchie, R.O. In Vitro fatigue behavior of human dentin with implications for life prediction. J. Biomed. Mater. Res. Part. A 2003, 66, 10–20. [Google Scholar] [CrossRef]
- Staninec, M.; Kim, P.; Marshall, G.W.; Ritchie, R.O.; Marshall, S.J. Fatigue of dentin-composite interfaces with four-point bend. Dent. Mater. 2008, 24, 799–803. [Google Scholar] [CrossRef]
- Pashley, D.H.; Carvalho, R.M.; Sano, H.; Nakajima, M.; Yoshiyama, M.; Shono, Y.; Fernandes, C.A.; Tay, F. The microtensile bond test: A review. J. Adhes. Dent. 1999, 1, 299–309. [Google Scholar] [PubMed]
- Sirisha, K.; Rambabu, T.; Shankar, Y.R.; Ravikumar, P. Validity of bond strength tests: A critical review: Part I. J. Conserv. Dent. 2014, 17, 305–311. [Google Scholar] [CrossRef]
- Chang, H.-S.; Noh, Y.-S.; Lee, Y.; Min, K.-S.; Bae, J.-M. Push-out bond strengths of fiber-reinforced composite posts with various resin cements according to the root level. J. Adv. Prosthodont. 2013, 5, 278–286. [Google Scholar] [CrossRef]
- İşman, E.; Karaarslan, E.Ş.; Okşayan, R.; Tunçdemir, A.R.; Üşümez, S.; Adanir, N.; Cebe, M.A. Inadequate shear bond strengths of self-etch, self-adhesive systems for secure orthodontic bonding. Dent. Mater. J. 2012, 31, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Erdemir, U.; Sancakli, H.; Yildiz, E.; Ozel, S.; Batur, Y.B. An in vitro comparison of different adhesive strategies on the micro push-out bond strength of a glass fiber post. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e626–e634. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimada, Y.; Yamaguchi, S.; Tagami, J. Micro-shear bond strength of dual-cured resin cement to glass ceramics. Dent. Mater. 2002, 18, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Meira, J.B.; Boaro, L.C.; Xavier, T.A. Adhesion to tooth structure: A critical review of “macro” test methods. Dent. Mater. 2010, 26, e38–e49. [Google Scholar] [CrossRef]
- Sirisha, K.; Rambabu, T.; Ravishankar, Y.; Ravikumar, P. Validity of bond strength tests: A critical review-Part II. J. Conserv. Dent. 2014, 17, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Viswanath, D.; Hegde, A.M.; Munshi, A.K. The removal of the smear layer using EGTA: A scanning electron microscopic study. J. Clin. Pediatr. Dent. 2003, 28, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zehnder, M. Root canal irrigants. J. Endod. 2006, 32, 389–398. [Google Scholar] [CrossRef]
- Kotb, R.M.; Elkateb, M.A.; Ahmed, A.M.; Kawana, K.Y.; El Meligy, O.A. Dentin Topographic Features following Chemomechanical Caries Removal in Primary Teeth. J. Clin. Pediatr. Dent. 2016, 40, 472–479. [Google Scholar] [CrossRef]
- Saikaew, P.; Sattabanasuk, V.; Harnirattisai, C.; Chowdhury, A.; Carvalho, R.; Sano, H. Role of the smear layer in adhesive dentistry and the clinical applications to improve bonding performance. Jpn. Dent. Sci. Rev. 2022, 58, 59–66. [Google Scholar] [CrossRef]
- Koibuchi, H.; Yasuda, N.; Nakabayashi, N. Bonding to dentin with a self-etching primer: The effect of smear layers. Dent. Mater. 2001, 17, 122–126. [Google Scholar] [CrossRef]
- Ayad, M.F.; Johnston, W.M.; Rosenstiel, S.F. Influence of dental rotary instruments on the roughness and wettability of human dentin surfaces. J. Prosthet. Dent. 2009, 102, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Peerzada, F.; Yiu, C.K.; Hiraishi, N.; Tay, F.R.; King, N.M. Effect of surface preparation on bond strength of resin luting cements to dentin. Oper. Dent. 2010, 35, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Sattabanasuk, V.; Vachiramon, V.; Qian, F.; Armstrong, S.R. Resin-dentin bond strength as related to different surface preparation methods. J. Dent. 2007, 35, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Saikaew, P.; Chowdhury, A.; Matsumoto, M.; Carvalho, R.M.; Sano, H. Effects of Double Application of a Resin Cement Primer and Different Diamond Burs on Cement-Dentin Bond Strength. J. Adhes. Dent. 2020, 22, 311–320. [Google Scholar]
- Cerqueira, L.A.C.; Costa, A.R.; Spohr, A.M.; Miyashita, E.; Miranzi, B.A.S.; Calabrez Filho, S.; Correr-Sobrinho, L.; Borges, G.A. Effect of Dentin Preparation Mode on the Bond Strength between Human Dentin and Different Resin Cements. Braz. Dent. J. 2018, 29, 268–274. [Google Scholar] [CrossRef]
- Bahari, M.; Oskoee, S.S.; Chaharom, M.E.E.; Kahnamoui, M.A.; Gholizadeh, S.; Davoodi, F. Effect of accelerated aging and double application on the dentin bond strength of universal adhesive system. Dent. Res. J. 2021, 18, 25. [Google Scholar]
- Rirattanapong, P.; Senawongse, P.; Harnirattisal, C.; Wunsiw, W. Effect of Smear Layers Created by Different Burs on Durability of Self-Etching Adhesive Bond to Dentin of Primary Teeth. J. Clin. Pediatr. Dent. 2015, 39, 224–230. [Google Scholar] [CrossRef]
- Sauro, S.; Faus-Matoses, V.; Makeeva, I.; Nuñez Martí, J.M.; Gonzalez Martínez, R.; García Bautista, J.A.; Faus-Llácer, V. Effects of Polyacrylic Acid Pre-Treatment on Bonded-Dentine Interfaces Created with a Modern Bioactive Resin-Modified Glass Ionomer Cement and Subjected to Cycling Mechanical Stress. Materials 2018, 11, 1884. [Google Scholar] [CrossRef]
- Ayad, M.F.; Maghrabi, A.A.; Saif, R.E.; García-Godoy, F. Influence of tooth preparation burs on the roughness and bond strength of adhesives to human dentin surfaces. Am. J. Dent. 2011, 24, 176–182. [Google Scholar]
- Muana, H.L.; Nassar, M.; Dargham, A.; Hiraishi, N.; Tagami, J. Effect of smear layer removal agents on the microhardness and roughness of radicular dentin. Saudi Dent. J. 2021, 33, 661–665. [Google Scholar] [CrossRef]
- Breschi, L.; Mazzoni, A.; Ruggeri, A.; Cadenaro, M.; Di Lenarda, R.; De Stefano Dorigo, E. Dental adhesion review: Aging and stability of the bonded interface. Dent. Mater. 2008, 24, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Kern, M. The role of host-derived dentinal matrix metalloproteinases in reducing dentin bonding of resin adhesives. Int. J. Oral Sci. 2009, 1, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Tjäderhane, L.; Checchi, V.; Di Lenarda, R.; Salo, T.; Tay, F.R.; Pashley, D.H.; Breschi, L. Role of dentin MMPs in caries progression and bond stability. J. Dent. Res. 2015, 94, 241–251. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, S.; Zhou, X.; Hannig, M.; Rupf, S.; Feng, J.; Peng, X.; Cheng, L. Modifying Adhesive Materials to Improve the Longevity of Resinous Restorations. Int. J. Mol. Sci. 2019, 20, 723. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Angeloni, V.; Comba, A.; Maravic, T.; Cadenaro, M.; Tezvergil-Mutluay, A.; Pashley, D.H.; Tay, F.R.; Breschi, L. Cross-linking effect on dentin bond strength and MMPs activity. Dent. Mater. 2018, 34, 288–295. [Google Scholar] [CrossRef]
- Scheffel, D.L.; Hebling, J.; Scheffel, R.H.; Agee, K.A.; Cadenaro, M.; Turco, G.; Breschi, L.; Mazzoni, A.; de Souza Costa, C.A.; Pashley, D.H. Stabilization of dentin matrix after cross-linking treatments, in vitro. Dent. Mater. 2014, 30, 227–233. [Google Scholar] [CrossRef]
- Venigalla, B.S.; Jyothi, P.; Kamishetty, S.; Reddy, S.; Cherukupalli, R.C.; Reddy, D.A. Resin bond strength to water versus ethanol-saturated human dentin pretreated with three different cross-linking agents. J. Conserv. Dent. JCD 2016, 19, 555. [Google Scholar] [CrossRef]
- Breschi, L.; Martin, P.; Mazzoni, A.; Nato, F.; Carrilho, M.; Tjäderhane, L.; Visintini, E.; Cadenaro, M.; Tay, F.R.; De Stefano Dorigo, E.; et al. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent. Mater. 2010, 26, 571–578. [Google Scholar] [CrossRef]
- Hardan, L.; Daood, U.; Bourgi, R.; Cuevas-Suárez, C.E.; Devoto, W.; Zarow, M.; Jakubowicz, N.; Zamarripa-Calderón, J.E.; Radwanski, M.; Orsini, G.; et al. Effect of Collagen Crosslinkers on Dentin Bond Strength of Adhesive Systems: A Systematic Review and Meta-Analysis. Cells 2022, 11, 2417. [Google Scholar] [CrossRef]
- Lingling, J.; Qianbing, W. Progress on matrix metalloproteinase inhibitors. Hua Xi Kou Qiang Yi Xue Za Zhi 2017, 35, 208–214. [Google Scholar]
- Mazzoni, A.; Nascimento, F.D.; Carrilho, M.; Tersariol, I.; Papa, V.; Tjäderhane, L.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Breschi, L. MMP activity in the hybrid layer detected with in situ zymography. J. Dent. Res. 2012, 91, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Moracho, N.; Learte, A.I.R.; Muñoz-Sáez, E.; Marchena, M.A.; Cid, M.A.; Arroyo, A.G.; Sánchez-Camacho, C. Emerging roles of MT-MMPs in embryonic development. Dev. Dyn. 2022, 251, 240–275. [Google Scholar] [CrossRef] [PubMed]
- de Andrade e Silva, S.M.; Carrilho, M.R.; Marquezini Junior, L.; Garcia, F.C.; Manso, A.P.; Alves, M.C.; de Carvalho, R.M. Effect of an additional hydrophilic versus hydrophobic coat on the quality of dentinal sealing provided by two-step etch-and-rinse adhesives. J. Appl. Oral Sci. 2009, 17, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Nishitani, Y.; Yoshiyama, M.; Donnelly, A.M.; Agee, K.A.; Sword, J.; Tay, F.R.; Pashley, D.H. Effects of resin hydrophilicity on dentin bond strength. J. Dent. Res. 2006, 85, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.A.; Yaman, P.; Dennison, J.B. Polymerization shrinkage and depth of cure of packable composites. Quintessence Int. 2005, 36, 25–31. [Google Scholar] [PubMed]
- Yoo, H.M.; Pereira, P.N. Effect of blood contamination with 1-step self-etching adhesives on microtensile bond strength to dentin. Oper. Dent. 2006, 31, 660–665. [Google Scholar] [CrossRef]
- Soetopo; Beech, D.R.; Hardwick, J.L. Mechanism of adhesion of polymers to acid-etched enamel. Effect of acid concentration and washing on bond strength. J. Oral Rehabil. 1978, 5, 69–80. [Google Scholar] [CrossRef]
- Taneja, S.; Kumari, M.; Bansal, S. Effect of saliva and blood contamination on the shear bond strength of fifth-, seventh-, and eighth-generation bonding agents: An in vitro study. J. Conserv. Dent. 2017, 20, 157–160. [Google Scholar] [CrossRef]
- Eiriksson, S.O.; Pereira, P.N.; Swift, E.J.; Heymann, H.O.; Sigurdsson, A. Effects of blood contamination on resin-resin bond strength. Dent. Mater. 2004, 20, 184–190. [Google Scholar] [CrossRef]
- Kanca, J., 3rd. Effect of resin primer solvents and surface wetness on resin composite bond strength to dentin. Am. J. Dent. 1992, 5, 213–215. [Google Scholar]
- Jacobsen, T.; Söderholm, K.J. Some effects of water on dentin bonding. Dent. Mater. 1995, 11, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Anzlovar, A.; Kiteska, B.; Cevc, P.; Kopač, I. The role of an interfacial interpenetrating polymer network formation on the adhesion of resin composite layers in immediate dentin sealing. Int. J. Adhes. Adhes. 2019, 90, 9–14. [Google Scholar] [CrossRef]
- Carneiro, D.; Generoso, G.; Ferreira, I.; Borges, B.; Freitas, C.; Silva, L. Resin–resin microtensile bond strength after different surface treatments to clean blood contamination. Rev. Port. Estomatol. Med. Dent. Cir. Maxilofac. 2015, 56, 215–220. [Google Scholar] [CrossRef]
- Fawzy, A.S.; El-Askary, F.S.; Amer, M.A. Effect of surface treatments on the tensile bond strength of repaired water-aged anterior restorative micro-fine hybrid resin composite. J. Dent. 2008, 36, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Eiriksson, S.O.; Pereira, P.N.; Swift, E.J., Jr.; Heymann, H.O.; Sigurdsson, A. Effects of saliva contamination on resin-resin bond strength. Dent. Mater. 2004, 20, 37–44. [Google Scholar] [CrossRef]
- Shahdad, S.A.; Kennedy, J.G. Bond strength of repaired anterior composite resins: An in vitro study. J. Dent. 1998, 26, 685–694. [Google Scholar] [CrossRef]
- Baier, R.E.; Shafrin, E.G.; Zisman, W.A. Adhesion: Mechanisms that assist or impede it. Science 1968, 162, 1360–1368. [Google Scholar] [CrossRef]
- Furuse, A.Y.; da Cunha, L.F.; Benetti, A.R.; Mondelli, J. Bond strength of resin-resin interfaces contaminated with saliva and submitted to different surface treatments. J. Appl. Oral Sci. 2007, 15, 501–505. [Google Scholar] [CrossRef]
- Brosh, T.; Pilo, R.; Bichacho, N.; Blutstein, R. Effect of combinations of surface treatments and bonding agents on the bond strength of repaired composites. J. Prosthet. Dent. 1997, 77, 122–126. [Google Scholar] [CrossRef]
- Bouschlicher, M.R.; Reinhardt, J.W.; Vargas, M.A. Surface treatment techniques for resin composite repair. Am. J. Dent. 1997, 10, 279–283. [Google Scholar]
- Barutcigil, K.; Barutcigil, Ç.; Kul, E.; Özarslan, M.M.; Buyukkaplan, U.S. Effect of Different Surface Treatments on Bond Strength of Resin Cement to a CAD/CAM Restorative Material. J. Prosthodont. 2019, 28, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Bottino, M.A.; Snellaert, A.; Bergoli, C.D.; Özcan, M.; Bottino, M.C.; Valandro, L.F. Effect of ceramic etching protocols on resin bond strength to a feldspar ceramic. Oper. Dent. 2015, 40, E40–E46. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, J.J.; Choi, J.Y.; Seo, J.M. Influence of nano-structured alumina coating on shear bond strength between Y-TZP ceramic and various dual-cured resin cements. J. Adv. Prosthodont. 2017, 9, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N.; Kleverlaan, C.J.; Feilzer, A.J. Selective infiltration-etching technique for a strong and durable bond of resin cements to zirconia-based materials. J. Prosthet. Dent. 2007, 98, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Mahshid, M.; Berijani, N.; Sadr, S.J.; Tabatabaian, F.; Homayoon, S.S. Effect of Coloring-by-Dipping on Microtensile Bond Strength of Zirconia to Resin Cement. J. Dent. 2015, 12, 414–423. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan-Chiang, Y.-S.; Chou, P.-C.; Hsiao, Y.-W.; Cheng, Y.-H.; Huang, Y.; Chiu, Y.-C.; Lin, Y.-J.; Mine, Y.; Feng, S.-W.; Lee, I.-T.; et al. Optimizing Dental Bond Strength: Insights from Comprehensive Literature Review and Future Implications for Clinical Practice. Biomedicines 2023, 11, 2995. https://doi.org/10.3390/biomedicines11112995
Fan-Chiang Y-S, Chou P-C, Hsiao Y-W, Cheng Y-H, Huang Y, Chiu Y-C, Lin Y-J, Mine Y, Feng S-W, Lee I-T, et al. Optimizing Dental Bond Strength: Insights from Comprehensive Literature Review and Future Implications for Clinical Practice. Biomedicines. 2023; 11(11):2995. https://doi.org/10.3390/biomedicines11112995
Chicago/Turabian StyleFan-Chiang, Yung-Shin, Peng-Chen Chou, Yu-Wen Hsiao, Yu-Hsuan Cheng, Yi Huang, Yu-Chieh Chiu, Yu-Ju Lin, Yuichi Mine, Sheng-Wei Feng, I-Ta Lee, and et al. 2023. "Optimizing Dental Bond Strength: Insights from Comprehensive Literature Review and Future Implications for Clinical Practice" Biomedicines 11, no. 11: 2995. https://doi.org/10.3390/biomedicines11112995
APA StyleFan-Chiang, Y.-S., Chou, P.-C., Hsiao, Y.-W., Cheng, Y.-H., Huang, Y., Chiu, Y.-C., Lin, Y.-J., Mine, Y., Feng, S.-W., Lee, I.-T., & Peng, T.-Y. (2023). Optimizing Dental Bond Strength: Insights from Comprehensive Literature Review and Future Implications for Clinical Practice. Biomedicines, 11(11), 2995. https://doi.org/10.3390/biomedicines11112995






