Clinical Perspectives of Gut Microbiota in Patients with Chronic Kidney Disease and End-Stage Kidney Disease: Where Do We Stand?
Abstract
:1. Introduction
2. The Kidney GM Crosstalk
- A.
- How do CKD and ESKD contribute to disturbed GM?
- B.
- How does disturbed GM impact CKD and ESKD Progression?
- C.
- How does disturbed GM contribute to CKD- and ESKD-related complications?
- ❖
- CKD- and ESKD-related cardiovascular disease
- ❖
- Cognitive psychiatric disorders
- ❖
- CKD—disorder of bone and minerals
- D.
- How does disturbed GM affect the production of key metabolic intermediates such as short-chain fatty acids?
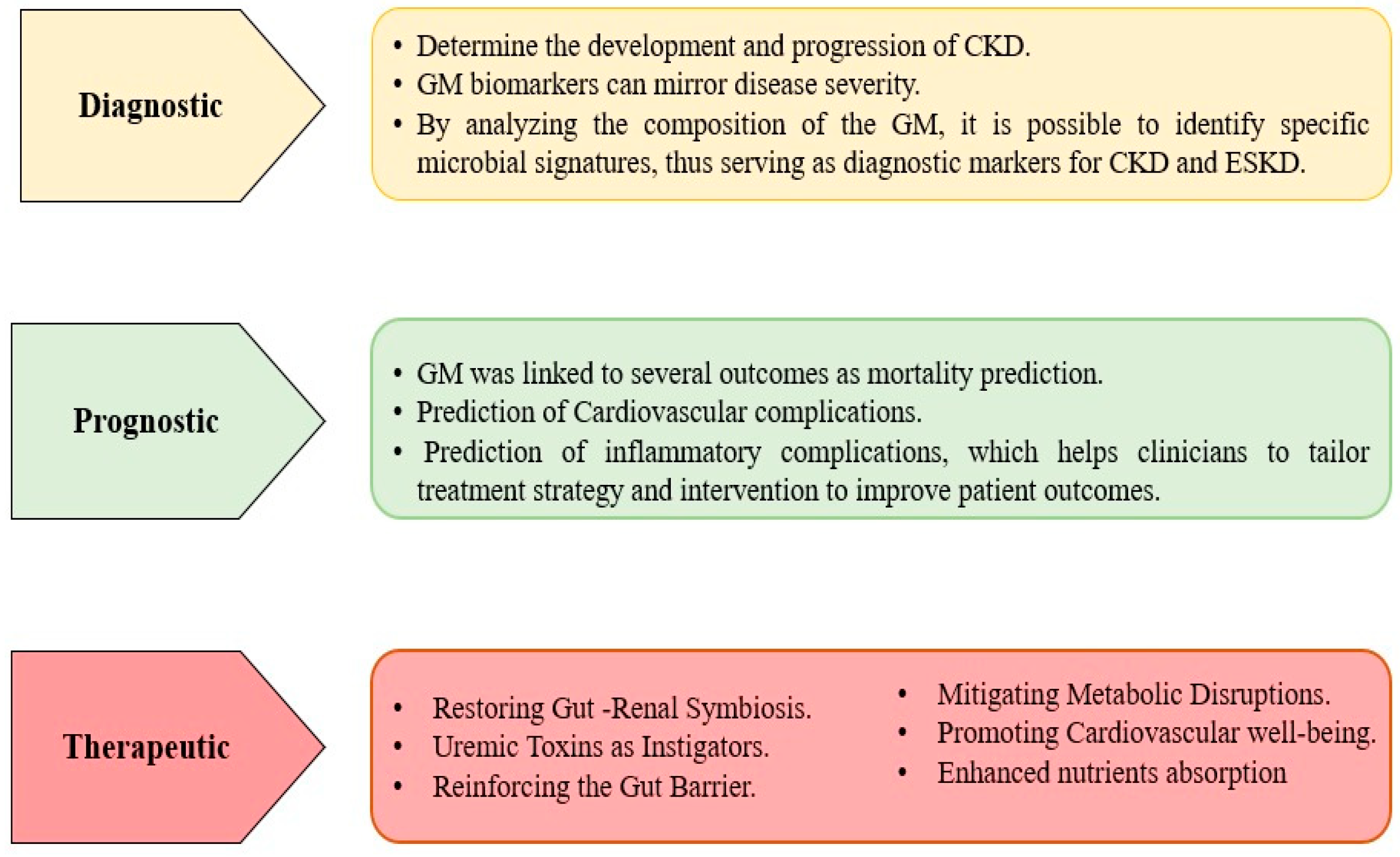
3. What Are the Clinical Applications for Implementing GM in Patients with CKD and ESKD?
- A.
- Diagnostic Applications:
- B.
- Prognostic Application:
- C.
- Therapeutic Applications:
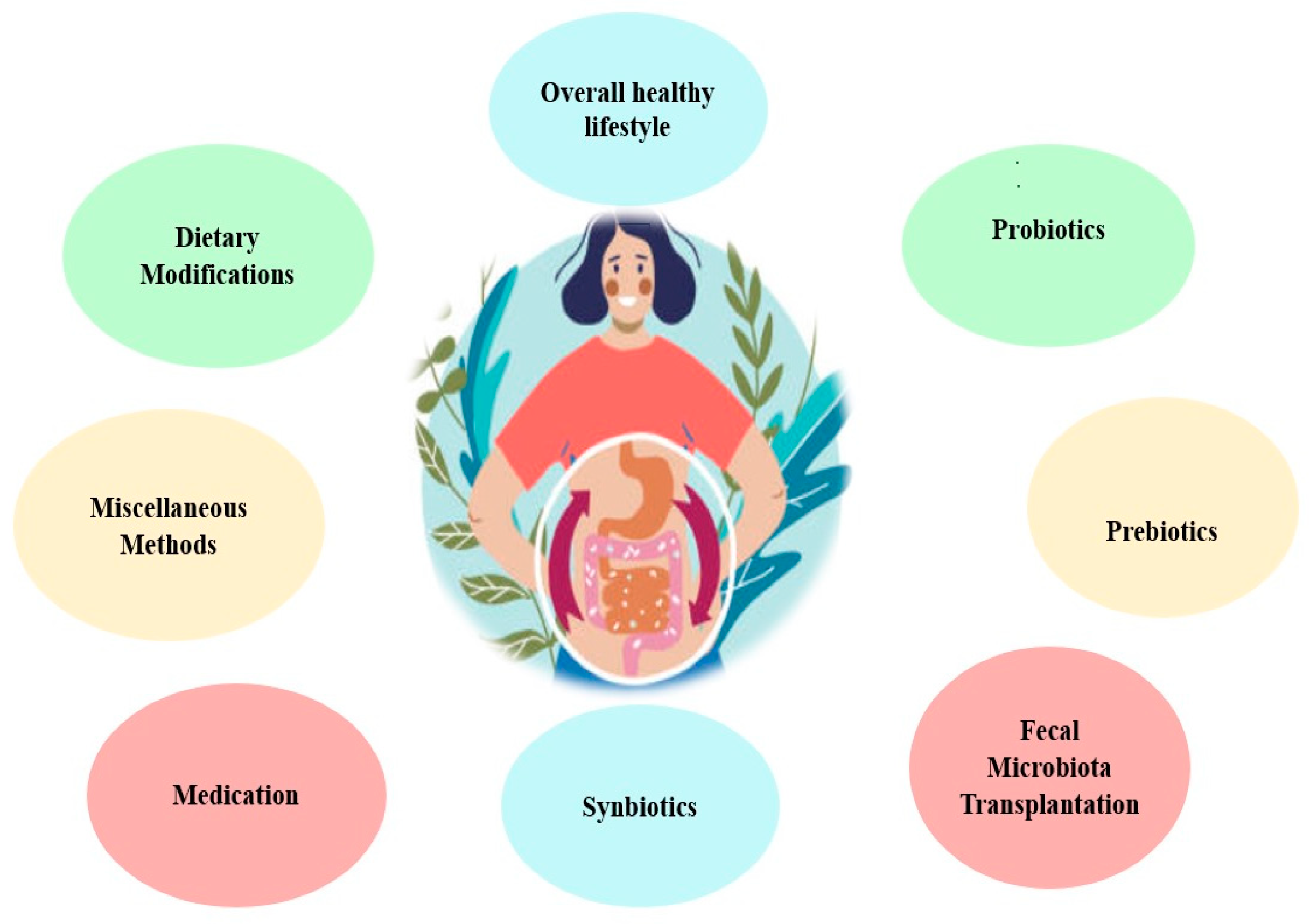
Methods by Which GM Balance Is Restored in CKD and ESKD
- ❖
- Maintaining an overall healthy lifestyle can positively enhance health. Regular physical activity, techniques for managing tension, enough sleep, and avoiding smoking and excess alcohol consumption may all contribute to a healthier digestive environment [103].
- ❖
- Dietary modifications: a personalized dietary plan is often made for CKD and ESKD patients. They are already on low protein intake, limited phosphorus, potassium-rich food, and fluid intake aiming to reduce uremic toxin precursor [104,105]. These modifications can indirectly impact GM [104,106]. Another dietary intervention is the high-fiber diet aimed to improve the reno-protective precursors [107].
- ❖
- Certain medications prescribed for the management of chronic renal disease can affect GM through multiple pathways, either by altering the composition of the gut microbiota or by eliminating both harmful and good bacteria [111]. Some medications harm the intestinal mucosa and alter the gut microbiome [112]. Additionally, immune suppressors tend to depress the immune response and alter the gut environment [113]. Even though these drugs may affect GM, their benefits for dealing with chronic renal disease typically outweigh their potential adverse effects [114].
- ❖
- Probiotics are primarily live bacteria, such as Bifidobacteria and Streptococci species [111]. Their principal therapeutic action is their ability to recalibrate the GM [115]. This equilibrium is reinstated through various mechanisms, including displacing harmful bacteria, fortifying gut barrier integrity, and adjusting the host’s immune response [116,117,118]. Research on probiotics suggested improved renal function and quality of life in CKD patients [119,120,121,122,123].
- ❖
- Prebiotics are indigestible food components that help stimulate the growth of specific bacteria in the colon [124]. Various prebiotics have been found to foster the expansion of advantageous bacterial strains such as Bifidobacteria and Lactobacilli species [125]. Simultaneously, these prebiotics appear to inhibit the growth of certain other bacterial clusters [125]. Prebiotics resist digestion until they reach the colon, where they’re fermented by native bacteria, producing short-chain fatty acids (SCFAs) [126]. These SCFAs enhance gut health and boost the immune response [81]. Research has shown that certain prebiotics can reduce the serum concentrations of specific uremic toxins in patients undergoing hemodialysis [127,128]. Furthermore, lactulose has been found to improve kidney function in animal models by modifying the gut microbiota, inhibiting the production of uremic toxins, and suppressing tubulointerstitial fibrosis [129,130].
- ❖
- Synbiotics are a combination of probiotics and prebiotics, used to potentiate the beneficial effects of probiotics. A study found that introducing synbiotics to patients with CKD lowered uremic toxins, specifically pCS [131]. Additionally, a randomized trial was conducted in 2023, which investigated the effects of synbiotics on non-dialyzed CKD patients [132] and reported that synbiotic regimens fostered the proliferation of beneficial bacteria in the gut [127]. It also notably decreased the serum levels of indoxyl sulfate, improved the glomerular filtration rate indicative of better kidney function, and attenuated inflammation [132]. Apart from minor side effects like increased flatulence, synbiotics were deemed to be a safe and effective therapeutic strategy to curb the levels of uremic toxins and inflammation in CKD patients [132].
- ❖
- Fecal Microbiota Transplantation (FMT) is a method that entails transferring fecal bacteria and other microscopic entities from a person in good health to another person [133]. The primary goal of FMT is to replace good bacteria that have been killed or suppressed, often using antibiotics, causing harmful bacteria, particularly Clostridium difficile, to overpopulate the colon [134]. The idea stems from the observation that CKD and ESKD patients often have altered GM, with an overgrowth of bacteria that produce uremic toxins, such as indoxyl sulfate and p-cresyl sulfate [135]. It is worth mentioning that modulation of gut microbiota is the principal mechanism in probiotics, prebiotics, synbiotics, and fecal microbiota transplantation. Early animal studies have provided some promising findings for treating CKD [136,137]. These findings suggest that FMT could potentially improve kidney function in patients with CKD and ESKD by reducing the levels of uremic toxins. However, it is important to note that these are preliminary findings, and more research is needed to determine the optimal protocol for FMT, including donor selection, preparation and administration of the fecal material, and long-term safety and efficacy monitoring.
- ❖
- Miscellaneous Methods include [138]:
- ▪
- Blocking LPS and inflammation via synthetic TLR4 antagonists and lipid A analogs.
- ▪
- The absorption of uremic toxins can be facilitated by oral adsorbents, dialyzers based on a carbon matrix; infusions of plasma-binding proteins like albumin, and the use of ibuprofen during dialysis.
- ▪
- Modulation of renal transporters via meclofenamate.
4. Evaluation of GM Modulation, Potential Risks, and Considerations
5. Applications and Limitation of GM Modulation in CKD and ESKD
6. Future Perspective and Further Research
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gebrayel, P.; Nicco, C.; Al Khodor, S.; Bilinski, J.; Caselli, E.; Comelli, E.M.; Egert, M.; Giaroni, C.; Karpinski, T.M.; Loniewski, I.; et al. Microbiota Medicine: Towards Clinical Revolution. J. Transl. Med. 2022, 20, 111. [Google Scholar] [CrossRef]
- Pantazi, A.C.; Mihai, C.M.; Balasa, A.L.; Chisnoiu, T.; Lupu, A.; Frecus, C.E.; Mihai, L.; Ungureanu, A.; Kassim, M.A.K.; Andrusca, A.; et al. Relationship between Gut Microbiota and Allergies in Children: A Literature Review. Nutrients 2023, 15, 2529. [Google Scholar] [CrossRef]
- Nori, W.; Akram, N.N.; Mueen Al-kaabi, M. Probiotics in Women and Pediatrics Health; A Narrative Review. Al-Anbar Med. J. 2023, 19, 10–16. [Google Scholar] [CrossRef]
- Lupu, V.V.; Adam Raileanu, A.; Mihai, C.M.; Morariu, I.D.; Lupu, A.; Starcea, I.M.; Frasinariu, O.E.; Mocanu, A.; Dragan, F.; Fotea, S. The Implication of the Gut Microbiome in Heart Failure. Cells 2023, 12, 1158. [Google Scholar] [CrossRef]
- Pantazi, A.C.; Balasa, A.L.; Mihai, C.M.; Chisnoiu, T.; Lupu, V.V.; Kassim, M.A.K.; Mihai, L.; Frecus, C.E.; Chirila, S.I.; Lupu, A.; et al. Development of Gut Microbiota in the First 1000 Days after Birth and Potential Interventions. Nutrients 2023, 15, 3647. [Google Scholar] [CrossRef]
- Lupu, V.V.; Ghiciuc, C.M.; Stefanescu, G.; Mihai, C.M.; Popp, A.; Sasaran, M.O.; Bozomitu, L.; Starcea, I.M.; Adam Raileanu, A.; Lupu, A. Emerging Role of the Gut Microbiome in Post-Infectious Irritable Bowel Syndrome: A Literature Review. World J. Gastroenterol. 2023, 29, 3241–3256. [Google Scholar] [CrossRef]
- Bozomitu, L.; Miron, I.; Adam Raileanu, A.; Lupu, A.; Paduraru, G.; Marcu, F.M.; Buga, A.M.L.; Rusu, D.C.; Dragan, F.; Lupu, V.V. The Gut Microbiome and Its Implication in the Mucosal Digestive Disorders. Biomedicines 2022, 10, 3117. [Google Scholar] [CrossRef]
- Lupu, V.V.; Trandafir, L.M.; Raileanu, A.A.; Mihai, C.M.; Morariu, I.D.; Starcea, I.M.; Mocanu, A.; Butnariu, L.I.; Stoleriu, G.; Salaru, D.L.; et al. Advances in Understanding the Human Gut Microbiota and Its Implication in Pediatric Celiac Disease—A Narrative Review. Nutrients 2023, 15, 2499. [Google Scholar] [CrossRef]
- Lupu, A.; Jechel, E.; Mihai, C.M.; Mitrofan, E.C.; Fotea, S.; Starcea, I.M.; Ioniuc, I.; Mocanu, A.; Ghica, D.C.; Popp, A.; et al. The Footprint of Microbiome in Pediatric Asthma—A Complex Puzzle for a Balanced Development. Nutrients 2023, 15, 3278. [Google Scholar] [CrossRef]
- Lupu, V.V.; Butnariu, L.I.; Fotea, S.; Morariu, I.D.; Badescu, M.C.; Starcea, I.M.; Salaru, D.L.; Popp, A.; Dragan, F.; Lupu, A.; et al. The Disease with a Thousand Faces and the Human Microbiome—A Physiopathogenic Intercorrelation in Pediatric Practice. Nutrients 2023, 15, 3359. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-Microbiota-Targeted Diets Modulate Human Immune Status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The Gut Microbiota and the Brain–Gut–Kidney Axis in Hypertension and Chronic Kidney Disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef]
- Suganya, K.; Son, T.; Kim, K.-W.; Koo, B.-S. Impact of Gut Microbiota: How It Could Play Roles beyond the Digestive System on Development of Cardiovascular and Renal Diseases. Microb. Pathog. 2021, 152, 104583. [Google Scholar] [CrossRef]
- Martinez, J.E.; Kahana, D.D.; Ghuman, S.; Wilson, H.P.; Wilson, J.; Kim, S.C.J.; Lagishetty, V.; Jacobs, J.P.; Sinha-Hikim, A.P.; Friedman, T.C. Unhealthy Lifestyle and Gut Dysbiosis: A Better Understanding of the Effects of Poor Diet and Nicotine on the Intestinal Microbiome. Front. Endocrinol. 2021, 12, 667066. [Google Scholar] [CrossRef]
- Chen, T.-H.; Cheng, C.-Y.; Huang, C.-K.; Ho, Y.-H.; Lin, J.-C. Exploring the Relevance between Gut Microbiota-Metabolites Profile and Chronic Kidney Disease with Distinct Pathogenic Factor. Microbiol. Spectr. 2023, 11, e02805-22. [Google Scholar] [CrossRef]
- Stanford, J.; Charlton, K.; Stefoska-Needham, A.; Zheng, H.; Bird, L.; Borst, A.; Fuller, A.; Lambert, K. Associations Among Plant-Based Diet Quality, Uremic Toxins, and Gut Microbiota Profile in Adults Undergoing Hemodialysis Therapy. J. Ren. Nutr. 2021, 31, 177–188. [Google Scholar] [CrossRef]
- Lin, X.; Liang, W.; Li, L.; Xiong, Q.; He, S.; Zhao, J.; Guo, X.; Xiang, S.; Zhang, P.; Wang, H.; et al. The Accumulation of Gut Microbiome–Derived Indoxyl Sulfate and P-Cresyl Sulfate in Patients with End-Stage Renal Disease. J. Ren. Nutr. 2022, 32, 578–586. [Google Scholar] [CrossRef]
- Feng, Z.; Wang, T.; Dong, S.; Jiang, H.; Zhang, J.; Raza, H.K.; Lei, G. Association between Gut Dysbiosis and Chronic Kidney Disease: A Narrative Review of the Literature. J. Int. Med. Res. 2021, 49, 030006052110532. [Google Scholar] [CrossRef]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Kassim, M.A.K.; Pantazi, A.C.; Nori, W.; Tuta, L.A.; Balasa, A.L.; Mihai, C.M.; Mihai, L.; Frecus, C.E.; Lupu, V.V.; Lupu, A.; et al. Non-Pharmacological Interventions for Pain Management in Hemodialysis: A Narrative Review. J. Clin. Med. 2023, 12, 5390. [Google Scholar] [CrossRef]
- Carney, E.F. The Impact of Chronic Kidney Disease on Global Health. Nat. Rev. Nephrol. 2020, 16, 251. [Google Scholar] [CrossRef]
- Cosola, C.; Rocchetti, M.T.; Sabatino, A.; Fiaccadori, E.; Di Iorio, B.R.; Gesualdo, L. Microbiota Issue in CKD: How Promising Are Gut-Targeted Approaches? J. Nephrol. 2019, 32, 27–37. [Google Scholar] [CrossRef]
- Vemuri, R.C.; Gundamaraju, R.; Shinde, T.; Eri, R. Therapeutic Interventions for Gut Dysbiosis and Related Disorders in the Elderly: Antibiotics, Probiotics or Faecal Microbiota Transplantation? Benef. Microbes 2017, 8, 179–192. [Google Scholar] [CrossRef]
- Garneata, L.; Stancu, A.; Dragomir, D.; Stefan, G.; Mircescu, G. Ketoanalogue-Supplemented Vegetarian Very Low–Protein Diet and CKD Progression. J. Am. Soc. Nephrol. 2016, 27, 2164–2176. [Google Scholar] [CrossRef] [PubMed]
- Arnold, R.; Pianta, T.J.; Pussell, B.A.; Kirby, A.; O’Brien, K.; Sullivan, K.; Holyday, M.; Cormack, C.; Kiernan, M.C.; Krishnan, A.V. Randomized, Controlled Trial of the Effect of Dietary Potassium Restriction on Nerve Function in CKD. Clin. J. Am. Soc. Nephrol. 2017, 12, 1569–1577. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Bellasi, A.; Pota, A.; Russo, L.; Di Iorio, B. Effects of Phosphorus-Restricted Diet and Phosphate-Binding Therapy on Outcomes in Patients with Chronic Kidney Disease. J. Nephrol. 2015, 28, 73–80. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vila, A.V.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.; Fu, J.; et al. Long-Term Dietary Patterns Are Associated with pro-Inflammatory and Anti-Inflammatory Features of the Gut Microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef] [PubMed]
- Beker, B.M.; Colombo, I.; Gonzalez-Torres, H.; Musso, C.G. Decreasing Microbiota-Derived Uremic Toxins to Improve CKD Outcomes. Clin. Kidney J. 2022, 15, 2214–2219. [Google Scholar] [CrossRef]
- Biruete, A.; Hill Gallant, K.M.; Lindemann, S.R.; Wiese, G.N.; Chen, N.X.; Moe, S.M. Phosphate Binders and Nonphosphate Effects in the Gastrointestinal Tract. J. Ren. Nutr. 2020, 30, 4–10. [Google Scholar] [CrossRef]
- Pergola, P.E.; Rosenbaum, D.P.; Yang, Y.; Chertow, G.M. A Randomized Trial of Tenapanor and Phosphate Binders as a Dual-Mechanism Treatment for Hyperphosphatemia in Patients on Maintenance Dialysis (AMPLIFY). J. Am. Soc. Nephrol. 2021, 32, 1465–1473. [Google Scholar] [CrossRef]
- Kim, S.M.; Song, I.H. The Clinical Impact of Gut Microbiota in Chronic Kidney Disease. Korean J. Intern. Med. 2020, 35, 1305–1316. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ghosh, S.; Banerjea, A.; De, R.; Hazra, A.; Mandal, S. Prescribing Patterns of Medicines in Chronic Kidney Disease Patients on Maintenance Hemodialysis. Indian. J. Pharmacol. 2016, 48, 586. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhao, W.; Lin, Z.; Wu, J.; Lin, H.; Li, Y.; Song, J.; Zhang, J.; Peng, H. The Effects of Hemodialysis and Peritoneal Dialysis on the Gut Microbiota of End-Stage Renal Disease Patients, and the Relationship Between Gut Microbiota and Patient Prognoses. Front. Cell Infect. Microbiol. 2021, 11, 579386. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Cai, J.; Luo, S.; Hong, X.; Xu, L.; Lin, H.; Chen, X.; Fu, W. Causal Effects of Gut Microbiota on the Risk of Chronic Kidney Disease: A Mendelian Randomization Study. Front. Cell Infect. Microbiol. 2023, 13, 1142140. [Google Scholar] [CrossRef] [PubMed]
- Ikee, R.; Yano, K.; Tsuru, T. Constipation in Chronic Kidney Disease: It Is Time to Reconsider. Ren. Replace. Ther. 2019, 5, 51. [Google Scholar] [CrossRef]
- Joossens, M.; Faust, K.; Gryp, T.; Nguyen, A.T.L.; Wang, J.; Eloot, S.; Schepers, E.; Dhondt, A.; Pletinck, A.; Vieira-Silva, S.; et al. Gut Microbiota Dynamics and Uraemic Toxins: One Size Does Not Fit All. Gut 2019, 68, 2257–2260. [Google Scholar] [CrossRef]
- Wehedy, E.; Shatat, I.F.; Al Khodor, S. The Human Microbiome in Chronic Kidney Disease: A Double-Edged Sword. Front. Med. 2022, 8, 790783. [Google Scholar] [CrossRef]
- Lohia, S.; Vlahou, A.; Zoidakis, J. Microbiome in Chronic Kidney Disease (CKD): An Omics Perspective. Toxins 2022, 14, 176. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut Microbiota Functions: Metabolism of Nutrients and Other Food Components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Kambale, R.M.; Ntagazibwa, J.N.; Kasengi, J.B.; Zigashane, A.B.; Francisca, I.N.; Mashukano, B.N.; Amani Ngaboyeka, G.; Bahizire, E.; Zech, F.; Bindels, L.B.; et al. Probiotics for Children with Uncomplicated Severe Acute Malnutrition (PruSAM Study): A Randomized Controlled Trial in the Democratic Republic of Congo. Am. J. Clin. Nutr. 2023, 117, 976–984. [Google Scholar] [CrossRef]
- Yang, J.; Lim, S.Y.; Ko, Y.S.; Lee, H.Y.; Oh, S.W.; Kim, M.G.; Cho, W.Y.; Jo, S.K. Intestinal Barrier Disruption and Dysregulated Mucosal Immunity Contribute to Kidney Fibrosis in Chronic Kidney Disease. Nephrol. Dial. Transplant. 2019, 34, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, G.; Banerjee, N.; Liang, Y.; Du, X.; Boor, P.J.; Hoffman, K.L.; Khan, M.F. Aberrant Gut Microbiome Contributes to Intestinal Oxidative Stress, Barrier Dysfunction, Inflammation and Systemic Autoimmune Responses in MRL/Lpr Mice. Front. Immunol. 2021, 12, 651191. [Google Scholar] [CrossRef] [PubMed]
- Koshida, K.; Ito, M.; Yakabe, K.; Takahashi, Y.; Tai, Y.; Akasako, R.; Kimizuka, T.; Takano, S.; Sakamoto, N.; Haniuda, K.; et al. Dysfunction of Foxp3+ Regulatory T Cells Induces Dysbiosis of Gut Microbiota via Aberrant Binding of Immunoglobulins to Microbes in the Intestinal Lumen. Int. J. Mol. Sci. 2023, 24, 8549. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.; McGregor, G.; Coggan, A.R.; Lewis, G.D.; Moe, S.M. Cardiovascular Functional Changes in Chronic Kidney Disease: Integrative Physiology, Pathophysiology and Applications of Cardiopulmonary Exercise Testing. Front. Physiol. 2020, 11, 572355. [Google Scholar] [CrossRef]
- Li, X.; Lindholm, B. Cardiovascular Risk Prediction in Chronic Kidney Disease. Am. J. Nephrol. 2022, 53, 730–739. [Google Scholar] [CrossRef]
- Onal, E.M.; Afsar, B.; Covic, A.; Vaziri, N.D.; Kanbay, M. Gut Microbiota and Inflammation in Chronic Kidney Disease and Their Roles in the Development of Cardiovascular Disease. Hypertens. Res. 2019, 42, 123–140. [Google Scholar] [CrossRef]
- Moris, D.; Spartalis, M.; Spartalis, E.; Karachaliou, G.-S.; Karaolanis, G.I.; Tsourouflis, G.; Tsilimigras, D.I.; Tzatzaki, E.; Theocharis, S. The Role of Reactive Oxygen Species in the Pathophysiology of Cardiovascular Diseases and the Clinical Significance of Myocardial Redox. Ann. Transl. Med. 2017, 5, 326. [Google Scholar] [CrossRef]
- Lin, C.-J.; Wu, V.; Wu, P.-C.; Wu, C.-J. Meta-Analysis of the Associations of p-Cresyl Sulfate (PCS) and Indoxyl Sulfate (IS) with Cardiovascular Events and All-Cause Mortality in Patients with Chronic Renal Failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef]
- Kanitsoraphan, C.; Rattanawong, P.; Charoensri, S.; Senthong, V. Trimethylamine N-Oxide and Risk of Cardiovascular Disease and Mortality. Curr. Nutr. Rep. 2018, 7, 207–213. [Google Scholar] [CrossRef]
- Heianza, Y.; Ma, W.; Manson, J.E.; Rexrode, K.M.; Qi, L. Gut Microbiota Metabolites and Risk of Major Adverse Cardiovascular Disease Events and Death: A Systematic Review and Meta-Analysis of Prospective Studies. J. Am. Heart Assoc. 2017, 6, e004947. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, I.; Tatebe, J.; Namba, S.; Koizumi, M.; Yamazaki, J.; Morita, T. Activation of Aryl Hydrocarbon Receptor Mediates Indoxyl Sulfate-Induced Monocyte Chemoattractant Protein-1 Expression in Human Umbilical Vein Endothelial Cells. Circ. J. 2013, 77, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodpoor, F.; Rahbar Saadat, Y.; Barzegari, A.; Ardalan, M.; Zununi Vahed, S. The Impact of Gut Microbiota on Kidney Function and Pathogenesis. Biomed. Pharmacother. 2017, 93, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-T.; Wu, P.-H.; Liang, S.-S.; Mubanga, M.; Yang, Y.-H.; Hsu, Y.-L.; Kuo, M.-C.; Hwang, S.-J.; Kuo, P.-L. Protein-Bound Uremic Toxins Are Associated with Cognitive Function among Patients Undergoing Maintenance Hemodialysis. Sci. Rep. 2019, 9, 20388. [Google Scholar] [CrossRef]
- Kwon, Y.E.; Choi, H.Y.; Kim, S.; Ryu, D.-R.; Oh, H.J. Fracture Risk in Chronic Kidney Disease: A Korean Population-Based Cohort Study. Kidney Res. Clin. Pract. 2019, 38, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Choi, H.I.; Bae, E.H.; Ma, S.K.; Kim, S.W. Paricalcitol Attenuates Indoxyl Sulfate-Induced Apoptosis through the Inhibition of MAPK, Akt, and NF-KB Activation in HK-2 Cells. Korean J. Intern. Med. 2019, 34, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Fujii, H.; Hamada, Y.; Yoshiya, K.; Fukagawa, M. Association Between Indoxyl Sulfate and Skeletal Resistance in Hemodialysis Patients. Ther. Apher. Dial. 2010, 14, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Nii-Kono, T.; Iwasaki, Y.; Uchida, M.; Fujieda, A.; Hosokawa, A.; Motojima, M.; Yamato, H.; Kurokawa, K.; Fukagawa, M. Indoxyl Sulfate Induces Skeletal Resistance to Parathyroid Hormone in Cultured Osteoblastic Cells. Kidney Int. 2007, 71, 738–743. [Google Scholar] [CrossRef]
- Magliocca, G.; Mone, P.; Di Iorio, B.R.; Heidland, A.; Marzocco, S. Short-Chain Fatty Acids in Chronic Kidney Disease: Focus on Inflammation and Oxidative Stress Regulation. Int. J. Mol. Sci. 2022, 23, 5354. [Google Scholar] [CrossRef]
- Mertowska, P.; Mertowski, S.; Wojnicka, J.; Korona-Głowniak, I.; Grywalska, E.; Błażewicz, A.; Załuska, W. A Link between Chronic Kidney Disease and Gut Microbiota in Immunological and Nutritional Aspects. Nutrients 2021, 13, 3637. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Liu, X.; Shao, J.; Liao, Y.-T.; Wang, L.-N.; Jia, Y.; Dong, P.; Liu, Z.; He, D.; Li, C.; Zhang, X. Regulation of Short-Chain Fatty Acids in the Immune System. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, X.; Yu, X.; Novák, P.; Gui, Q.; Yin, K. Enhancing Intestinal Barrier Efficiency: A Novel Metabolic Diseases Therapy. Front. Nutr. 2023, 10, 1120168. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xu, H.; Tu, X.; Gao, Z. The Role of Short-Chain Fatty Acids of Gut Microbiota Origin in Hypertension. Front. Microbiol. 2021, 12, 730809. [Google Scholar] [CrossRef]
- Zheng, L.; Luo, M.; Zhou, H.; Chen, J. Natural Products from Plants and Microorganisms: Novel Therapeutics for Chronic Kidney Disease via Gut Microbiota Regulation. Front. Pharmacol. 2023, 13, 1068613. [Google Scholar] [CrossRef] [PubMed]
- Voroneanu, L.; Burlacu, A.; Brinza, C.; Covic, A.; Balan, G.G.; Nistor, I.; Popa, C.; Hogas, S.; Covic, A. Gut Microbiota in Chronic Kidney Disease: From Composition to Modulation towards Better Outcomes—A Systematic Review. J. Clin. Med. 2023, 12, 1948. [Google Scholar] [CrossRef]
- Lim, Y.J.; Sidor, N.A.; Tonial, N.C.; Che, A.; Urquhart, B.L. Uremic Toxins in the Progression of Chronic Kidney Disease and Cardiovascular Disease: Mechanisms and Therapeutic Targets. Toxins 2021, 13, 142. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, M.; Fang, X.; Teng, F.; Tan, X.; Li, X.; Wang, M.; Long, Y.; Xu, Y. Gut Microbiota-Derived Trimethylamine N-Oxide and Kidney Function: A Systematic Review and Meta-Analysis. Adv. Nutr. 2021, 12, 1286–1304. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.; Kuffel, G.; Thomas-White, K.; Wolfe, A.J.; Vellanki, K.; Leehey, D.J.; Bansal, V.K.; Brubaker, L.; Flanigan, R.; Koval, J.; et al. Diversity of the Midstream Urine Microbiome in Adults with Chronic Kidney Disease. Int. Urol. Nephrol. 2018, 50, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Xie, S.; Lv, D.; Wang, P.; He, H.; Zhang, T.; Zhou, Y.; Lin, Q.; Zhou, H.; Jiang, J.; et al. Alteration of the Gut Microbiota in Chinese Population with Chronic Kidney Disease. Sci. Rep. 2017, 7, 2870. [Google Scholar] [CrossRef]
- Barrios, C.; Beaumont, M.; Pallister, T.; Villar, J.; Goodrich, J.K.; Clark, A.; Pascual, J.; Ley, R.E.; Spector, T.D.; Bell, J.T.; et al. Gut-Microbiota-Metabolite Axis in Early Renal Function Decline. PLoS ONE 2015, 10, e0134311. [Google Scholar] [CrossRef]
- Fernandez-Prado, R.; Esteras, R.; Perez-Gomez, M.; Gracia-Iguacel, C.; Gonzalez-Parra, E.; Sanz, A.; Ortiz, A.; Sanchez-Niño, M. Nutrients Turned into Toxins: Microbiota Modulation of Nutrient Properties in Chronic Kidney Disease. Nutrients 2017, 9, 489. [Google Scholar] [CrossRef]
- Devlin, A.S.; Marcobal, A.; Dodd, D.; Nayfach, S.; Plummer, N.; Meyer, T.; Pollard, K.S.; Sonnenburg, J.L.; Fischbach, M.A. Modulation of a Circulating Uremic Solute via Rational Genetic Manipulation of the Gut Microbiota. Cell Host Microbe 2016, 20, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Salgado, J.; Vehaskari, V.M.; Stewart, T.; Ferris, M.; Zhang, Q.; Wang, G.; Blanchard, E.E.; Taylor, C.M.; Kallash, M.; Greenbaum, L.A.; et al. Intestinal Microbiota in Pediatric Patients with End Stage Renal Disease: A Midwest Pediatric Nephrology Consortium Study. Microbiome 2016, 4, 50. [Google Scholar] [CrossRef] [PubMed]
- Uda, A.; Shigemura, K.; Kitagawa, K.; Osawa, K.; Onuma, K.; Yan, Y.; Nishioka, T.; Fujisawa, M.; Yano, I.; Miyara, T. Risk Factors for the Acquisition of Enterococcus Faecium Infection and Mortality in Patients with Enterococcal Bacteremia: A 5-Year Retrospective Analysis in a Tertiary Care University Hospital. Antibiotics 2021, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-Y.; Wu, P.-H.; Lin, Y.-T.; Hung, S.-C. Gut Dysbiosis and Mortality in Hemodialysis Patients. NPJ Biofilms Microbiomes 2021, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, F.; Or-Rashid, M.H.; Al Mamun, A.; Rahaman, M.S.; Islam, M.M.; Meem, A.F.K.; Sutradhar, P.R.; Mitra, S.; Mimi, A.A.; et al. The Gut Microbiota (Microbiome) in Cardiovascular Disease and Its Therapeutic Regulation. Front. Cell Infect. Microbiol. 2022, 12, 903570. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Zhang, Q.; He, C.; Fu, C.; Wei, Q. The Role of the Gut Microbiota in Health and Cardiovascular Diseases. Mol. Biomed. 2022, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Mihai, S.; Codrici, E.; Popescu, I.D.; Enciu, A.-M.; Albulescu, L.; Necula, L.G.; Mambet, C.; Anton, G.; Tanase, C. Inflammation-Related Mechanisms in Chronic Kidney Disease Prediction, Progression, and Outcome. J. Immunol. Res. 2018, 2018, 2180373. [Google Scholar] [CrossRef] [PubMed]
- Widhani, A.; Djauzi, S.; Suyatna, F.D.; Dewi, B.E. Changes in Gut Microbiota and Systemic Inflammation after Synbiotic Supplementation in Patients with Systemic Lupus Erythematosus: A Randomized, Double-Blind, Placebo-Controlled Trial. Cells 2022, 11, 3419. [Google Scholar] [CrossRef] [PubMed]
- Seikrit, C.; Schimpf, J.I.; Wied, S.; Stamellou, E.; Izcue, A.; Pabst, O.; Rauen, T.; Lenaerts, K.; Floege, J. Intestinal Permeability in Patients with IgA Nephropathy and Other Glomerular Diseases: An Observational Study. J. Nephrol. 2022, 36, 463–474. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Chertow, G.M.; Devarajan, P.; Levin, A.; Andreoli, S.P.; Bangalore, S.; Warady, B.A. Chronic Inflammation in Chronic Kidney Disease Progression: Role of Nrf2. Kidney Int. Rep. 2021, 6, 1775–1787. [Google Scholar] [CrossRef]
- Kakey, M.I.S.; Abdoulrahman, K.K. Estimation of Liver Parameters and Oxidative Stress in Chronic Renal Failure Patients on Hemodialysis in Erbil Governorate. AIP Conf. Proc. 2017, 1888, 020029. [Google Scholar] [CrossRef]
- Tecklenborg, J.; Clayton, D.; Siebert, S.; Coley, S.M. The Role of the Immune System in Kidney Disease. Clin. Exp. Immunol. 2018, 192, 142–150. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Ławiński, J.; Olszewski, R.; Ciałkowska-Rysz, A.; Gluba-Brzózka, A. The Impact of CKD on Uremic Toxins and Gut Microbiota. Toxins 2021, 13, 252. [Google Scholar] [CrossRef]
- Kinashi, Y.; Hase, K. Partners in Leaky Gut Syndrome: Intestinal Dysbiosis and Autoimmunity. Front. Immunol. 2021, 12, 673708. [Google Scholar] [CrossRef]
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef]
- Masenga, S.K.; Hamooya, B.; Hangoma, J.; Hayumbu, V.; Ertuglu, L.A.; Ishimwe, J.; Rahman, S.; Saleem, M.; Laffer, C.L.; Elijovich, F.; et al. Recent Advances in Modulation of Cardiovascular Diseases by the Gut Microbiota. J. Hum. Hypertens. 2022, 36, 952–959. [Google Scholar] [CrossRef]
- Trøseid, M.; Andersen, G.Ø.; Broch, K.; Hov, J.R. The Gut Microbiome in Coronary Artery Disease and Heart Failure: Current Knowledge and Future Directions. EBioMedicine 2020, 52, 102649. [Google Scholar] [CrossRef]
- Tsafack, P.B.; Li, C.; Tsopmo, A. Food Peptides, Gut Microbiota Modulation, and Antihypertensive Effects. Molecules 2022, 27, 8806. [Google Scholar] [CrossRef] [PubMed]
- Costacurta, M.; Basilicata, M.; Marrone, G.; Di Lauro, M.; Campolattano, V.; Bollero, P.; Docimo, R.; Di Daniele, N.; Noce, A. The Impact of Chronic Kidney Disease on Nutritional Status and Its Possible Relation with Oral Diseases. Nutrients 2022, 14, 2002. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Lu, N.; Li, Q.; Wen, H.; Zhang, X.Q.; Zhang, M. Gut Microbiota and Drug-Related Liver Injury: Challenges and Perspectives. Adv. Gut Microbiome Res. 2023, 2023, 5442597. [Google Scholar] [CrossRef]
- Niu, M.-W.; Chen, P. Gut Microbiota and Drug-Induced Liver Injury: An Update. Chin. Med. J. 2020, 133, 494–495. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Maiti, T.K.; Mahajan, D.; Das, B. Human Gut Microbiota and Drug Metabolism. Microb. Ecol. 2023, 86, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, X.; Niu, D.; Zhang, S.; Wang, H.; Zhang, X.; Nan, F.; Jiang, S.; Wang, B. Gut Microbiota Induces Hepatic Steatosis by Modulating the T Cells Balance in High Fructose Diet Mice. Sci. Rep. 2023, 13, 6701. [Google Scholar] [CrossRef]
- Bishehsari, F.; Magno, E.; Swanson, G.; Desai, V.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. Alcohol and Gut-Derived Inflammation. Alcohol. Res. 2017, 38, 163–171. [Google Scholar]
- Li, F.; McClain, C.J.; Feng, W. Microbiome Dysbiosis and Alcoholic Liver Disease. Liver Res. 2019, 3, 218–226. [Google Scholar] [CrossRef]
- Lim, D.-W.; Wang, J.-H. Gut Microbiome: The Interplay of an “Invisible Organ” with Herbal Medicine and Its Derived Compounds in Chronic Metabolic Disorders. Int. J. Environ. Res. Public Health 2022, 19, 13076. [Google Scholar] [CrossRef] [PubMed]
- Nori, W.; Kassim, M.A.K.; Pantazi, A.C. Probiotics Role in Reducing GIT Cancer-Related Therapy Side Effects. Al-Rafidain J. Med. Sci. 2023, 5, 114–115. [Google Scholar] [CrossRef]
- Conlon, M.; Bird, A. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef]
- Clegg, D.J.; Headley, S.A.; Germain, M.J. Impact of Dietary Potassium Restrictions in CKD on Clinical Outcomes: Benefits of a Plant-Based Diet. Kidney Med. 2020, 2, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Apetrii, M.; Timofte, D.; Voroneanu, L.; Covic, A. Nutrition in Chronic Kidney Disease—The Role of Proteins and Specific Diets. Nutrients 2021, 13, 956. [Google Scholar] [CrossRef]
- Hsu, C.-K.; Su, S.-C.; Chang, L.-C.; Shao, S.-C.; Yang, K.-J.; Chen, C.-Y.; Chen, Y.-T.; Wu, I.-W. Effects of Low Protein Diet on Modulating Gut Microbiota in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis of International Studies. Int. J. Med. Sci. 2021, 18, 3839–3850. [Google Scholar] [CrossRef]
- Yang, H.-L.; Feng, P.; Xu, Y.; Hou, Y.-Y.; Ojo, O.; Wang, X.-H. The Role of Dietary Fiber Supplementation in Regulating Uremic Toxins in Patients With Chronic Kidney Disease: A Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 2021, 31, 438–447. [Google Scholar] [CrossRef]
- Raj Krishnamurthy, V.M.; Wei, G.; Baird, B.C.; Murtaugh, M.; Chonchol, M.B.; Raphael, K.L.; Greene, T.; Beddhu, S. High Dietary Fiber Intake Is Associated with Decreased Inflammation and All-Cause Mortality in Patients with Chronic Kidney Disease. Kidney Int. 2012, 81, 300–306. [Google Scholar] [CrossRef]
- Kalmokoff, M.; Zwicker, B.; O’Hara, M.; Matias, F.; Green, J.; Shastri, P.; Green-Johnson, J.; Brooks, S.P.J. Temporal Change in the Gut Community of Rats Fed High Amylose Cornstarch Is Driven by Endogenous Urea Rather than Strictly on Carbohydrate Availability. J. Appl. Microbiol. 2013, 114, 1516–1528. [Google Scholar] [CrossRef]
- Zupcic, A.; Slezak, P.; Radloff, J. The Gastrointestinal Microbiota as a Potential Cause and Target in Chronic Kidney Disease Accentuating Treatment and Intervention Strategies. Appl. Sci. 2023, 13, 3212. [Google Scholar] [CrossRef]
- Kieffer, D.A.; Piccolo, B.D.; Vaziri, N.D.; Liu, S.; Lau, W.L.; Khazaeli, M.; Nazertehrani, S.; Moore, M.E.; Marco, M.L.; Martin, R.J.; et al. Resistant Starch Alters Gut Microbiome and Metabolomic Profiles Concurrent with Amelioration of Chronic Kidney Disease in Rats. Am. J. Physiol.-Ren. Physiol. 2016, 310, F857–F871. [Google Scholar] [CrossRef] [PubMed]
- Trautvetter, U.; Camarinha-Silva, A.; Jahreis, G.; Lorkowski, S.; Glei, M. High Phosphorus Intake and Gut-Related Parameters—Results of a Randomized Placebo-Controlled Human Intervention Study. Nutr. J. 2018, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.; Ruff, W.E.; Longbrake, E.E. Influence of Immunomodulatory Drugs on the Gut Microbiota. Transl. Res. 2021, 233, 144–161. [Google Scholar] [CrossRef] [PubMed]
- de Araújo, É.M.R.; Meneses, G.C.; Carioca, A.A.F.; Martins, A.M.C.; Daher, E.D.F.; da Silva Junior, G.B. Use of Probiotics in Patients with Chronic Kidney Disease on Hemodialysis: A Randomized Clinical Trial. Braz. J. Nephrol. 2022, 45, 152–161. [Google Scholar] [CrossRef]
- Favero, C.; Ortiz, A.; Sanchez-Niño, M.D. Probiotics for Kidney Disease. Clin. Kidney J. 2022, 15, 1981–1986. [Google Scholar] [CrossRef]
- Mandal, S.M. New Insights into the Bioactivity of Peptides from Probiotics. Front. Biosci. 2016, 8, 779. [Google Scholar] [CrossRef]
- Gou, H.-Z.; Zhang, Y.-L.; Ren, L.-F.; Li, Z.-J.; Zhang, L. How Do Intestinal Probiotics Restore the Intestinal Barrier? Front. Microbiol. 2022, 13, 929346. [Google Scholar] [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef]
- Ranganathan, N.; Ranganathan, P.; Friedman, E.A.; Joseph, A.; Delano, B.; Goldfarb, D.S.; Tam, P.; Venketeshwer, R.A.; Anteyi, E.; Guido Musso, C. Pilot Study of Probiotic Dietary Supplementation for Promoting Healthy Kidney Function in Patients with Chronic Kidney Disease. Adv. Ther. 2010, 27, 634–647. [Google Scholar] [CrossRef]
- Koppe, L.; Mafra, D.; Fouque, D. Probiotics and Chronic Kidney Disease. Kidney Int. 2015, 88, 958–966. [Google Scholar] [CrossRef]
- Fagundes, R.A.B.; Soder, T.F.; Grokoski, K.C.; Benetti, F.; Mendes, R.H. Probiotics in the Treatment of Chronic Kidney Disease: A Systematic Review. Braz. J. Nephrol. 2018, 40, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Merkling, T.; Metzger, M.; Koppe, L.; Laville, M.; Boutron-Ruault, M.-C.; Frimat, L.; Combe, C.; Massy, Z.A.; Stengel, B.; et al. Probiotic Intake and Inflammation in Patients with Chronic Kidney Disease: An Analysis of the CKD-REIN Cohort. Front. Nutr. 2022, 9, 772596. [Google Scholar] [CrossRef]
- Dai, Y.; Quan, J.; Xiong, L.; Luo, Y.; Yi, B. Probiotics Improve Renal Function, Glucose, Lipids, Inflammation and Oxidative Stress in Diabetic Kidney Disease: A Systematic Review and Meta-Analysis. Ren. Fail. 2022, 44, 862–880. [Google Scholar] [CrossRef] [PubMed]
- Brüssow, H. Probiotics and Prebiotics in Clinical Tests: An Update. F1000Research 2019, 8, 1157. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Meijers, B.K.I.; De Preter, V.; Verbeke, K.; Vanrenterghem, Y.; Evenepoel, P. P-Cresyl Sulfate Serum Concentrations in Haemodialysis Patients Are Reduced by the Prebiotic Oligofructose-Enriched Inulin. Nephrol. Dial. Transplant. 2010, 25, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhao, J.; Qin, Y.; Wang, Y.; Zhang, Y.; Sun, S. Probiotics, Prebiotics, and Synbiotics Improve Uremic, Inflammatory, and Gastrointestinal Symptoms in End-Stage Renal Disease with Dialysis: A Network Meta-Analysis of Randomized Controlled Trials. Front. Nutr. 2022, 9, 850425. [Google Scholar] [CrossRef]
- Sueyoshi, M.; Fukunaga, M.; Mei, M.; Nakajima, A.; Tanaka, G.; Murase, T.; Narita, Y.; Hirata, S.; Kadowaki, D. Effects of Lactulose on Renal Function and Gut Microbiota in Adenine-Induced Chronic Kidney Disease Rats. Clin. Exp. Nephrol. 2019, 23, 908–919. [Google Scholar] [CrossRef]
- Tayebi-Khosroshahi, H.; Habibzadeh, A.; Niknafs, B.; Ghotaslou, R.; Yeganeh Sefidan, F.; Ghojazadeh, M.; Moghaddaszadeh, M.; Parkhide, S. The Effect of Lactulose Supplementation on Fecal Microflora of Patients with Chronic Kidney Disease; a Randomized Clinical Trial. J. Renal Inj. Prev. 2016, 5, 162–167. [Google Scholar] [CrossRef]
- McFarlane, C.; Krishnasamy, R.; Stanton, T.; Savill, E.; Snelson, M.; Mihala, G.; Kelly, J.T.; Morrison, M.; Johnson, D.W.; Campbell, K.L. Synbiotics Easing Renal Failure by Improving Gut Microbiology II (SYNERGY II): A Feasibility Randomized Controlled Trial. Nutrients 2021, 13, 4481. [Google Scholar] [CrossRef] [PubMed]
- Mitrović, M.; Stanković-Popović, V.; Tolinački, M.; Golić, N.; Soković Bajić, S.; Veljović, K.; Nastasijević, B.; Soldatović, I.; Svorcan, P.; Dimković, N. The Impact of Synbiotic Treatment on the Levels of Gut-Derived Uremic Toxins, Inflammation, and Gut Microbiome of Chronic Kidney Disease Patients—A Randomized Trial. J. Ren. Nutr. 2023, 33, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Samuthpongtorn, C.; Kantagowit, P.; Pittayanon, R.; Patcharatrakul, T.; Gonlachanvit, S. Tu1063: Fecal microbiota transplantation in irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. Gastroenterology 2022, 162, S-868. [Google Scholar] [CrossRef]
- Kelly, C.R.; Khoruts, A.; Staley, C.; Sadowsky, M.J.; Abd, M.; Alani, M.; Bakow, B.; Curran, P.; McKenney, J.; Tisch, A.; et al. Effect of Fecal Microbiota Transplantation on Recurrence in Multiply Recurrent Clostridium difficile Infection. Ann. Intern. Med. 2016, 165, 609. [Google Scholar] [CrossRef]
- Evenepoel, P.; Meijers, B.K.I.; Bammens, B.R.M.; Verbeke, K. Uremic Toxins Originating from Colonic Microbial Metabolism. Kidney Int. 2009, 76, S12–S19. [Google Scholar] [CrossRef] [PubMed]
- Barba, C.; Soulage, C.O.; Caggiano, G.; Glorieux, G.; Fouque, D.; Koppe, L. Effects of Fecal Microbiota Transplantation on Composition in Mice with CKD. Toxins 2020, 12, 741. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Wang, X.; Liu, P.; Wang, L.; Li, Y.; Wang, X.; Ren, F. Fecal Microbiota Transplantation Restores Normal Fecal Composition and Delays Malignant Development of Mild Chronic Kidney Disease in Rats. Front. Microbiol. 2022, 13, 1037257. [Google Scholar] [CrossRef]
- Sturov, N.V.; Popov, S.V.; Belikov, I.I. Gut Microbiota and the Ways to Correct It in Chronic Kidney Disease. Indian. J. Nephrol. 2023, 33, 162–169. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N.-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef]
- Kaesler, N.; Baid-Agrawal, S.; Grams, S.; Nadal, J.; Schmid, M.; Schneider, M.P.; Eckardt, K.-U.; Floege, J.; Bergmann, M.M.; Schlieper, G.; et al. Low Adherence to CKD-Specific Dietary Recommendations Associates with Impaired Kidney Function, Dyslipidemia, and Inflammation. Eur. J. Clin. Nutr. 2021, 75, 1389–1397. [Google Scholar] [CrossRef]
- Simon, E.; Călinoiu, L.F.; Mitrea, L.; Vodnar, D.C. Probiotics, Prebiotics, and Synbiotics: Implications and Beneficial Effects against Irritable Bowel Syndrome. Nutrients 2021, 13, 2112. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S129–S134. [Google Scholar] [CrossRef]
- Dore, M.P.; Bibbò, S.; Fresi, G.; Bassotti, G.; Pes, G.M. Side Effects Associated with Probiotic Use in Adult Patients with Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2019, 11, 2913. [Google Scholar] [CrossRef]
- Lenoir-Wijnkoop, I.; Merenstein, D.; Korchagina, D.; Broholm, C.; Sanders, M.E.; Tancredi, D. Probiotics Reduce Health Care Cost and Societal Impact of Flu-Like Respiratory Tract Infections in the USA: An Economic Modeling Study. Front. Pharmacol. 2019, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- Wynn, A.B.; Beyer, G.; Richards, M.; Ennis, L.A. Procedure, Screening, and Cost of Fecal Microbiota Transplantation. Cureus 2023, 15, e35116. [Google Scholar] [CrossRef] [PubMed]
- Thanush, D.; Basavaraj, H.C.; Gowrav, M.P. Current Regulation and Initial Considerations for Successful Development and Commercialization of Microbiome Therapies. Adv. Gut Microbiome Res. 2023, 2023, 6657515. [Google Scholar] [CrossRef]
- Ianiro, G.; Punčochář, M.; Karcher, N.; Porcari, S.; Armanini, F.; Asnicar, F.; Beghini, F.; Blanco-Míguez, A.; Cumbo, F.; Manghi, P.; et al. Variability of Strain Engraftment and Predictability of Microbiome Composition after Fecal Microbiota Transplantation across Different Diseases. Nat. Med. 2022, 28, 1913–1923. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of Gut Bacteria on Human Health and Diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Thongprayoon, C.; Hatch, S.T.; Kaewput, W.; Sharma, K.; Ungprasert, P.; Wijarnpreecha, K.; D’Costa, M.; Mao, M.A.; Cheungpasitporn, W. The Effects of Probiotics on Renal Function and Uremic Toxins in Patients with Chronic Kidney Disease; a Meta-Analysis of Randomized Controlled Trials. J. Nephropathol. 2018, 7, 106–114. [Google Scholar] [CrossRef]
- Nguyen, T.T.U.; Kim, H.W.; Kim, W. Effects of Probiotics, Prebiotics, and Synbiotics on Uremic Toxins, Inflammation, and Oxidative Stress in Hemodialysis Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 4456. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, C.; Ramos, C.I.; Johnson, D.W.; Campbell, K.L. Prebiotic, Probiotic, and Synbiotic Supplementation in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Ren. Nutr. 2019, 29, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Takkavatakarn, K.; Wuttiputinun, T.; Phannajit, J.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Protein-Bound Uremic Toxin Lowering Strategies in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. J. Nephrol. 2021, 34, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhong, J.; Yang, H.; Wang, D.; Zhang, Y.; Yang, Y.; Xing, G.; Kon, V. Biotic Supplements in Patients with Chronic Kidney Disease: Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 2022, 32, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Wang, X.; Li, R.; Zhang, Z.Y.; Fang, J.; Zhang, X. Effects of Probiotic Preparations on Inflammatory Cytokines in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. Curr. Pharm. Biotechnol. 2021, 22, 1338–1349. [Google Scholar] [CrossRef]
- Tao, S.; Tao, S.; Cheng, Y.; Liu, J.; Ma, L.; Fu, P. Effects of Probiotic Supplements on the Progression of Chronic Kidney Disease: A Meta-analysis. Nephrology 2019, 24, 1122–1130. [Google Scholar] [CrossRef]
- Jia, L.; Jia, Q.; Yang, J.; Jia, R.; Zhang, H. Efficacy of Probiotics Supplementation on Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2018, 43, 1623–1635. [Google Scholar] [CrossRef]
- Jia, L.; Dong, X.; Li, X.; Jia, R.; Zhang, H.-L. Benefits of Resistant Starch Type 2 for Patients with End-Stage Renal Disease under Maintenance Hemodialysis: A Systematic Review and Meta-Analysis. Int. J. Med. Sci. 2021, 18, 811–820. [Google Scholar] [CrossRef]
- Chen, C.; Wang, J.; Li, J.; Zhang, W.; Ou, S. Probiotics, Prebiotics, and Synbiotics for Patients on Dialysis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Ren. Nutr. 2023, 33, 126–139. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Qin, Y.; Yu, Z.; Zhang, Y.; Ning, X.; Sun, S. The Specific Alteration of Gut Microbiota in Diabetic Kidney Diseases—A Systematic Review and Meta-Analysis. Front. Immunol. 2022, 13, 908219. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.J.; Guo, J.; Wang, Q.; Wang, L.; Wang, Y.; Zhang, F.; Huang, W.-J.; Zhang, W.; Liu, W.J.; Wang, Y. Probiotics, Prebiotics, and Synbiotics for the Improvement of Metabolic Profiles in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Crit. Rev. Food Sci. Nutr. 2021, 61, 577–598. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, H.; Guo, J.; Zhang, M.; Zheng, H.; Liu, Y.; Liu, W. Gut Microbiota-Derived Trimethylamine N-Oxide Is Associated with the Risk of All-Cause and Cardiovascular Mortality in Patients with Chronic Kidney Disease: A Systematic Review and Dose-Response Meta-Analysis. Ann. Med. 2023, 55, 2215542. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef]
- Mocanu, A.; Bogos, R.A.; Lazaruc, T.I.; Trandafir, L.M.; Lupu, V.V.; Ioniuc, I.; Alecsa, M.; Ivanov, A.; Lupu, A.; Starcea, I.M. Exploring a Complex Interplay: Kidney–Gut Axis in Pediatric Chronic Kidney Disease. Nutrients 2023, 15, 3609. [Google Scholar] [CrossRef]
- Berding, K.; Vlckova, K.; Marx, W.; Schellekens, H.; Stanton, C.; Clarke, G.; Jacka, F.; Dinan, T.G.; Cryan, J.F. Diet and the Microbiota–Gut–Brain Axis: Sowing the Seeds of Good Mental Health. Adv. Nutr. 2021, 12, 1239–1285. [Google Scholar] [CrossRef]
- Bryniarski, M.A.; Hamarneh, F.; Yacoub, R. The Role of Chronic Kidney Disease-Associated Dysbiosis in Cardiovascular Disease. Exp. Biol. Med. 2019, 244, 514–525. [Google Scholar] [CrossRef]
- Hernández-Calderón, P.; Wiedemann, L.; Benítez-Páez, A. The Microbiota Composition Drives Personalized Nutrition: Gut Microbes as Predictive Biomarkers for the Success of Weight Loss Diets. Front. Nutr. 2022, 9, 1006747. [Google Scholar] [CrossRef]
- Stanigut, A.M.; Pana, C.; Enciu, M.; Deacu, M.; Cimpineanu, B.; Tuta, L.A. Hypoxia-Inducible Factors and Diabetic Kidney Disease—How Deep Can We Go? Int. J. Mol. Sci. 2022, 23, 10413. [Google Scholar] [CrossRef]
- Giannese, D.; D’Alessandro, C.; Panichi, V.; Pellegrino, N.; Cupisti, A. Nutritional Treatment as a Synergic Intervention to Pharmacological Therapy in CKD Patients. Nutrients 2023, 15, 2715. [Google Scholar] [CrossRef]
- Belo, L.; Carvalho, M. Chronic Kidney Disease: Underlying Molecular Mechanisms—A Special Issue Overview. Int. J. Mol. Sci. 2023, 24, 12363. [Google Scholar] [CrossRef] [PubMed]
- Strianese, O.; Rizzo, F.; Ciccarelli, M.; Galasso, G.; D’Agostino, Y.; Salvati, A.; Del Giudice, C.; Tesorio, P.; Rusciano, M.R. Precision and Personalized Medicine: How Genomic Approach Improves the Management of Cardiovascular and Neurodegenerative Disease. Genes 2020, 11, 747. [Google Scholar] [CrossRef] [PubMed]

| Potential Contraindications | Proposed Side Effect | References |
|---|---|---|
| Compromised immunity state | Introducing new GM may disrupt the delicate gut balance and potentially lead to infections. | Thursby et al. [139]; 2017 |
| Medication interaction | Those patients are often on multiple medications, such as immunosuppressants and antibiotics, which impact GM composition and may interact with any introduced microbial modulation. | Chakraborty et al. [32]; 2016 |
| Fluid and electrolyte imbalance | Altering the GM in cases with these imbalances potentially worsens the condition. | Rapa et al. [140]; 2020 |
| Dialysis consideration | The impact of GM therapies on dialysis efficiency or complications is not well understood. | Tang et al. [141]; 2015 |
| Method Modulating GM | Pros | Cons | References |
|---|---|---|---|
| Dietary interventions | Effective, non-invasive, and generally well-tolerated have additional benefits, such as improving cardiovascular health. |
| Kaesler et al. [142]; 2021 |
| Probiotics and/or prebiotics | Safe for most individuals. |
| Simon et al. [143]; 2021 Doron et al. [144]; 2015 Dore et al. [145]; 2019 Lenoir-Wijnkoop et al. [146]; 2019 |
| Fecal Microbiota Transplantation | Safe when it performed under appropriate medical supervision and with proper screening protocols for donors. |
| Wynn et al. [147]; 2023 |
| References | Study Modality Number and Type of Studies Examined | Number of Participants and Their Criteria | Key Findings |
|---|---|---|---|
| Thongprayoon et al. [151]; 2018 | Meta-analysis was conducted on five randomized controlled trials (RCT) | 161 participants with chronic kidney cases (CKD) | Beneficial effects of probiotics on uremic toxins in CKD patients. |
| Nguyen et al. [152]; 2021 | Systematic Review and Meta-analysis on 23 RCT | 931 participants On hemodialysis patients | Supplementation with probiotics, prebiotics, and synbiotics significantly decreased circulating levels of various uremic toxins and inflammatory biomarkers. A potential therapeutic benefit in alleviating uremic toxin levels, oxidative stress, and inflammation in hemodialysis patients. |
| McFarlane et al. [153]; 2019 | Systematic Review and Meta-analysis On 16 RCT | 645 participants adults and children with CKD. | Prebiotics supplementations have slightly reduced serum urea concentration. However, the evidence was limited. |
| Yu et al. [128]; 2022 | Network Meta-analysis on 25 RCT | 1106 participants in ESKD With Dialysis | Prebiotics were found to be effective in reducing certain inflammatory markers and uremic toxins. Synbiotics were effective in reducing CRP and endotoxin levels. Probiotics were beneficial in alleviating gastrointestinal symptoms. This study provides better clinical decisions in treating ESRD patients. |
| Takkavatakarn et al. [154]; 2021 | Systematic Review and Meta-analysis on 38 articles including observational and RCTs. | 2492 participants with CKD on dialysis | Protein-bound uremic toxins, including indoxyl sulfate and p-cresyl sulfate, are linked with increased cardiovascular risks in CKD. Strategies such as prebiotics, synbiotics, and AST-120 effectively reduce these toxins. |
| Liu et al. [155]; 2022 | Systematic Review and Meta-analysis on 23 RCT | 842 participants with CKD | Probiotics favorably influenced markers of creatinine, oxidant stress, inflammation, and certain uremic toxins in CKD patients. |
| Yang et al. [107]; 2021 | Meta-analysis on 10 RCT | 292 participants With CKD | Dietary fiber supplementation can significantly reduce levels of specific uremic toxins in CKD patients. This provides evidence for the clinical recommendation in practice. |
| Liu et al. [156]; 2020 | Systematic Review and Meta-analysis on 16 RCT | 605 participants with CKD | Probiotics significantly decreased serum levels of certain inflammatory cytokines in CKD patients, such as CRP and IL-6. They did not significantly affect serum uremic toxin levels, including creatine, urea, uric acid, PCS, and IS. The results help treatment decisions in clinical practice. |
| Tao et al. [157]; 2019 | Meta-analysis on 10 RCT | 359 cases with CKD to assess progression | The study suggests that probiotics can reduce urea levels in non-dialysis CKD patients. |
| Jia et al. [158]; 2018 | Systematic Review and Meta-analysis on 8 RCT | 261 CKD patients (stage 3 to 5) with and without dialysis | Dysbiosis of the intestinal microbiota may accelerate CKD progression by increasing urea toxin levels. Probiotics have been recognized to maintain the physiological balance. |
| Jia et al. [159]; 2021 | Systematic Review and Meta-analysis on 5 RCT | 179 CKD cases | A significant reduction in blood urea nitrogen, serum creatinine, and interleukin (IL)-6 levels in the RS2 group. The findings suggest that RS2 might improve residual renal function in MHD patients and reduce proinflammatory responses. |
| Chen et al. [160]; 2023 | Meta-analysis on 18 RCT | 237 cases on Dialysis | Probiotics, prebiotics, and synbiotics supplements could reduce levels of C-reactive protein, interleukin 6, and indoxyl sulfate and increase high-density lipoprotein cholesterol compared to the control group. |
| Wang et al. [161]; 2022 | Meta-Analysis examined 16 case-control or cross-sectional studies | 1022 participants (578 patients with Diabetic KD and 444 Healthy controls) | Patients with diabetic kidney disease (DKD) had significantly decreased bacterial richness. The gut microbiota of patients with DKD had specific features characterized by the expansion of genera like Escherichia, Citrobacter, and Klebsiella, and depletion of Roseburia. These microbial taxa might be closely related to DKD and could serve as potential targets for DKD management. |
| Zheng et al. [162]; 2021 | Meta-Analysis examined 13 RCT | 671 CKD cases | Microbial therapies significantly reduced levels of C-reactive protein, malondialdehyde, total cholesterol, and low-density lipoprotein cholesterol. Increased glutathione levels, total antioxidant capacity, and high-density lipoprotein cholesterol in CKD patients compared to placebo groups. The findings support the potential use of probiotic, prebiotic, and synbiotic supplements in improving cardiovascular risk factors in CKD patients. |
| Dai et al. [123]; 2022 | Meta-Analysis examined 10 RCT | 552 participants with diabetic KD | Probiotics can delay renal function injury, improve glucose and lipid metabolism, and reduce inflammation and oxidative stress in DKD patients. |
| Li et al. [163]; 2023 | Meta-Analysis examined 21 cohort, case-control, nested case-control, or analytic cross-sectional studies | 15,637 participants that were non-CKD vs. non-black dialysis patients. | Non-dialysis CKD patients and non-black dialysis patients with the highest circulating TMAO concentration had an increased risk of all-cause mortality. Non-black dialysis patients with the highest TMAO concentration also had an increased risk of cardiovascular mortality. Increased circulating TMAO concentrations are associated with higher mortality risks in specific CKD patient groups. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantazi, A.C.; Kassim, M.A.K.; Nori, W.; Tuta, L.A.; Mihai, C.M.; Chisnoiu, T.; Balasa, A.L.; Mihai, L.; Lupu, A.; Frecus, C.E.; et al. Clinical Perspectives of Gut Microbiota in Patients with Chronic Kidney Disease and End-Stage Kidney Disease: Where Do We Stand? Biomedicines 2023, 11, 2480. https://doi.org/10.3390/biomedicines11092480
Pantazi AC, Kassim MAK, Nori W, Tuta LA, Mihai CM, Chisnoiu T, Balasa AL, Mihai L, Lupu A, Frecus CE, et al. Clinical Perspectives of Gut Microbiota in Patients with Chronic Kidney Disease and End-Stage Kidney Disease: Where Do We Stand? Biomedicines. 2023; 11(9):2480. https://doi.org/10.3390/biomedicines11092480
Chicago/Turabian StylePantazi, Alexandru Cosmin, Mustafa Ali Kassim Kassim, Wassan Nori, Liliana Ana Tuta, Cristina Maria Mihai, Tatiana Chisnoiu, Adriana Luminita Balasa, Larisia Mihai, Ancuta Lupu, Corina Elena Frecus, and et al. 2023. "Clinical Perspectives of Gut Microbiota in Patients with Chronic Kidney Disease and End-Stage Kidney Disease: Where Do We Stand?" Biomedicines 11, no. 9: 2480. https://doi.org/10.3390/biomedicines11092480
APA StylePantazi, A. C., Kassim, M. A. K., Nori, W., Tuta, L. A., Mihai, C. M., Chisnoiu, T., Balasa, A. L., Mihai, L., Lupu, A., Frecus, C. E., Lupu, V. V., Chirila, S. I., Badescu, A. G., Hangan, L.-T., & Cambrea, S. C. (2023). Clinical Perspectives of Gut Microbiota in Patients with Chronic Kidney Disease and End-Stage Kidney Disease: Where Do We Stand? Biomedicines, 11(9), 2480. https://doi.org/10.3390/biomedicines11092480










