Olfactory Bulbectomy Model of Depression Lowers Responding for Food in Male and Female Rats: The Modulating Role of Caloric Restriction and Response Requirement
Abstract
1. Introduction
2. Material and Methods
2.1. Animals
2.2. Olfactory Bulbectomy Surgery
2.3. Food Self-Administration Apparatus and Protocol
2.4. Statistical Analysis
3. Results
3.1. Experiment 1 (SD)
3.1.1. Male SD Rats
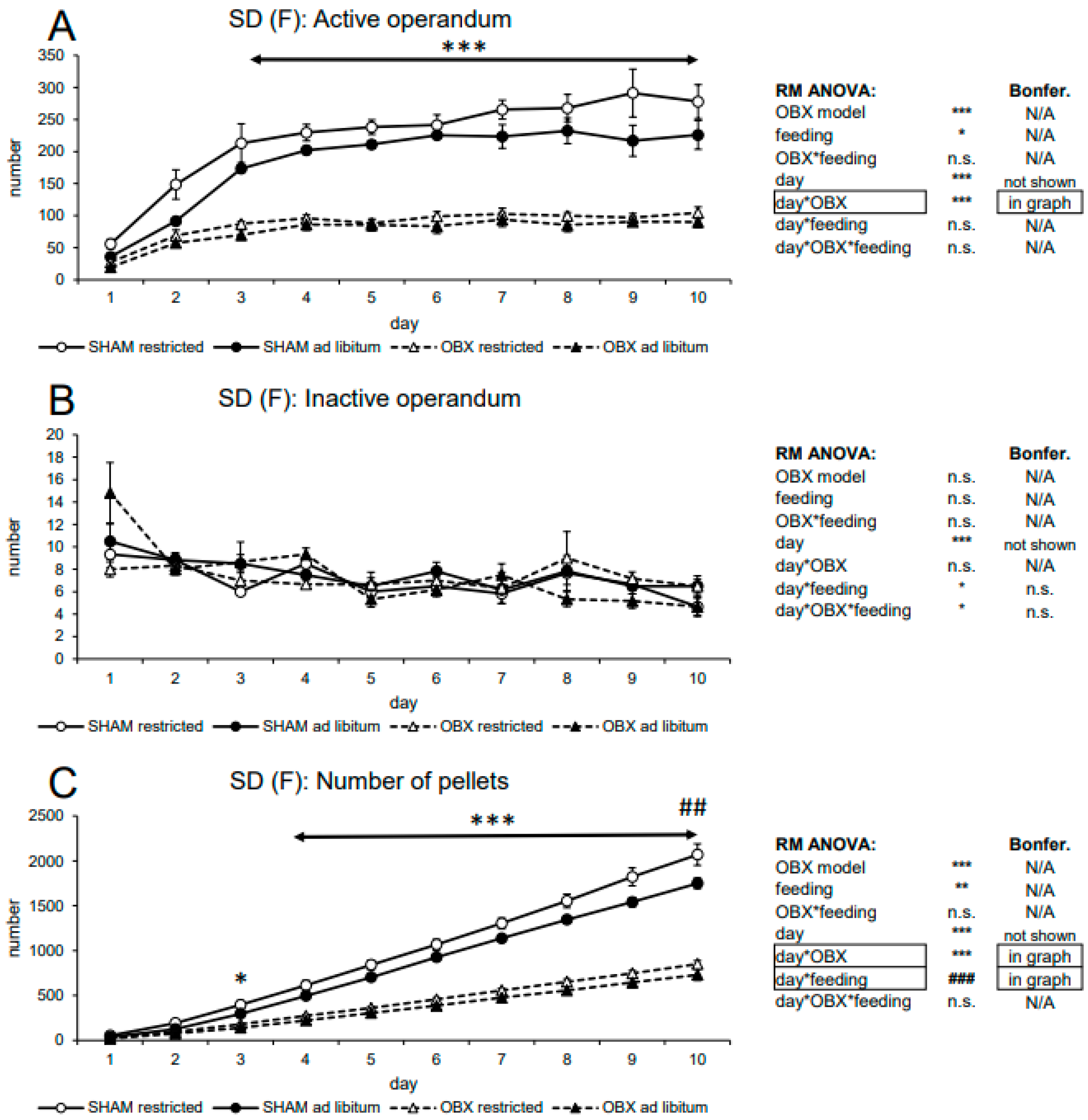
3.1.2. Female SD Rats
3.1.3. Evaluation of Sex Differences in SD Rats
3.2. Experiment 2 (WI)
3.2.1. Male WI Rats
3.2.2. Female WI Rats
3.2.3. Evaluation of Sex Differences in WI Rats
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Leri, F.; Rizvi, S.J. Anhedonia as a Central Factor in Depression: Neural Mechanisms Revealed from Preclinical to Clinical Evidence. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110289. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Ge, H.; Sun, M.; Gao, Y. Selecting an Appropriate Animal Model of Depression. Int. J. Mol. Sci. 2019, 20, 4827. [Google Scholar] [CrossRef] [PubMed]
- Harro, J. Animal Models of Depression: Pros and Cons. Cell Tissue Res. 2019, 377, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Czeh, B.; Fuchs, E.; Wiborg, O.; Simon, M. Animal Models of Major Depression and Their Clinical Implications. Prog. Neuropsychopharmacol. Biol. Psychiatry 2015, 64, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Leonard, B.E. The Olfactory Bulbectomised Rat as a Model of Depression. Neurosci. Biobehav. Rev. 2005, 29, 627–647. [Google Scholar] [CrossRef] [PubMed]
- Harkin, A.; Kelly, J.P.; Leonard, B.E. A Review of the Relevance and Validity of Olfactory Bulbectomy as a Model of Depression. Clin. Neurosci. Res. 2003, 3, 253–262. [Google Scholar] [CrossRef]
- Abelaira, H.M.; Réus, G.Z.; Quevedo, J. Animal Models as Tools to Study the Pathophysiology of Depression. Rev. Bras. Psiquiatr. 2013, 35 (Suppl. S2), S112–S120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Tao, Y.; Wang, T.; Zhou, J.; Yang, Y.; Cheng, L.; Zhu, H.; Zhang, W.; Huang, F.; Wu, X. Long-Term Stability and Characteristics of Behavioral, Biochemical, and Molecular Markers of Three Different Rodent Models for Depression. Brain Behav. 2020, 10, e01508. [Google Scholar] [CrossRef] [PubMed]
- Morales-Medina, J.C.; Iannitti, T.; Freeman, A.; Caldwell, H.K. The Olfactory Bulbectomized Rat as a Model of Depression: The Hippocampal Pathway. Behav. Brain Res. 2017, 317, 562–575. [Google Scholar] [CrossRef]
- Rajkumar, R.; Dawe, G.S. OBscure but Not OBsolete: Perturbations of the Frontal Cortex in Common between Rodent Olfactory Bulbectomy Model and Major Depression. J. Chem. Neuroanat. 2018, 91, 63–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Noda, Y.; Tsunekawa, H.; Zhou, Y.; Miyazaki, M.; Senzaki, K.; Nabeshima, T. Behavioural and Neurochemical Features of Olfactory Bulbectomized Rats Resembling Depression with Comorbid Anxiety. Behav. Brain Res. 2007, 178, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Filip, M.; Frankowska, M.; Jastrzebska, J.; Wydra, K.; Przegalinski, E. Preclinical Studies on Comorbidity between Depression and Psychostimulant Addiction. Pharmacol. Rep. 2013, 65, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- Lieb, R. Epidemiological Perspectives on Comorbidity Between Substance Use Disorders and Other Mental Disorders. In Co-Occurring Addictive and Psychiatric Disorders: A Practice-Based Handbook from a European Perspective; Dom, G., Moggi, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 3–12. ISBN 978-3-642-45375-5. [Google Scholar]
- Torrens, M.; Rossi, P. Mood Disorders and Addiction. In Co-Occurring Addictive and Psychiatric Disorders. A Practice-Based Handbook from a European Perspective; Dom, G., Moggi, F., Eds.; Springer: New York, NY, USA, 2015; pp. 103–117. ISBN 978-3-642-45375-5. [Google Scholar]
- Ortiz-Gomez, L.D.; Lopez-Canul, B.; Arankowsky-Sandoval, G. Factors Associated with Depression and Suicide Attempts in Patients Undergoing Rehabilitation for Substance Abuse. J. Affect. Disord. 2014, 169, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.V.; Masini, C.V.; Primeaux, S.D.; Garrett, J.L.; Zellner, A.; Stogner, K.S.; Duncan, A.A.; Crystal, J.D. Intravenous Self-Administration of Amphetamine Is Increased in a Rat Model of Depression. Synapse 2002, 46, 4–10. [Google Scholar] [CrossRef]
- Babinska, Z.; Ruda-Kucerova, J. Differential Characteristics of Ketamine Self-Administration in the Olfactory Bulbectomy Model of Depression in Male Rats. Exp. Clin. Psychopharmacol. 2017, 25, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, J.; Pistovcakova, J.; Vrskova, D.; Dusek, L.; Sulcova, A. The Effects of Methamphetamine Self-Administration on Behavioural Sensitization in the Olfactory Bulbectomy Rat Model of Depression. Int. J. Neuropsych. 2012, 15, 1503–1511. [Google Scholar] [CrossRef] [PubMed]
- Amchova, P.; Kucerova, J.; Giugliano, V.; Babinska, Z.; Zanda, M.; Scherma, M.; Dusek, L.; Fadda, P.; Micale, V.; Sulcova, A.; et al. Enhanced Self-Administration of the CB1 Receptor Agonist WIN55,212-2 in Olfactory Bulbectomized Rats: Evaluation of Possible Serotonergic and Dopaminergic Underlying Mechanisms. Front. Pharmacol. 2014, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Frankowska, M.; Jastrzebska, J.; Nowak, E.; Bialko, M.; Przegalinski, E.; Filip, M. The Effects of N-Acetylcysteine on Cocaine Reward and Seeking Behaviors in a Rat Model of Depression. Behav. Brain Res. 2014, 266, 108–118. [Google Scholar] [CrossRef]
- Vieyra-Reyes, P.; Mineur, Y.S.; Picciotto, M.R.; Tunez, I.; Vidaltamayo, R.; Drucker-Colin, R. Antidepressant-like Effects of Nicotine and Transcranial Magnetic Stimulation in the Olfactory Bulbectomy Rat Model of Depression. Brain Res. Bull. 2008, 77, 13–18. [Google Scholar] [CrossRef]
- Siska, F.; Amchova, P.; Kuruczova, D.; Tizabi, Y.; Ruda-Kucerova, J. Effects of Low-Dose Alcohol Exposure in Adolescence on Subsequent Alcohol Drinking in Adulthood in a Rat Model of Depression. World J. Biol. Psychiatry 2021, 22, 757–769. [Google Scholar] [CrossRef]
- Babinska, Z.; Ruda-Kucerova, J.; Amchova, P.; Merhautova, J.; Dusek, L.; Sulcova, A. Olfactory Bulbectomy Increases Reinstatement of Methamphetamine Seeking after a Forced Abstinence in Rats. Behav. Brain Res. 2016, 297, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Lucas, G.; Rymar, V.V.; Du, J.; Mnie-Filali, O.; Bisgaard, C.; Manta, S.; Lambas-Senas, L.; Wiborg, O.; Haddjeri, N.; Piñeyro, G.; et al. Serotonin(4) (5-HT(4)) Receptor Agonists Are Putative Antidepressants with a Rapid Onset of Action. Neuron 2007, 55, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Padilla, K.M.; Quintanar-Setephano, A.; López-Vallejo, F.; Berumen, L.C.; Miledi, R.; García-Alcocer, G. Behavioral Changes Induced through Adenosine A2A Receptor Ligands in a Rat Depression Model Induced by Olfactory Bulbectomy. Brain Behav. 2018, 8, e00952. [Google Scholar] [CrossRef] [PubMed]
- Primeaux, S.D.; Wilson, M.A.; Wilson, S.P.; Guth, A.N.; Lelutiu, N.B.; Holmes, P.V. Herpes Virus-Mediated Preproenkephalin Gene Transfer in the Ventral Striatum Mimics Behavioral Changes Produced by Olfactory Bulbectomy in Rats. Brain Res. 2003, 988, 43–55. [Google Scholar] [CrossRef]
- Romeas, T.; Morissette, M.C.; Mnie-Filali, O.; Pineyro, G.; Boye, S.M. Simultaneous Anhedonia and Exaggerated Locomotor Activation in an Animal Model of Depression. Psychopharmacology 2009, 205, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.-S.; Park, S.-S.; Lee, J.-M.; Kim, T.-W.; Kim, Y.-P. Treadmill Exercise Improves Depression-like Symptoms by Enhancing Serotonergic Function through Upregulation of 5-HT1A Expression in the Olfactory Bulbectomized Rats. J. Exerc. Rehabil. 2017, 13, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xu, H.; Liu, Y.; He, H.; Li, G. Vanillin-Induced Amelioration of Depression-like Behaviors in Rats by Modulating Monoamine Neurotransmitters in the Brain. Psychiatry Res. 2015, 225, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Du, Q.; Liu, C.; Yang, Y.; Wang, J.; Duan, S.; Duan, J. Rhodioloside Ameliorates Depressive Behavior via Up-Regulation of Monoaminergic System Activity and Anti-Inflammatory Effect in Olfactory Bulbectomized Rats. Int. Immunopharmacol. 2016, 36, 300–304. [Google Scholar] [CrossRef]
- Gupta, D.; Radhakrishnan, M.; Thangaraj, D.; Kurhe, Y. Antidepressant and Anti-Anxiety like Effects of 4i (N-(3-Chloro-2-Methylphenyl) Quinoxalin-2-Carboxamide), a Novel 5-HT3 Receptor Antagonist in Acute and Chronic Neurobehavioral Rodent Models. Eur. J. Pharmacol. 2014, 735, 59–67. [Google Scholar] [CrossRef]
- Jindal, A.; Mahesh, R.; Bhatt, S. Etazolate, a Phosphodiesterase-4 Enzyme Inhibitor Produces Antidepressant-like Effects by Blocking the Behavioral, Biochemical, Neurobiological Deficits and Histological Abnormalities in Hippocampus Region Caused by Olfactory Bulbectomy. Psychopharmacology 2014, 105, 63–70. [Google Scholar] [CrossRef]
- Jindal, A.; Mahesh, R.; Bhatt, S. Type 4 Phosphodiesterase Enzyme Inhibitor, Rolipram Rescues Behavioral Deficits in Olfactory Bulbectomy Models of Depression: Involvement of Hypothalamic-Pituitary-Adrenal Axis, CAMP Signaling Aspects and Antioxidant Defense System. Pharmacol. Biochem. Behav. 2015, 132, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Rinwa, P.; Kumar, A. Panax Quinquefolium Involves Nitric Oxide Pathway in Olfactory Bulbectomy Rat Model. Physiol. Behav. 2014, 129, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Chambliss, H.O.; Van Hoomissen, J.D.; Holmes, P.V.; Bunnell, B.N.; Dishman, R.K. Effects of Chronic Activity Wheel Running and Imipramine on Masculine Copulatory Behavior after Olfactory Bulbectomy. Physiol. Behav. 2004, 82, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Slattery, D.A.; Markou, A.; Cryan, J.F. Evaluation of Reward Processes in an Animal Model of Depression. Psychopharmacology 2007, 190, 555–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stepanichev, M.; Markov, D.; Pasikova, N.; Gulyaeva, N. Behavior and the Cholinergic Parameters in Olfactory Bulbectomized Female Rodents: Difference between Rats and Mice. Behav. Brain Res. 2016, 297, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Otmakhova, N.A.; Gurevich, E.V.; Katkov, Y.A.; Nesterova, I.V.; Bobkova, N.V. Dissociation of Multiple Behavioral Effects between Olfactory Bulbectomized C57Bl/6J and DBA/2J Mice. Physiol. Behav. 1992, 52, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.H.; Wang, S. Strain Differences in the Chronic Mild Stress Animal Model of Depression. Behav. Brain Res. 2010, 213, 94–102. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, C.M.; Clarke, G.; Gibney, S.; Dinan, T.G.; Cryan, J.F. Strain Differences in the Neurochemical Response to Chronic Restraint Stress in the Rat: Relevance to Depression. Pharmacol. Biochem. Behav. 2011, 97, 690–699. [Google Scholar] [CrossRef]
- Gieryk, A.; Ziolkowska, B.; Solecki, W.; Kubik, J.; Przewlocki, R. Forebrain PENK and PDYN Gene Expression Levels in Three Inbred Strains of Mice and Their Relationship to Genotype-Dependent Morphine Reward Sensitivity. Psychopharmacology 2010, 208, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Brand, T.; Spanagel, R.; Schneider, M. Decreased Reward Sensitivity in Rats from the Fischer344 Strain Compared to Wistar Rats Is Paralleled by Differences in Endocannabinoid Signaling. PLoS ONE 2012, 7, e31169. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deiana, S.; Fattore, L.; Spano, M.S.; Cossu, G.; Porcu, E.; Fadda, P.; Fratta, W. Strain and Schedule-Dependent Differences in the Acquisition, Maintenance and Extinction of Intravenous Cannabinoid Self-Administration in Rats. Neuropharmacology 2007, 52, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Marusich, J.A.; McCuddy, W.T.; Beckmann, J.S.; Gipson, C.D.; Bardo, M.T. Strain Differences in Self-Administration of Methylphenidate and Sucrose Pellets in a Rat Model of ADHD. Behav. Pharmacol. 2011, 22, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Manduca, A.; Campolongo, P.; Palmery, M.; Vanderschuren, L.J.M.J.; Cuomo, V.; Trezza, V. Social Play Behavior, Ultrasonic Vocalizations and Their Modulation by Morphine and Amphetamine in Wistar and Sprague-Dawley Rats. Psychopharmacology 2014, 231, 1661–1673. [Google Scholar] [CrossRef] [PubMed]
- Paré, W.P. Investigatory Behavior of a Novel Conspecific by Wistar Kyoto, Wistar and Sprague-Dawley Rats. Brain Res. Bull. 2000, 53, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Ruda-Kucerova, J.; Zanda, M.T.; Amchova, P.; Fratta, W.; Fattore, L. Sex and Feeding Status Differently Affect Natural Reward Seeking Behavior in Olfactory Bulbectomized Rats. Front. Behav. Neurosci. 2018, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Stock, H.S.; Ford, K.; Wilson, M.A. Gender and Gonadal Hormone Effects in the Olfactory Bulbectomy Animal Model of Depression. Pharmacol. Biochem. Behav. 2000, 67, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Dalla, C.; Pitychoutis, P.M.; Kokras, N.; Papadopoulou-Daifoti, Z. Sex Differences in Response to Stress and Expression of Depressive-Like Behaviours in the Rat. In Biological Basis of Sex Differences in Psychopharmacology; Neill, J.C., Kulkarni, J., Eds.; Current Topics in Behavioral Neurosciences; Springer: Berlin/Heidelberg, Germany, 2011; pp. 97–118. ISBN 978-3-642-20006-9. [Google Scholar]
- Fattore, L.; Spano, M.S.; Altea, S.; Angius, F.; Fadda, P.; Fratta, W. Cannabinoid Self-Administration in Rats: Sex Differences and the Influence of Ovarian Function. Br. J. Pharmacol. 2007, 152, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Fattore, L.; Spano, M.; Altea, S.; Fadda, P.; Fratta, W. Drug- and Cue-Induced Reinstatement of Cannabinoid-Seeking Behaviour in Male and Female Rats: Influence of Ovarian Hormones. Br. J. Pharmacol. 2010, 160, 724–735. [Google Scholar] [CrossRef] [PubMed]
- Castelli, M.P.; Fadda, P.; Casu, A.; Spano, M.S.; Casti, A.; Fratta, W.; Fattore, L. Male and Female Rats Differ in Brain Cannabinoid CB1 Receptor Density and Function and in Behavioural Traits Predisposing to Drug Addiction: Effect of Ovarian Hormones. Curr. Pharm. Des. 2014, 20, 2100–2113. [Google Scholar] [CrossRef] [PubMed]
- Pisanu, A.; Lo Russo, G.; Talani, G.; Bratzu, J.; Siddi, C.; Sanna, F.; Diana, M.; Porcu, P.; De Luca, M.A.; Fattore, L. Effects of the Phenethylamine 2-Cl-4,5-MDMA and the Synthetic Cathinone 3,4-MDPHP in Adolescent Rats: Focus on Sex Differences. Biomedicines 2022, 10, 2336. [Google Scholar] [CrossRef]
- Ruda-Kucerova, J.; Amchova, P.; Havlickova, T.; Jerabek, P.; Babinska, Z.; Kacer, P.; Syslova, K.; Sulcova, A.; Sustkova-Fiserova, M. Reward Related Neurotransmitter Changes in a Model of Depression: An in Vivo Microdialysis Study. World J. Biol. Psychiatry 2015, 16, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P.; Wrynn, A.S.; Leonard, B.E. The Olfactory Bulbectomized Rat as a Model of Depression: An Update. Pharmacol. Ther. 1997, 74, 299–316. [Google Scholar] [CrossRef] [PubMed]
- Cabeza de Vaca, S.; Carr, K.D. Food restriction enhances the central rewarding effect of abused drugs. J. Neurosci. 1998, 18, 7502–7510. [Google Scholar] [CrossRef] [PubMed]
- Fattore, L.; Cossu, G.; Martellotta, C.M.; Fratta, W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology 2001, 156, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Ruda-Kucerova, J.; Amchova, P.; Babinska, Z.; Dusek, L.; Micale, V.; Sulcova, A. Sex Differences in the Reinstatement of Methamphetamine Seeking after Forced Abstinence in Sprague-Dawley Rats. Front. Psychiatry 2015, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.J.; Zlebnik, N.E.; Navin, S.F.; Carroll, M.E. Responding during Signaled Availability and Nonavailability of Iv Cocaine and Food in Rats: Age and Sex Differences. Psychopharmacology 2011, 215, 785–799. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Contini, A.; Sanna, F.; Maccioni, P.; Colombo, G.; Argiolas, A. Comparison between Male and Female Rats in a Model of Self-Administration of a Chocolate-Flavored Beverage: Behavioral and Neurochemical Studies. Behav. Brain Res. 2018, 344, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, J.; Frankowska, M.; Suder, A.; Wydra, K.; Nowak, E.; Filip, M.; Przegaliński, E. Effects of Escitalopram and Imipramine on Cocaine Reinforcement and Drug-Seeking Behaviors in a Rat Model of Depression. Brain Res. 2017, 1673, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Gawlińska, K.; Jastrzębska, J.; Gamberini, S.; Gawliński, D.; Pieniążek, R.; Suder, A.; Wydra, K.; Frankowska, M. The Impact of GABAB Receptors and Their Pharmacological Stimulation on Cocaine Reinforcement and Drug-Seeking Behaviors in a Rat Model of Depression. Eur. J. Pharmacol. 2020, 883, 173324. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.P.; Leonard, B.E. Effects of Chronic Desipramine on Waiting Behaviour for a Food Reward in Olfactory Bulbectomized Rats. J. Psychopharmacol. 1996, 10, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Jastrzębska, J.; Frankowska, M.; Smaga, I.; Hubalewska-Mazgaj, M.; Suder, A.; Pieniążek, R.; Przegaliński, E.; Filip, M. Evaluation of the 5-HT2C Receptor Drugs RO 60-0175, WAY 161503 and Mirtazepine in a Preclinical Model of Comorbidity of Depression and Cocaine Addiction. Pharmacol. Rep. 2023, 75, 99–118. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fattore, L.; Amchova, P.; Fadda, P.; Ruda-Kucerova, J. Olfactory Bulbectomy Model of Depression Lowers Responding for Food in Male and Female Rats: The Modulating Role of Caloric Restriction and Response Requirement. Biomedicines 2023, 11, 2481. https://doi.org/10.3390/biomedicines11092481
Fattore L, Amchova P, Fadda P, Ruda-Kucerova J. Olfactory Bulbectomy Model of Depression Lowers Responding for Food in Male and Female Rats: The Modulating Role of Caloric Restriction and Response Requirement. Biomedicines. 2023; 11(9):2481. https://doi.org/10.3390/biomedicines11092481
Chicago/Turabian StyleFattore, Liana, Petra Amchova, Paola Fadda, and Jana Ruda-Kucerova. 2023. "Olfactory Bulbectomy Model of Depression Lowers Responding for Food in Male and Female Rats: The Modulating Role of Caloric Restriction and Response Requirement" Biomedicines 11, no. 9: 2481. https://doi.org/10.3390/biomedicines11092481
APA StyleFattore, L., Amchova, P., Fadda, P., & Ruda-Kucerova, J. (2023). Olfactory Bulbectomy Model of Depression Lowers Responding for Food in Male and Female Rats: The Modulating Role of Caloric Restriction and Response Requirement. Biomedicines, 11(9), 2481. https://doi.org/10.3390/biomedicines11092481







