Evaluating the Causal Association between Inflammatory Bowel Disease and Risk of Atherosclerotic Cardiovascular Disease: Univariable and Multivariable Mendelian Randomization Study
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Selection of Genetic Variants for IBD
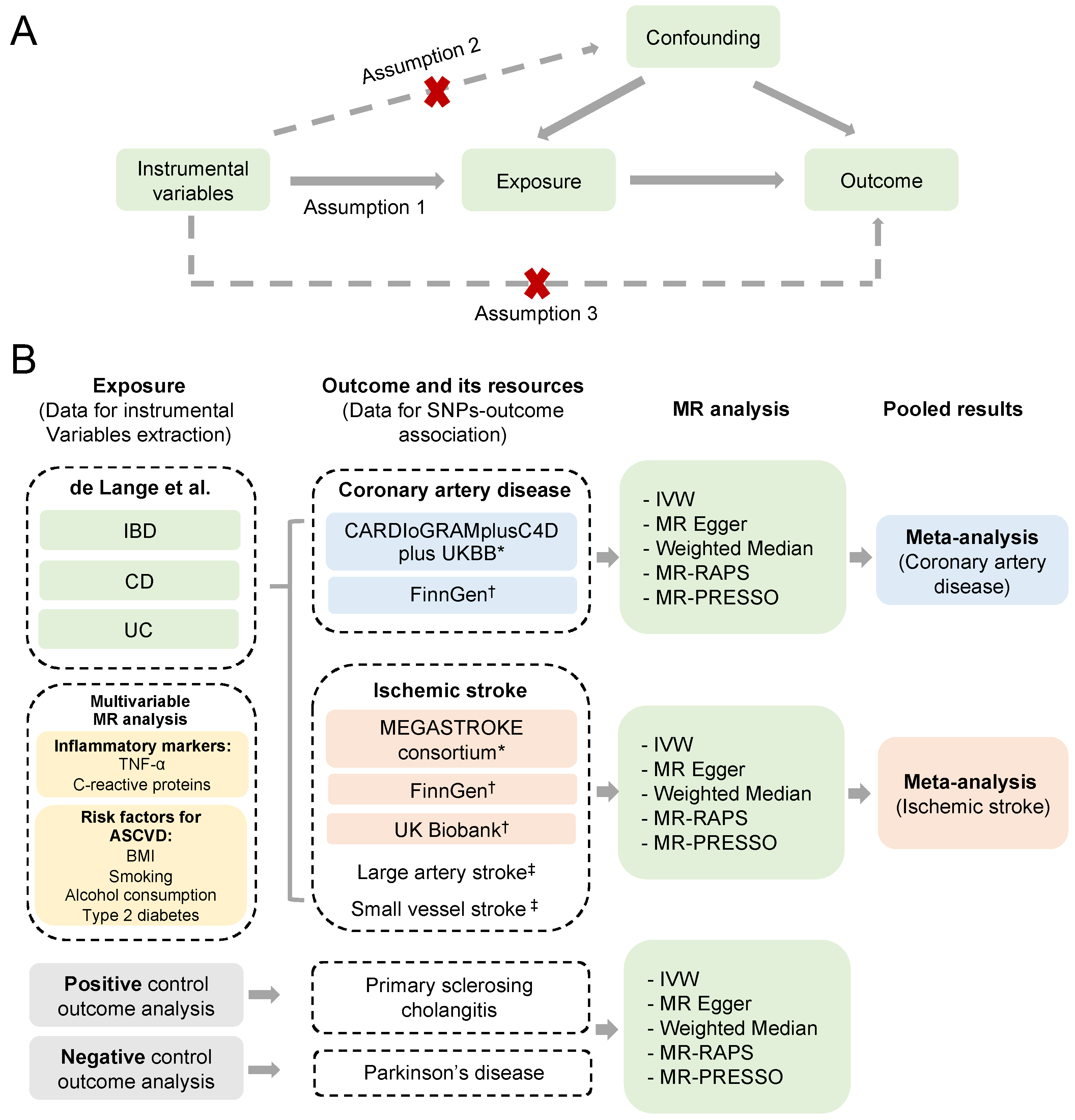
2.3. Summary Statistics for Atherosclerotic Cardiovascular Disease
2.4. Summary Statistics for Negative and Positive Control Outcomes
2.5. Statistical Analyses
3. Results
3.1. Genetic Instruments
3.2. Main Analysis
3.3. Replication and Meta-Analysis
3.4. Multivariable MR Analysis
3.5. Positive and Negative Control Outcome Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liu, J.; Wang, M.; Zhang, X.; Zhou, M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019, 16, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Bigeh, A.; Sanchez, A.; Maestas, C.; Gulati, M. Inflammatory bowel disease and the risk for cardiovascular disease: Does all inflammation lead to heart disease? Trends Cardiovasc. Med. 2020, 30, 463–469. [Google Scholar] [CrossRef]
- Rungoe, C.; Basit, S.; Ranthe, M.F.; Wohlfahrt, J.; Langholz, E.; Jess, T. Risk of ischaemic heart disease in patients with inflammatory bowel disease: A nationwide Danish cohort study. Gut 2013, 62, 689–694. [Google Scholar] [CrossRef]
- Fang, L.; Gao, H.; Gao, X.; Wu, W.; Miao, Y.; Zhang, H.; Guleng, B.; Zhang, H.; Wang, Y.; Li, M.; et al. Risks of Cardiovascular Events in Patients With Inflammatory Bowel Disease in China: A Retrospective Multicenter Cohort Study. Inflamm. Bowel Dis. 2022, 28 (Suppl. S2), S52–S58. [Google Scholar] [CrossRef]
- Singh, S.; Singh, H.; Loftus, E.V., Jr.; Pardi, D.S. Risk of cerebrovascular accidents and ischemic heart disease in patients with inflammatory bowel disease: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2014, 12, 382–393.e381: Quiz e322. [Google Scholar] [CrossRef]
- Feng, W.; Chen, G.; Cai, D.; Zhao, S.; Cheng, J.; Shen, H. Inflammatory Bowel Disease and Risk of Ischemic Heart Disease: An Updated Meta-Analysis of Cohort Studies. J. Am. Heart Assoc. 2017, 6, e005892. [Google Scholar] [CrossRef]
- Sleutjes, J.A.M.; van der Woude, C.J.; Verploegh, P.J.P.; Aribas, E.; Kavousi, M.; Roeters van Lennep, J.E.; de Vries, A.C. Cardiovascular risk profiles in patients with Inflammatory Bowel Disease differ from matched controls from the general population. Eur. J. Prev. Cardiol. 2023, corrected proof. [Google Scholar] [CrossRef]
- Sun, J.; Halfvarson, J.; Appelros, P.; Bergman, D.; Ebrahimi, F.; Roelstraete, B.; Olén, O.; Ludvigsson, J.F. Long-term Risk of Stroke in Patients With Inflammatory Bowel Disease: A Population-Based, Sibling-Controlled Cohort Study, 1969–2019. Neurology 2023, 101, e653–e664. [Google Scholar] [CrossRef]
- Davey Smith, G.; Phillips, A.N. Correlation without a cause: An epidemiological odyssey. Int. J. Epidemiol. 2020, 49, 4–14. [Google Scholar] [CrossRef]
- Bochud, M. On the use of Mendelian randomization to infer causality in observational epidemiology. Eur. Heart J. 2008, 29, 2456–2457. [Google Scholar] [CrossRef]
- van der Harst, P.; Verweij, N. Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ. Res. 2018, 122, 433–443. [Google Scholar] [CrossRef]
- Malik, R.; Chauhan, G.; Traylor, M.; Sargurupremraj, M.; Okada, Y.; Mishra, A.; Rutten-Jacobs, L.; Giese, A.K.; van der Laan, S.W.; Gretarsdottir, S.; et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 2018, 50, 524–537. [Google Scholar] [CrossRef]
- de Lange, K.M.; Moutsianas, L.; Lee, J.C.; Lamb, C.A.; Luo, Y.; Kennedy, N.A.; Jostins, L.; Rice, D.L.; Gutierrez-Achury, J.; Ji, S.G.; et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat. Genet. 2017, 49, 256–261. [Google Scholar] [CrossRef]
- Davey Smith, G.; Hemani, G. Mendelian randomization: Genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 2014, 23, R89–R98. [Google Scholar] [CrossRef]
- Davies, N.M.; Holmes, M.V.; Davey Smith, G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ 2018, 362, k601. [Google Scholar] [CrossRef]
- Kurki, M.I.; Karjalainen, J.; Palta, P.; Sipilä, T.P.; Kristiansson, K.; Donner, K.; Reeve, M.P.; Laivuori, H.; Aavikko, M.; Kaunisto, M.A.; et al. FinnGen: Unique genetic insights from combining isolated population and national health register data. medRxiv 2022. [Google Scholar] [CrossRef]
- Burgess, S.; Davey Smith, G.; Davies, N.M.; Dudbridge, F.; Gill, D.; Glymour, M.M.; Hartwig, F.P.; Holmes, M.V.; Minelli, C.; Relton, C.L.; et al. Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 2019, 4, 186. [Google Scholar] [CrossRef]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Yarmolinsky, J.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Higgins, J.P.T.; Timpson, N.J.; Dimou, N.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomization: The STROBE-MR Statement. JAMA 2021, 326, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Brion, M.-J.A.; Shakhbazov, K.; Visscher, P.M. Calculating statistical power in Mendelian randomization studies. Int. J. Epidemiol. 2013, 42, 1497–1501. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.-G.; Juran, B.D.; Mucha, S.; Folseraas, T.; Jostins, L.; Melum, E.; Kumasaka, N.; Atkinson, E.J.; Schlicht, E.M.; Liu, J.Z.; et al. Genome-wide association study of primary sclerosing cholangitis identifies new risk loci and quantifies the genetic relationship with inflammatory bowel disease. Nat. Genet. 2017, 49, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A.; et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Fausa, O.; Schrumpf, E.; Elgjo, K. Relationship of inflammatory bowel disease and primary sclerosing cholangitis. Semin. Liver Dis. 1991, 11, 31–39. [Google Scholar] [CrossRef]
- Karlsen, T.H.; Folseraas, T.; Thorburn, D.; Vesterhus, M. Primary sclerosing cholangitis—A comprehensive review. J. Hepatol. 2017, 67, 1298–1323. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Burgess, S. Mendelian randomization with invalid instruments: Effect estimation and bias detection through Egger regression. Int. J. Epidemiol. 2015, 44, 512–525. [Google Scholar] [CrossRef]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, J.; Hemani, G.; Bowden, J.; Small, D.S. Statistical inference in two-sample summary-data Mendelian randomization using robust adjusted profile score. Ann. Stat. 2020, 48, 1742–1769. [Google Scholar] [CrossRef]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Hemani, G.; Bowden, J.; Davey Smith, G. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum. Mol. Genet. 2018, 27, R195–R208. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, E.; Spiller, W.; Bowden, J. Testing and correcting for weak and pleiotropic instruments in two-sample multivariable Mendelian randomization. Stat. Med. 2021, 40, 5434–5452. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Reinisch, W.; Colombel, J.F.; Mantzaris, G.J.; Kornbluth, A.; Diamond, R.; Rutgeerts, P.; Tang, L.K.; Cornillie, F.J.; Sandborn, W.J. Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn’s disease in the SONIC trial. Gut 2014, 63, 88–95. [Google Scholar] [CrossRef]
- Kirchgesner, J.; Beaugerie, L.; Carrat, F.; Andersen, N.N.; Jess, T.; Schwarzinger, M. Increased risk of acute arterial events in young patients and severely active IBD: A nationwide French cohort study. Gut 2018, 67, 1261–1268. [Google Scholar] [CrossRef]
- Aarestrup, J.; Jess, T.; Kobylecki, C.J.; Nordestgaard, B.G.; Allin, K.H. Cardiovascular Risk Profile among Patients with Inflammatory Bowel Disease: A Population-based Study of More than 100,000 Individuals. J. Crohn’s Colitis 2019, 13, 319–323. [Google Scholar] [CrossRef]
- Barnes, E.L.; Beery, R.M.; Schulman, A.R.; McCarthy, E.P.; Korzenik, J.R.; Winter, R.W. Hospitalizations for Acute Myocardial Infarction Are Decreased among Patients with Inflammatory Bowel Disease Using a Nationwide Inpatient Database. Inflamm. Bowel Dis. 2016, 22, 2229–2237. [Google Scholar] [CrossRef]
- Bewtra, M.; Kaiser, L.M.; Tenhave, T.; Lewis, J.D. Crohn’s Disease and Ulcerative Colitis Are Associated with Elevated Standardized Mortality Ratios. Inflamm. Bowel Dis. 2013, 19, 599–613. [Google Scholar] [CrossRef]
- Huang, W.S.; Tseng, C.H.; Chen, P.C.; Tsai, C.H.; Lin, C.L.; Sung, F.C.; Kao, C.H. Inflammatory bowel diseases increase future ischemic stroke risk: A Taiwanese population-based retrospective cohort study. Eur. J. Intern. Med. 2014, 25, 561–565. [Google Scholar] [CrossRef]
- Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Fleming, P.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.; et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; MacDonald, T.M.; Walker, B.R. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann. Intern. Med. 2004, 141, 764–770. [Google Scholar] [CrossRef]
- Bili, A.; Tang, X.; Pranesh, S.; Bozaite, R.; Morris, S.J.; Antohe, J.L.; Kirchner, H.L.; Wasko, M.C. Tumor necrosis factor α inhibitor use and decreased risk for incident coronary events in rheumatoid arthritis. Arthritis. Care Res. 2014, 66, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.D.; Hadid, H.; Schairer, J.; Imam, W.; Jafri, S.M. Effect of Inflammatory Bowel Disease-Related Characteristics and Treatment Interventions on Cardiovascular Disease Incidence. Am. J. Med. Sci. 2015, 350, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Ridker, P.M.; Libby, P.; MacFadyen, J.G.; Thuren, T.; Ballantyne, C.; Fonseca, F.; Koenig, W.; Shimokawa, H.; Everett, B.M.; Glynn, R.J. Modulation of the interleukin-6 signalling pathway and incidence rates of atherosclerotic events and all-cause mortality: Analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Heart J. 2018, 39, 3499–3507. [Google Scholar] [CrossRef]
- Ridker, P.M. Anticytokine Agents: Targeting Interleukin Signaling Pathways for the Treatment of Atherothrombosis. Circ. Res. 2019, 124, 437–450. [Google Scholar] [CrossRef]
| Phenotype | Data Sources | No. of Cases | No. of Controls | Population |
|---|---|---|---|---|
| Exposures | ||||
| Inflammatory bowel disease | de Lange et al. [16] | 25,042 | 34,915 | European |
| Crohn’s disease | de Lange et al. [16] | 12,194 | 28,072 | European |
| Ulcerative colitis | de Lange et al. [16] | 12,366 | 33,609 | European |
| Outcomes | ||||
| Coronary artery disease | CARDIoGRAMplusC4D plus UKBB * [14] | 122,733 | 424,528 | Majority European |
| FinnGen † [19] | 31,640 | 187,152 | European | |
| Ischemic stroke | MEGASTROKE consortium * [15] | 34,217 | 406,111 | European |
| FinnGen † | 10,551 | 208,241 | European | |
| UKBB † | 3314 | 357,880 | European | |
| Large artery stroke | MEGASTROKE consortium * | 4373 | 406,111 | European |
| Small vessel stroke | MEGASTROKE consortium * | 5386 | 192,662 | European |
| Positive and negative control outcome | ||||
| Primary sclerosing cholangitis | International PSC Study Group [23] | 2187 | 12,019 | Majority European |
| Parkinson’s disease | International Parkinson’s Disease Genomics Consortium [24] | 33,674 | 449,056 | European |
| Exposure | Outcome | IVW | MR Egger | Weighted Median | MR-RAPS | MR-PRESSO | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | ||
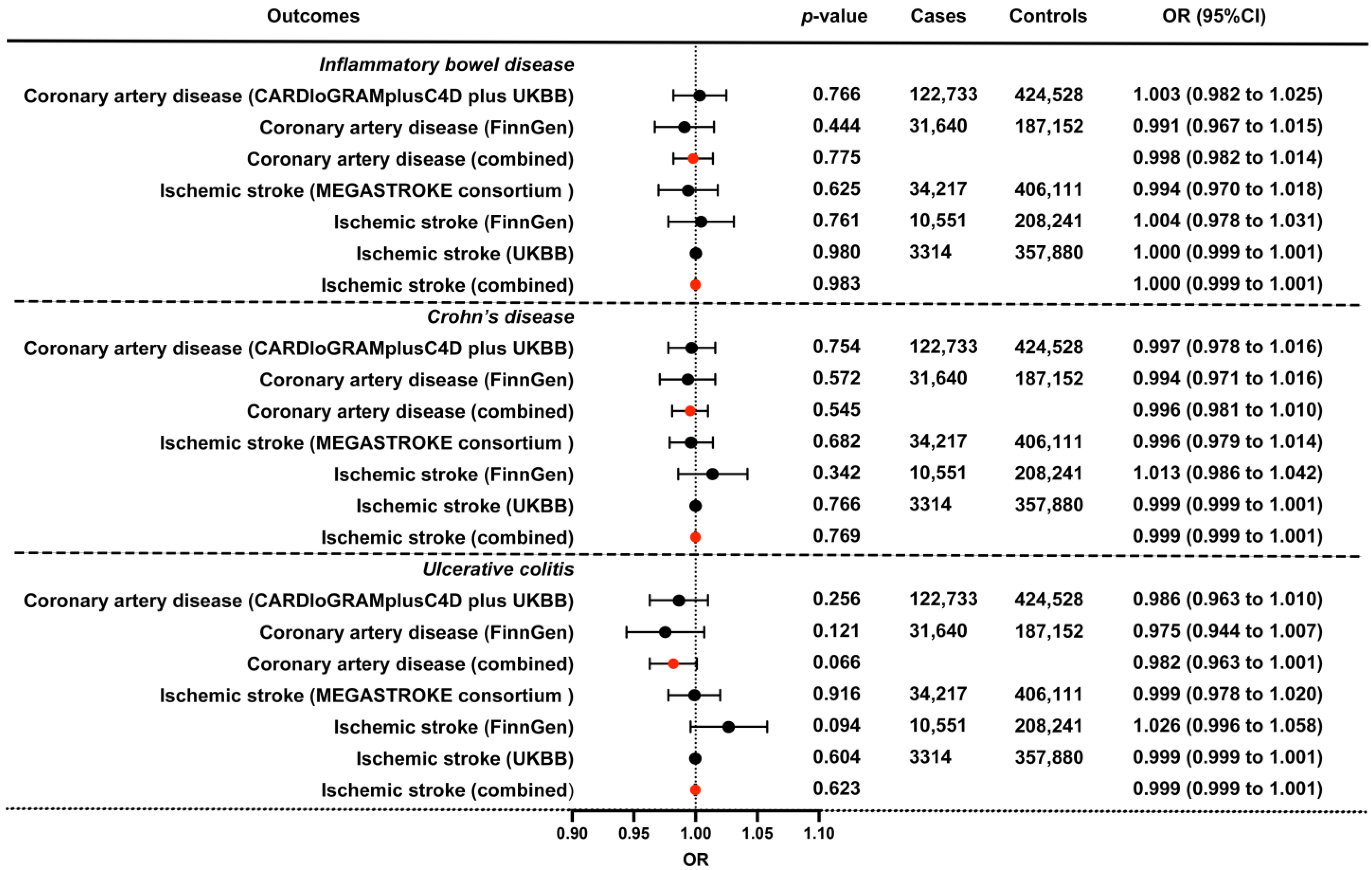
| IBD | Coronary artery disease | 1.003 (0.982 to 1.025) | 0.766 | 0.962 (0.908 to 1.018) | 0.181 | 1.002 (0.979 to 1.027) | 0.839 | 1.001 (0.981 to 1.020) | 0.943 | 1.002 (0.988 to 1.016) | 0.806 |
| Ischemic stroke * | 0.994 (0.97 to 1.018) | 0.625 | 0.969 (0.913 to 1.029) | 0.312 | 0.983 (0.952 to 1.016) | 0.314 | 0.988 (0.966 to 1.010) | 0.290 | 0.992 (0.973 to 1.01) | 0.390 | |
| Ischemic stroke * (large artery atherosclerosis) | 1.013 (0.955 to 1.074) | 0.668 | 1.047 (0.904 to 1.212) | 0.542 | 1.05 (0.972 to 1.135) | 0.215 | 1.025 (0.967 to 1.086) | 0.408 | 1.018 (0.973 to 1.065) | 0.443 | |
| Ischemic stroke * (small-vessel) | 1.021 (0.976 to 1.067) | 0.369 | 0.937 (0.84 to 1.046) | 0.251 | 0.974 (0.906 to 1.048) | 0.481 | 1.011 (0.967 to 1.057) | 0.638 | 1.026 (0.987 to 1.066) | 0.204 | |
| CD | Coronary artery disease | 0.997 (0.978 to 1.016) | 0.754 | 0.976 (0.926 to 1.028) | 0.362 | 0.996 (0.978 to 1.015) | 0.702 | 0.998 (0.980 to 1.016) | 0.835 | 0.996 (0.983 to 1.008) | 0.474 |
| Ischemic stroke * | 0.996 (0.979 to 1.014) | 0.682 | 0.959 (0.916 to 1.004) | 0.075 | 1.003 (0.976 to 1.031) | 0.805 | 0.996 (0.977 to 1.014) | 0.651 | 0.998 (0.982 to 1.015) | 0.852 | |
| Ischemic stroke * (large artery atherosclerosis) | 1.044 (1.003 to 1.087) | 0.037 | 1.037 (0.932 to 1.155) | 0.507 | 1.08 (1.013 to 1.151) | 0.018 | 1.05 (1.004 to 1.097) | 0.032 | 1.041 (1.001 to 1.082) | 0.046 | |
| Ischemic stroke * (small-vessel) | 1.012 (0.979 to 1.047) | 0.477 | 0.908 (0.825 to 0.999) | 0.050 | 0.995 (0.942 to 1.052) | 0.869 | 1.014 (0.977 to 1.053) | 0.459 | 1.015 (0.983 to 1.049) | 0.367 | |
| UC | Coronary artery disease | 0.986 (0.963 to 1.010) | 0.256 | 0.967 (0.902 to 1.037) | 0.355 | 0.99 (0.968 to 1.014) | 0.416 | 0.988 (0.967 to 1.01) | 0.275 | 0.994 (0.979 to 1.009) | 0.414 |
| Ischemic stroke * | 0.999 (0.978 to 1.020) | 0.916 | 0.968 (0.909 to 1.03) | 0.309 | 0.982 (0.951 to 1.015) | 0.289 | 0.997 (0.975 to 1.02) | 0.808 | 1.001 (0.983 to 1.02) | 0.903 | |
| Ischemic stroke * (large artery atherosclerosis) | 1.036 (0.974 to 1.101) | 0.263 | 1.018 (0.848 to 1.223) | 0.847 | 1.086 (0.998 to 1.181) | 0.054 | 1.054 (0.991 to 1.122) | 0.096 | 1.053 (1 to 1.109) | 0.055 | |
| Ischemic stroke * (small-vessel) | 1.033 (0.98 to 1.090) | 0.224 | 0.963 (0.823 to 1.128) | 0.645 | 1.006 (0.936 to 1.081) | 0.876 | 1.028 (0.974 to 1.085) | 0.314 | 1.021 (0.973 to 1.072) | 0.400 | |
| Exposure | Outcome | IVW | MR Egger | Weighted Median | MR-RAPS | MR-PRESSO | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | ||
| IBD | Coronary artery disease (FinnGen) | 0.991 (0.967 to 1.015) | 0.444 | 0.987 (0.947 to 1.030) | 0.551 | 0.980 (0.947 to 1.014) | 0.251 | 0.988 (0.965 to 1.011) | 0.300 | 0.987 (0.968 to 1.007) | 0.217 |
| Ischemic stroke (FinnGen) | 1.004 (0.978 to 1.031) | 0.761 | 1.028 (0.982 to 1.076) | 0.233 | 1.021 (0.976 to 1.068) | 0.361 | 1.006 (0.979 to 1.033) | 0.659 | 0.979 (0.953 to 1.005) | 0.111 | |
| Ischemic stroke (UKBB) | 1.000 (0.999 to 1.001) | 0.98 | 0.998 (0.997 to 0.999) | 0.026 | 0.999 (0.999 to 1.001) | 0.393 | 0.999 (0.999 to 1.001) | 0.938 | 0.999 (0.999 to 1.001) | 0.935 | |
| CD | Coronary artery disease (FinnGen) | 0.994 (0.971 to 1.016) | 0.572 | 0.972 (0.915 to 1.033) | 0.362 | 1.001 (0.974 to 1.029) | 0.925 | 0.989 (0.965 to 1.012) | 0.344 | 0.990 (0.970 to 1.010) | 0.333 |
| Ischemic stroke (FinnGen) | 1.013 (0.986 to 1.042) | 0.342 | 0.993 (0.922 to 1.070) | 0.862 | 1.010 (0.972 to 1.050) | 0.602 | 1.015 (0.987 to 1.043) | 0.311 | 0.976 (0.950 to 1.002) | 0.076 | |
| Ischemic stroke (UKBB) | 0.999 (0.999 to 1.001) | 0.766 | 0.999 (0.998 to 1.001) | 0.226 | 1.000 (0.999 to 1.001) | 0.524 | 0.999 (0.999 to 1.001) | 0.721 | 0.999 (0.999 to 1.001) | 0.690 | |
| UC | Coronary artery disease (FinnGen) | 0.975 (0.944 to 1.007) | 0.121 | 0.945 (0.844 to 1.057) | 0.317 | 0.973 (0.938 to 1.010) | 0.157 | 0.979 (0.949 to 1.010) | 0.186 | 0.986 (0.961 to 1.012) | 0.304 |
| Ischemic stroke (FinnGen) | 1.026 (0.996 to 1.058) | 0.094 | 0.935 (0.852 to 1.025) | 0.16 | 1.008 (0.962 to 1.056) | 0.742 | 1.022 (0.989 to 1.055) | 0.195 | 1.005 (0.971 to 1.040) | 0.785 | |
| Ischemic stroke (UKBB) | 0.999 (0.999 to 1.001) | 0.604 | 0.998 (0.997 to 1.001) | 0.155 | 0.999 (0.998 to 1.001) | 0.251 | 0.999 (0.999 to 1.001) | 0.611 | 0.999 (0.999 to 1.001) | 0.927 | |
| Exposure | Outcome | Method | OR (95%CI) | p-Value |
|---|---|---|---|---|
| TNF-α | Coronary artery disease (CARDIoGRAMplusC4D plus UKBB) | IVW | 0.993 (0.941 to 1.047) | 0.787 |
| Coronary artery disease (FinnGen) | IVW | 1.225 (0.913 to 1.644) | 0.176 | |
| Ischemic stroke (MEGASTROKE) | IVW | 1.000 (0.999 to 1.001) | 0.927 | |
| Ischemic stroke (large artery atherosclerosis) (MEGASTROKE) | IVW | 0.987 (0.943 to 1.032) | 0.559 | |
| Ischemic stroke (small vessel) (MEGASTROKE) | IVW | 0.945 (0.864 to 1.033) | 0.214 | |
| Ischemic stroke (FinnGen) | IVW | 1.026 (0.835 to 1.261) | 0.809 | |
| Ischemic stroke (UKBB) | IVW | 1.056 (0.790 to 1.411) | 0.715 | |
| CRP | Coronary artery disease (CARDIoGRAMplusC4D plus UKBB) | IVW | 0.908 (0.828 to 0.996) | 0.041 |
| Coronary artery disease (FinnGen) | IVW | 0.969 (0.869 to 1.079) | 0.563 | |
| Ischemic stroke (MEGASTROKE) | IVW | 1.024 (0.957 to 1.096) | 0.486 | |
| Ischemic stroke (large artery atherosclerosis) (MEGASTROKE) | IVW | 0.999 (0.998 to 1.000) | 0.150 | |
| Ischemic stroke (small vessel) (MEGASTROKE) | IVW | 1.09 (0.920 to 1.291) | 0.321 | |
| Ischemic stroke (FinnGen) | IVW | 0.989 (0.848 to 1.154) | 0.889 | |
| Ischemic stroke (UKBB) | IVW | 0.984 (0.914 to 1.060) | 0.678 | |
| BMI | Coronary artery disease (CARDIoGRAMplusC4D plus UKBB) | IVW | 1.507 (1.406 to 1.615) | 3.20 × 10−31 |
| Coronary artery disease (FinnGen) | IVW | 1.002 (1.001 to 1.004) | 0.004 | |
| Ischemic stroke (MEGASTROKE) | IVW | 1.314 (1.101 to 1.568) | 0.003 | |
| Ischemic stroke (large artery atherosclerosis) (MEGASTROKE) | IVW | 1.337 (1.232 to 1.451) | 3.71 × 10−12 | |
| Ischemic stroke (small vessel) (MEGASTROKE) | IVW | 1.162 (1.077 to 1.254) | 1.10 × 10−4 | |
| Ischemic stroke (FinnGen) | IVW | 1.120 (0.954 to 1.315) | 0.167 | |
| Ischemic stroke (UKBB) | IVW | 1.155 (1.047 to 1.275) | 0.004 | |
| Alcoholic drinks per week | Coronary artery disease (CARDIoGRAMplusC4D plus UKBB) | IVW | 1.273 (0.972 to 1.667) | 0.079 |
| Coronary artery disease (FinnGen) | IVW | 0.799 (0.522 to 1.223) | 0.302 | |
| Ischemic stroke (MEGASTROKE) | IVW | 1.124 (0.901 to 1.402) | 0.300 | |
| Ischemic stroke (large artery atherosclerosis) (MEGASTROKE) | IVW | 1.606 (0.944 to 2.734) | 0.081 | |
| Ischemic stroke (small vessel) (MEGASTROKE) | IVW | 0.713 (0.411 to 1.237) | 0.229 | |
| Ischemic stroke (FinnGen) | IVW | 1.136 (0.812 to 1.591) | 0.456 | |
| Ischemic stroke (UKBB) | IVW | 1.003 (0.997 to 1.008) | 0.326 | |
| Smoking initiation | Coronary artery disease (CARDIoGRAMplusC4D plus UKBB) | IVW | 1.242 (1.127 to 1.368) | 1.15 × 10−5 |
| Coronary artery disease (FinnGen) | IVW | 1.181 (1.007 to 1.385) | 0.041 | |
| Ischemic stroke (MEGASTROKE) | IVW | 1.137 (1.027 to 1.258) | 0.013 | |
| Ischemic stroke (large artery atherosclerosis) (MEGASTROKE) | IVW | 1.575 (1.283 to 1.935) | 1.47 × 10−5 | |
| Ischemic stroke (small vessel) (MEGASTROKE) | IVW | 1.219 (1.093 to 1.36) | 3.63 × 10−4 | |
| Ischemic stroke (FinnGen) | IVW | 1.195 (0.941 to 1.517) | 0.145 | |
| Ischemic stroke (UKBB) | IVW | 1.002 (1.000 to 1.004) | 0.067 | |
| Type 2 diabetes | Coronary artery disease (CARDIoGRAMplusC4D plus UKBB) | IVW | 1.014 (0.998 to 1.03) | 0.090 |
| Coronary artery disease (FinnGen) | IVW | 1.009 (0.948 to 1.074) | 0.767 | |
| Ischemic stroke (MEGASTROKE) | IVW | 0.997 (0.981 to 1.014) | 0.729 | |
| Ischemic stroke (large artery atherosclerosis) (MEGASTROKE) | IVW | 1.000 (1.000 to 1.000) | 0.290 | |
| Ischemic stroke (small vessel) (MEGASTROKE) | IVW | 0.996 (0.986 to 1.005) | 0.397 | |
| Ischemic stroke (FinnGen) | IVW | 1.001 (0.983 to 1.020) | 0.895 | |
| Ischemic stroke (UKBB) | IVW | 1.003 (0.970 to 1.038) | 0.853 |
| Exposure | Outcome | Method | OR (95%CI) | p-Value |
|---|---|---|---|---|
| IBD, adjusted for BMI and smoking initiation | Coronary artery disease (CARDIoGRAMplusC4D plus UKBB) | IVW | 1.009 (0.982 to 1.038) | 0.507 |
| Coronary artery disease (FinnGen) | IVW | 0.998 (0.964 to 1.033) | 0.921 | |
| Ischemic stroke (MEGASTROKE) | IVW | 1.000 (0.969 to 1.032) | 0.991 | |
| Ischemic stroke (large artery atherosclerosis) (MEGASTROKE) | IVW | 0.993 (0.922 to 1.069) | 0.847 | |
| Ischemic stroke (small vessel) (MEGASTROKE) | IVW | 1.014 (0.948 to 1.085) | 0.688 | |
| Ischemic stroke (FinnGen) | IVW | 0.98 (0.941 to 1.02) | 0.322 | |
| Ischemic stroke (UKBB) | IVW | 1.000 (1.000 to 1.001) | 0.590 | |
| CD, adjusted for BMI and smoking initiation | Coronary artery disease (CARDIoGRAMplusC4D plus UKBB) | IVW | 1.009 (0.988 to 1.030) | 0.410 |
| Coronary artery disease (FinnGen) | IVW | 1.015 (0.989 to 1.042) | 0.267 | |
| Ischemic stroke (MEGASTROKE) | IVW | 1.002 (0.979 to 1.026) | 0.844 | |
| Ischemic stroke (large artery atherosclerosis) (MEGASTROKE) | IVW | 1.043 (0.988 to 1.101) | 0.128 | |
| Ischemic stroke (small vessel) (MEGASTROKE) | IVW | 1.025 (0.975 to 1.078) | 0.339 | |
| Ischemic stroke (FinnGen) | IVW | 0.996 (0.965 to 1.028) | 0.792 | |
| Ischemic stroke (UKBB) | IVW | 1.000 (1.000 to 1.001) | 0.846 | |
| UC, adjusted for BMI and smoking initiation | Coronary artery disease (CARDIoGRAMplusC4D plus UKBB) | IVW | 1.013 (0.985 to 1.040) | 0.367 |
| Coronary artery disease (FinnGen) | IVW | 1.017 (0.982 to 1.053) | 0.343 | |
| Ischemic stroke (MEGASTROKE) | IVW | 1.010 (0.979 to 1.042) | 0.537 | |
| Ischemic stroke (large artery atherosclerosis) (MEGASTROKE) | IVW | 0.994 (0.923 to 1.071) | 0.879 | |
| Ischemic stroke (small vessel) (MEGASTROKE) | IVW | 1.040 (0.971 to 1.113) | 0.266 | |
| Ischemic stroke (FinnGen) | IVW | 1.019 (0.979 to 1.061) | 0.362 | |
| Ischemic stroke (UKBB) | IVW | 1.000 (0.999 to 1.001) | 0.859 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Qin, Z.; Cai, Z.; Liu, Z.; Chen, Y.-L.; Yin, X.; Yin, Y.; Peng, X.; Zhang, B. Evaluating the Causal Association between Inflammatory Bowel Disease and Risk of Atherosclerotic Cardiovascular Disease: Univariable and Multivariable Mendelian Randomization Study. Biomedicines 2023, 11, 2543. https://doi.org/10.3390/biomedicines11092543
Liu B, Qin Z, Cai Z, Liu Z, Chen Y-L, Yin X, Yin Y, Peng X, Zhang B. Evaluating the Causal Association between Inflammatory Bowel Disease and Risk of Atherosclerotic Cardiovascular Disease: Univariable and Multivariable Mendelian Randomization Study. Biomedicines. 2023; 11(9):2543. https://doi.org/10.3390/biomedicines11092543
Chicago/Turabian StyleLiu, Baike, Zijian Qin, Zhaolun Cai, Zheran Liu, Yun-Lin Chen, Xiaonan Yin, Yuan Yin, Xingchen Peng, and Bo Zhang. 2023. "Evaluating the Causal Association between Inflammatory Bowel Disease and Risk of Atherosclerotic Cardiovascular Disease: Univariable and Multivariable Mendelian Randomization Study" Biomedicines 11, no. 9: 2543. https://doi.org/10.3390/biomedicines11092543
APA StyleLiu, B., Qin, Z., Cai, Z., Liu, Z., Chen, Y.-L., Yin, X., Yin, Y., Peng, X., & Zhang, B. (2023). Evaluating the Causal Association between Inflammatory Bowel Disease and Risk of Atherosclerotic Cardiovascular Disease: Univariable and Multivariable Mendelian Randomization Study. Biomedicines, 11(9), 2543. https://doi.org/10.3390/biomedicines11092543






