Change in Splenic Volume as a Surrogate Marker for Immunotherapy Response in Patients with Advanced Urothelial and Renal Cell Carcinoma—Evaluation of a Novel Approach of Fully Automated Artificial Intelligence Based Splenic Segmentation
Abstract
:1. Introduction
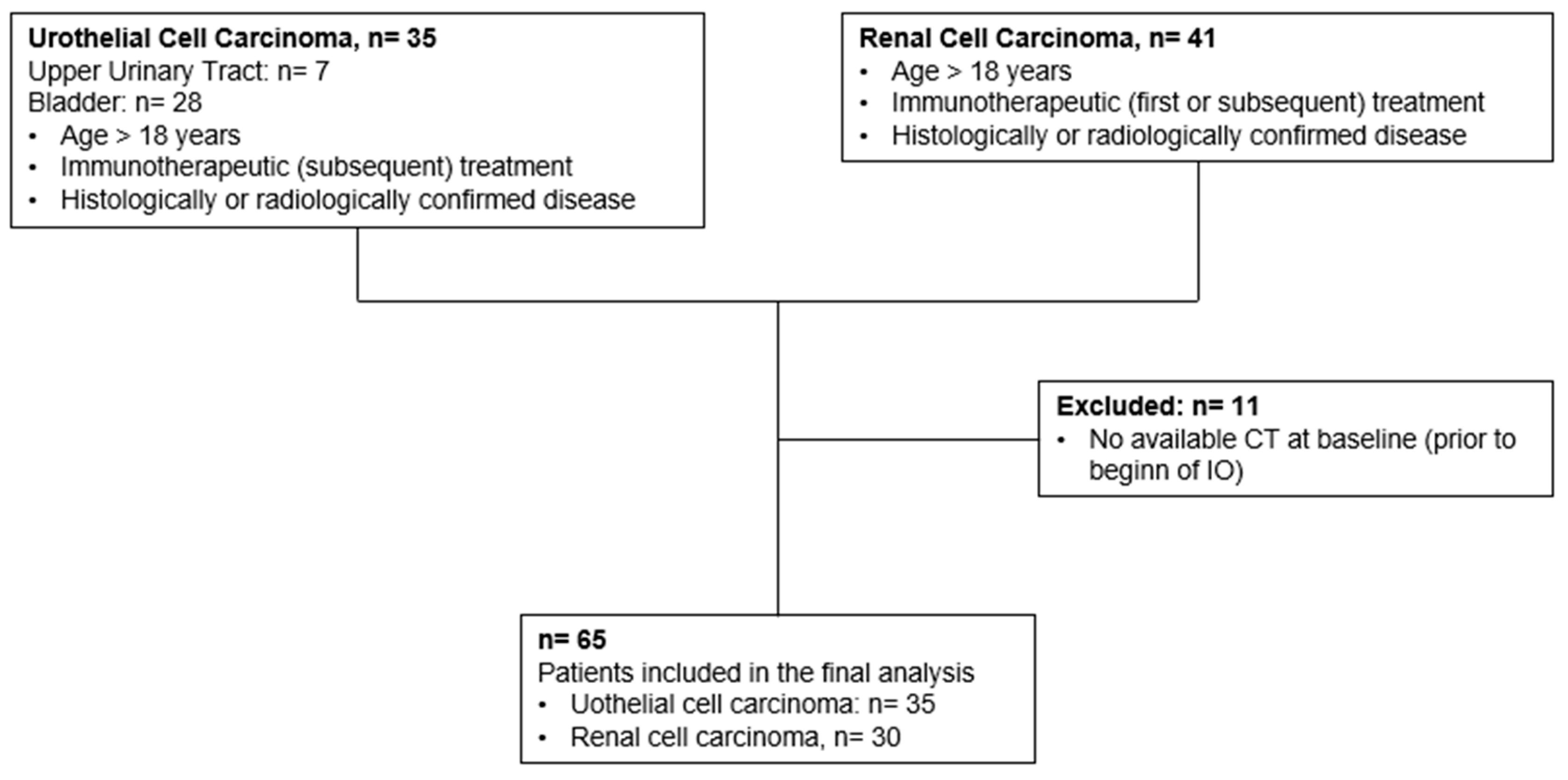
2. Materials and Methods
2.1. Patients
2.2. Splenic Volume Assessment
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Patients with Advanced Urothelial Carcinoma
3.2. Baseline Characteristics of Patients with Advanced Renal Cell Carcinoma
3.3. Change in Splenic Volume after Initiation of Immunotherapy
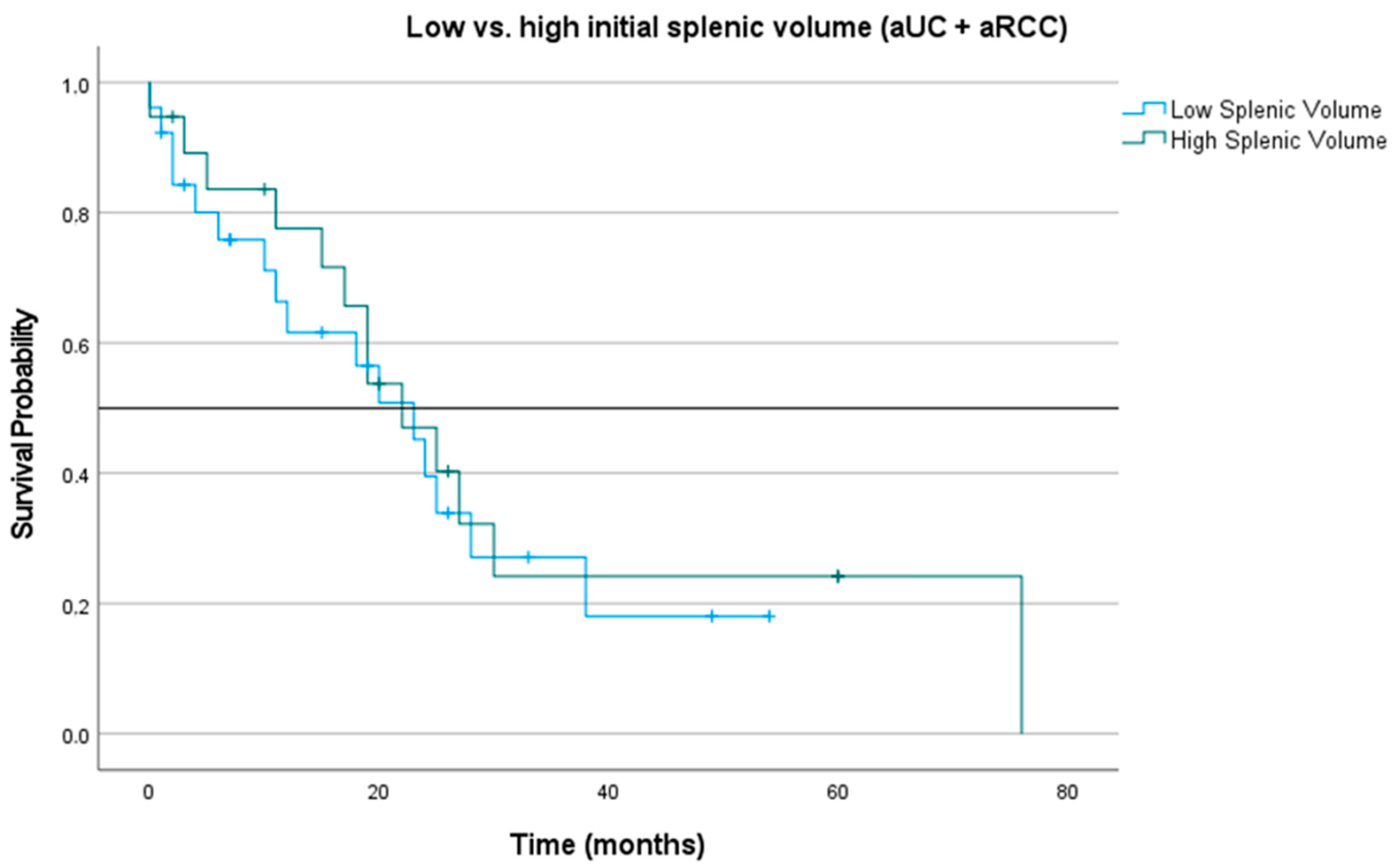
3.4. Impact of Splenic Volume at Treatment Initiation and during Three-Month Follow-Up on Overall Survival
3.5. Multivariate Survival Analysis for Overall Survival and Progression-Free Survival Based on Initial Splenic Volume and Change in 3Month Follow Up Splenic Volume
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Powles, T.; Bellmunt, J.; Comperat, E.; De Santis, M.; Huddart, R.; Loriot, Y.; Necchi, A.; Valderrama, B.P.; Ravaud, A.; Shariat, S.F.; et al. Bladder cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Motzer, R.J. Systemic Therapy for Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2017, 376, 354–366. [Google Scholar] [CrossRef]
- Rosellini, M.; Marchetti, A.; Mollica, V.; Rizzo, A.; Santoni, M.; Massari, F. Prognostic and predictive biomarkers for immunotherapy in advanced renal cell carcinoma. Nat. Rev. Urol. 2023, 20, 133–157. [Google Scholar] [CrossRef] [PubMed]
- Rebuzzi, S.E.; Banna, G.L.; Murianni, V.; Damassi, A.; Giunta, E.F.; Fraggetta, F.; De Giorgi, U.; Cathomas, R.; Rescigno, P.; Brunelli, M.; et al. Prognostic and Predictive Factors in Advanced Urothelial Carcinoma Treated with Immune Checkpoint Inhibitors: A Review of the Current Evidence. Cancers 2021, 13, 5517. [Google Scholar] [CrossRef]
- Mori, K.; Pradere, B.; Moschini, M.; Mostafaei, H.; Laukhtina, E.; Schuettfort, V.M.; Sari Motlagh, R.; Soria, F.; Teoh, J.Y.C.; Egawa, S.; et al. First-line immune-checkpoint inhibitor combination therapy for chemotherapy-eligible patients with metastatic urothelial carcinoma: A systematic review and meta-analysis. Eur. J. Cancer 2021, 151, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Bisno, D.I.; Patel, H.V.; Ghodoussipour, S.; Saraiya, B.; Mayer, T.; Singer, E.A. Immune Checkpoint Inhibitors in the Management of Urothelial Carcinoma. J. Cancer Immunol. 2021, 3, 115–136. [Google Scholar] [CrossRef]
- Balar, A.V.; Castellano, D.; O’Donnell, P.H.; Grivas, P.; Vuky, J.; Powles, T.; Plimack, E.R.; Hahn, N.M.; de Wit, R.; Pang, L.; et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Garcia, J.A.; Faltas, B.; Grivas, P.; Barata, P.; Shoag, J.E. Immune Checkpoint Inhibitors and Long-term Survival of Patients With Metastatic Urothelial Cancer. JAMA Netw. Open 2023, 6, e237444. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, F.; Hwang, E.C.; Lam, T.B.; Bex, A.; Yuan, Y.; Marconi, L.S.; Ljungberg, B. Targeted therapy for metastatic renal cell carcinoma. Cochrane Database Syst. Rev. 2020, 10, CD012796. [Google Scholar] [CrossRef]
- Brown, L.C.; Zhu, J.; Desai, K.; Kinsey, E.; Kao, C.; Lee, Y.H.; Pabla, S.; Labriola, M.K.; Tran, J.; Dragnev, K.H.; et al. Evaluation of tumor microenvironment and biomarkers of immune checkpoint inhibitor response in metastatic renal cell carcinoma. J. Immunother. Cancer 2022, 10, e005249. [Google Scholar] [CrossRef]
- Markel, J.E.; Noore, J.; Emery, E.J.; Bobnar, H.J.; Kleinerman, E.S.; Lindsey, B.A. Using the Spleen as an In VivoSystemic Immune Barometer Alongside Osteosarcoma Disease Progression and Immunotherapy withα-PD-L1. Sarcoma 2018, 2018, 8694397. [Google Scholar] [CrossRef] [PubMed]
- Knudson, K.M.; Hicks, K.C.; Alter, S.; Schlom, J.; Gameiro, S.R. Mechanisms involved in IL-15 superagonist enhancement of anti-PD-L1 therapy. J. Immunother. Cancer 2019, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Roy, M.D.; Golas, J.; Vitsky, A.; Ram, S.; Kumpf, S.W.; Martin, M.; Barletta, F.; Meier, W.A.; Hooper, A.T.; et al. Myocarditis in Cynomolgus Monkeys Following Treatment with Immune Checkpoint Inhibitors. Clin. Cancer Res. 2019, 25, 4735–4748. [Google Scholar] [CrossRef] [PubMed]
- Susok, L.; Reinert, D.; Lukas, C.; Stockfleth, E.; Gambichler, T. Volume increase of spleen in melanoma patients undergoing immune checkpoint blockade. Immunotherapy 2021, 13, 885–891. [Google Scholar] [CrossRef]
- Castagnoli, F.; Doran, S.; Lunn, J.; Minchom, A.; O’Brien, M.; Popat, S.; Messiou, C.; Koh, D.M. Splenic volume as a predictor of treatment response in patients with non-small cell lung cancer receiving immunotherapy. PLoS ONE 2022, 17, e0270950. [Google Scholar] [CrossRef]
- Müller, L.; Kloeckner, R.; Mähringer-Kunz, A.; Stoehr, F.; Düber, C.; Arnhold, G.; Gairing, S.J.; Foerster, F.; Weinmann, A.; Galle, P.R.; et al. Fully automated AI-based splenic segmentation for predicting survival and estimating the risk of hepatic decompensation in TACE patients with HCC. Eur. Radiol. 2022, 32, 6302–6313. [Google Scholar] [CrossRef]
- Müller, L.; Gairing, S.J.; Kloeckner, R.; Foerster, F.; Weinmann, A.; Mittler, J.; Stoehr, F.; Emrich, T.; Düber, C.; Galle, P.R.; et al. Baseline Splenic Volume Outweighs Immuno-Modulated Size Changes with Regard to Survival Outcome in Patients with Hepatocellular Carcinoma under Immunotherapy. Cancers 2022, 14, 3574. [Google Scholar] [CrossRef]
- Galland, L.; Lecuelle, J.; Favier, L.; Fraisse, C.; Lagrange, A.; Kaderbhai, C.; Truntzer, C.; Ghiringhelli, F. Splenic Volume as a Surrogate Marker of Immune Checkpoint Inhibitor Efficacy in Metastatic Non Small Cell Lung Cancer. Cancers 2021, 13, 3020. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Müller, D.; Kramer, F. MIScnn: A framework for medical image segmentation with convolutional neural networks and deep learning. BMC Med. Imaging 2021, 21, 12. [Google Scholar] [CrossRef]
- Nioche, C.; Orlhac, F.; Boughdad, S.; Reuzé, S.; Goya-Outi, J.; Robert, C.; Pellot-Barakat, C.; Soussan, M.; Frouin, F.; Buvat, I. LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res. 2018, 78, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Birkenkamp-Demtröder, K.; Christensen, E.; Nordentoft, I.; Knudsen, M.; Taber, A.; Høyer, S.; Lamy, P.; Agerbæk, M.; Jensen, J.B.; Dyrskjøt, L. Monitoring Treatment Response and Metastatic Relapse in Advanced Bladder Cancer by Liquid Biopsy Analysis. Eur. Urol. 2018, 73, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Vandekerkhove, G.; Lavoie, J.M.; Annala, M.; Murtha, A.J.; Sundahl, N.; Walz, S.; Sano, T.; Taavitsainen, S.; Ritch, E.; Fazli, L.; et al. Plasma ctDNA is a tumor tissue surrogate and enables clinical-genomic stratification of metastatic bladder cancer. Nat. Commun. 2021, 12, 184. [Google Scholar] [CrossRef] [PubMed]
- Bajorin, D.F.; Dodd, P.M.; Mazumdar, M.; Fazzari, M.; McCaffrey, J.A.; Scher, H.I.; Herr, H.; Higgins, G.; Boyle, M.G. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J. Clin. Oncol. 1999, 17, 3173–3181. [Google Scholar] [CrossRef]
- Bellmunt, J.; Choueiri, T.K.; Fougeray, R.; Schutz, F.A.; Salhi, Y.; Winquist, E.; Culine, S.; von der Maase, H.; Vaughn, D.J.; Rosenberg, J.E. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J. Clin. Oncol. 2010, 28, 1850–1855. [Google Scholar] [CrossRef]
- Park, K.J.; Lee, J.L.; Yoon, S.K.; Heo, C.; Park, B.W.; Kim, J.K. Radiomics-based prediction model for outcomes of PD-1/PD-L1 immunotherapy in metastatic urothelial carcinoma. Eur. Radiol. 2020, 30, 5392–5403. [Google Scholar] [CrossRef]
- Rundo, F.; Bersanelli, M.; Urzia, V.; Friedlaender, A.; Cantale, O.; Calcara, G.; Addeo, A.; Banna, G.L. Three-Dimensional Deep Noninvasive Radiomics for the Prediction of Disease Control in Patients With Metastatic Urothelial Carcinoma treated With Immunotherapy. Clin. Genitourin. Cancer 2021, 19, 396–404. [Google Scholar] [CrossRef]
- Bamias, A.; Merseburger, A.; Loriot, Y.; James, N.; Choy, E.; Castellano, D.; Lopez-Rios, F.; Calabrò, F.; Kramer, M.; de Velasco, G.; et al. New prognostic model in patients with advanced urothelial carcinoma treated with second-line immune checkpoint inhibitors. J. Immunother. Cancer 2023, 11, e005977. [Google Scholar] [CrossRef]
- Nuffer, Z.; Marini, T.; Rupasov, A.; Kwak, S.; Bhatt, S. The Best Single Measurement for Assessing Splenomegaly in Patients with Cirrhotic Liver Morphology. Acad. Radiol. 2017, 24, 1510–1516. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, S.S.; Choi, W.M.; Kim, K.M.; Sung, Y.S.; Lee, S.; Lee, S.J.; Yoon, J.S.; Suk, H.I. An index based on deep learning-measured spleen volume on CT for the assessment of high-risk varix in B-viral compensated cirrhosis. Eur. Radiol. 2021, 31, 3355–3365. [Google Scholar] [CrossRef]

| Characteristics (Numerical) | n = 35 | |
|---|---|---|
| Age at start of IO | ||
| Median (IQR) | 65.00 (57.50; 73.50) | |
| Time period initial diagnosis until metastasis (months) | ||
| Median (IQR) | 11.00 (5.0; 31.00) | |
| Period from start of IO until progress (days) | ||
| Median (IQR) | 143.00 (109.25; 255.25) | |
| Overall Survival/Follow-Up (months) | ||
| Median (IQR) | 11.50 (6.25; 24.25) | |
| Splenic Volume at baseline (start of IO) | ||
| Median (IQR) | 191.84 (152.16; 273.44) | |
| Splenic Volume at 3-month follow-up | ||
| Median (IQR) | 236.51 (181.55; 255.35) | |
| Splenic Volume at 9-month follow-up | ||
| Median (IQR) | 202.74 (135.49; 253;44) | |
| IQR: interquartile range | ||
| Characteristics (categorical) | n = 35 | % |
| Synchronous Metastasis | ||
| Yes | 4 | 11.4 |
| None | 6 | 17.1 |
| Unknown/data missing | 25 | 71.4 |
| Sex | ||
| Men | 24 | 68.6 |
| Female | 11 | 31.4 |
| Unknown/data missing | ||
| Neoadjuvant * intravesical treatment with BCG | ||
| yes | 1 | 2.9 |
| no | 19 | 54.3 |
| Unknown/data missing | 15 | 42.9 |
| Neoadjuvant * intravesical treatment with Mitomycin C | ||
| yes | 6 | 17.1 |
| no | 14 | 40 |
| Unknown/data missing | 15 | 42.9 |
| Neoadjuvant * chemotherapy | ||
| yes | 2 | 5.7 |
| no | 24 | 68.6 |
| Unknown/data missing | 9 | 25.7 |
| Adjuvant/Palliative chemotherapy | ||
| yes | 30 | 85.7 |
| no | 2 | 5.7 |
| Unknown/data missing | 3 | 8.6 |
| Deceased | ||
| Yes | 18 | 51.4 |
| None/Unknown | 17 | 48.6 |
| IO: immunotherapy | ||
| Characteristics (Numerical) | n = 30 | |
|---|---|---|
| Age at time of initial diagnosis | ||
| Median (IQR) | 65.00 (57.00; 72.00) | |
| Age at start of IO | ||
| Median (IQR) | 66.00 (59.00; 72.00) | |
| Overall Survival/Follow-Up (months) | ||
| Median (IQR) | 25.00 (13.00; 40.00) | |
| Splenic Volume at baseline (start of IO) | ||
| Median (IQR) | 281.72 (241.89; 312.85) | |
| Splenic Volume at 3-month follow-up | ||
| Median (IQR) | 298.23 (222.16; 328.43) | |
| Splenic Volume at 9-month follow-up | ||
| Median (IQR) | 260.71 (209.90; 293;12) | |
| IQR: interquartile range | ||
| Characteristics (categorical) | n = 30 | % |
| Sex | ||
| Men | 26 | 86.7 |
| Female | 4 | 13.3 |
| Histology of RCC | ||
| Clear Cell RCC | 24 | 80 |
| Papillary RCC | 2 | 6.7 |
| Other | 4 | 13.3 |
| Initial local treatment | ||
| Nephrectomy | 17 | 56.7 |
| Partial nephrectomy | 3 | 10 |
| No surgery/other | 10 | 33.3 |
| Regional lymph nodes at the time of initial diagnosis | ||
| Negative (N0) | 6 | 20 |
| Positive (N1) | 8 | 26.7 |
| Not Assessable (Nx) | 11 | 36.7 |
| Unknown/data missing | 5 | 16.7 |
| Distant Metastasis at the time of initial diagnosis | ||
| Negative (M0) | 3 | 10 |
| Positive (M1) | 11 | 36.6 |
| Unknown/data missing | 16 | 53.3 |
| IMDC Score * | ||
| 1 | 13 | 43.3 |
| 2 | 8 | 26.6 |
| 3 | 5 | 16.7 |
| 4 | 2 | 6.7 |
| Unknown/data missing | 2 | 6.7 |
| Synchronous lymph node metastasis | ||
| yes | 10 | 33.3 |
| no | 20 | 66.7 |
| Synchronous distant metastasis | ||
| yes | 11 | 36.7 |
| no | 19 | 63.3 |
| Distribution of metastases | ||
| Lung | 18 | 60 |
| Liver | 3 | 10 |
| Bones | 9 | 30 |
| Lymph nodes | 13 | 43.3 |
| IO at first-line treatment | ||
| Yes | 16 | |
| None/Other | 14 | |
| IO at second-line treatment | ||
| Yes | 14 | |
| None/Other | 16 | |
| IO at third-line treatment | ||
| Yes | 7 | |
| None/Other | 23 | |
| Deceased | ||
| Yes | 10 | 33.3 |
| None/Unknown | 20 | 66.6 |
| IO: immunotherapy | ||
| Patient Characteristic | Cox Proportional Hazards Regression for Survival | |||
|---|---|---|---|---|
| Overall Survival | Progression-Free Survival | |||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Initial low SV * | 2.21 (0.75–6.51) | 0.151 | 1.99 (0.76–5.20) | 0.163 |
| Age at start of IO | 0.98 (0.92–1.04) | 0.505 | 0.96 (0.92–1.01) | 0.105 |
| Time span from initial diagnosis to recurrence | 0.99 (0.99–1.00) | 0.095 | 0.99 (0.99–1.00) | 0.208 |
| Time span from recurrence to start of IO | 1.00 (0.99–1.00) | 0.185 | 1.00 (0.99–1.00) | 0.228 |
| Patient Characteristic | Cox Proportional Hazards Regression for Overall Survival | |||
|---|---|---|---|---|
| Based on Initial Splenic Volume | Based on Follow-Up Splenic Volume | |||
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Initial low SV * | 0.25 (0.04–1.78) | 0.167 | ||
| low SV at 3-month follow-up * | 0.07 (0.01–0.91) | 0.041 | ||
| Age at start of IO | 0.94 (0.82–1.08) | 0.396 | 0.97 (0.90–1.03) | 0.286 |
| T-Stage (pathologically) | 0.48 (0.08–2.94) | 0.425 | 0.13 (0.01–1.95) | 0.141 |
| N-Stage (pathologically) | 0.21 (0.01–3.72) | 0.289 | 3.61 (0.24–54.51) | 0.356 |
| IMDC Score | 0.36 (0.11–1.34) | 0.129 | ||
| Charlson Comorbidity Index | 2.66 (0.41–17.22) | 0.305 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duwe, G.; Müller, L.; Ruckes, C.; Fischer, N.D.; Frey, L.J.; Börner, J.H.; Rölz, N.; Haack, M.; Sparwasser, P.; Jorg, T.; et al. Change in Splenic Volume as a Surrogate Marker for Immunotherapy Response in Patients with Advanced Urothelial and Renal Cell Carcinoma—Evaluation of a Novel Approach of Fully Automated Artificial Intelligence Based Splenic Segmentation. Biomedicines 2023, 11, 2482. https://doi.org/10.3390/biomedicines11092482
Duwe G, Müller L, Ruckes C, Fischer ND, Frey LJ, Börner JH, Rölz N, Haack M, Sparwasser P, Jorg T, et al. Change in Splenic Volume as a Surrogate Marker for Immunotherapy Response in Patients with Advanced Urothelial and Renal Cell Carcinoma—Evaluation of a Novel Approach of Fully Automated Artificial Intelligence Based Splenic Segmentation. Biomedicines. 2023; 11(9):2482. https://doi.org/10.3390/biomedicines11092482
Chicago/Turabian StyleDuwe, Gregor, Lukas Müller, Christian Ruckes, Nikita Dhruva Fischer, Lisa Johanna Frey, Jan Hendrik Börner, Niklas Rölz, Maximilian Haack, Peter Sparwasser, Tobias Jorg, and et al. 2023. "Change in Splenic Volume as a Surrogate Marker for Immunotherapy Response in Patients with Advanced Urothelial and Renal Cell Carcinoma—Evaluation of a Novel Approach of Fully Automated Artificial Intelligence Based Splenic Segmentation" Biomedicines 11, no. 9: 2482. https://doi.org/10.3390/biomedicines11092482
APA StyleDuwe, G., Müller, L., Ruckes, C., Fischer, N. D., Frey, L. J., Börner, J. H., Rölz, N., Haack, M., Sparwasser, P., Jorg, T., Neumann, C. C. M., Tsaur, I., Höfner, T., Haferkamp, A., Hahn, F., Mager, R., & Brandt, M. P. (2023). Change in Splenic Volume as a Surrogate Marker for Immunotherapy Response in Patients with Advanced Urothelial and Renal Cell Carcinoma—Evaluation of a Novel Approach of Fully Automated Artificial Intelligence Based Splenic Segmentation. Biomedicines, 11(9), 2482. https://doi.org/10.3390/biomedicines11092482







