The Role of Hypoxia on the Trimethylation of H3K27 in Podocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Podocyte Cell Culture
2.2. Reverse Transcription and Real-Time PCR
2.3. Western Blot Analysis
2.4. Immunofluorescence Staining of Cultured Differentiated Podocytes
2.5. Animal Studies
2.6. Double-Immunofluorescence Staining of Paraffin Kidney Sections
2.7. Statistics
3. Results
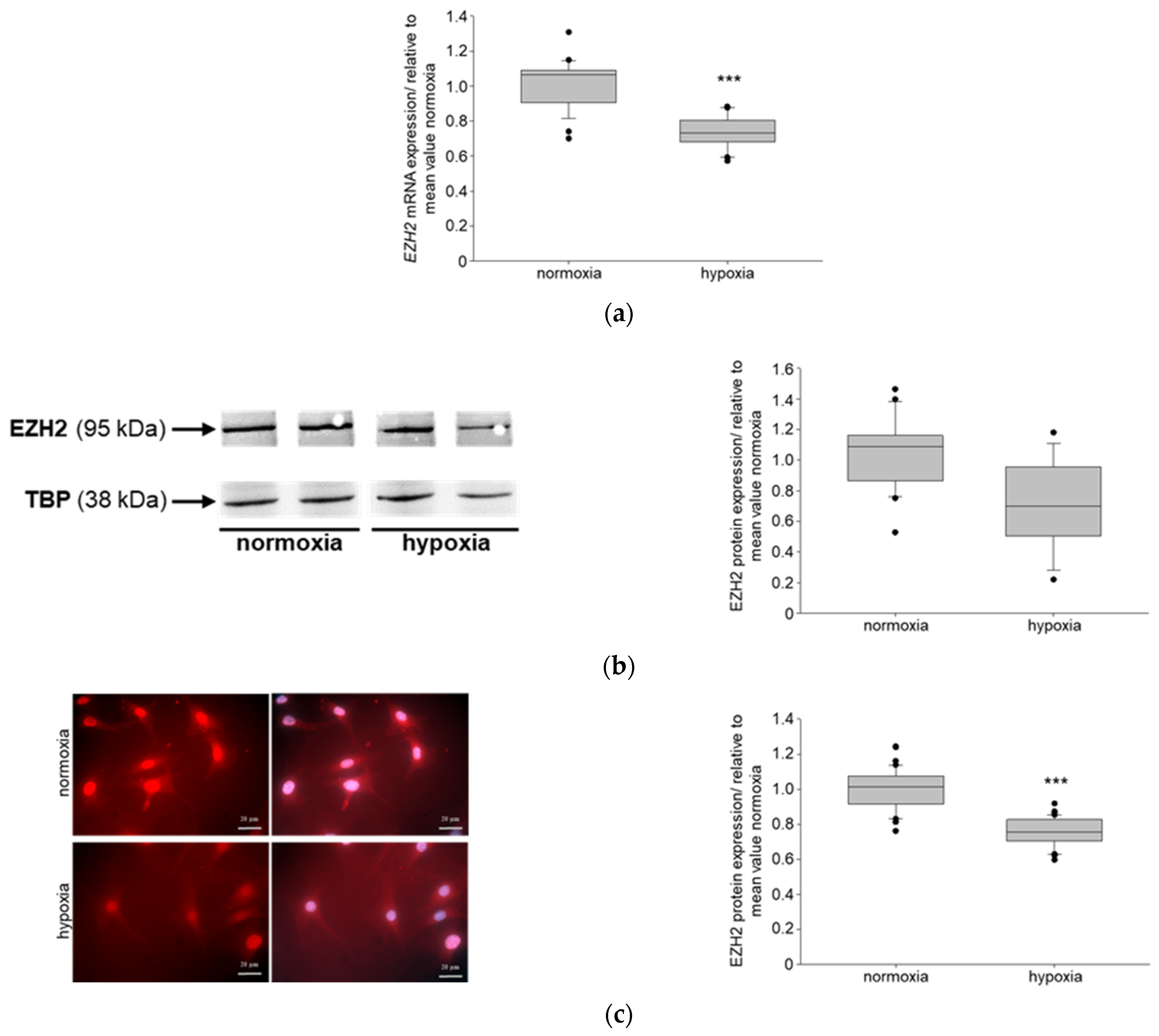
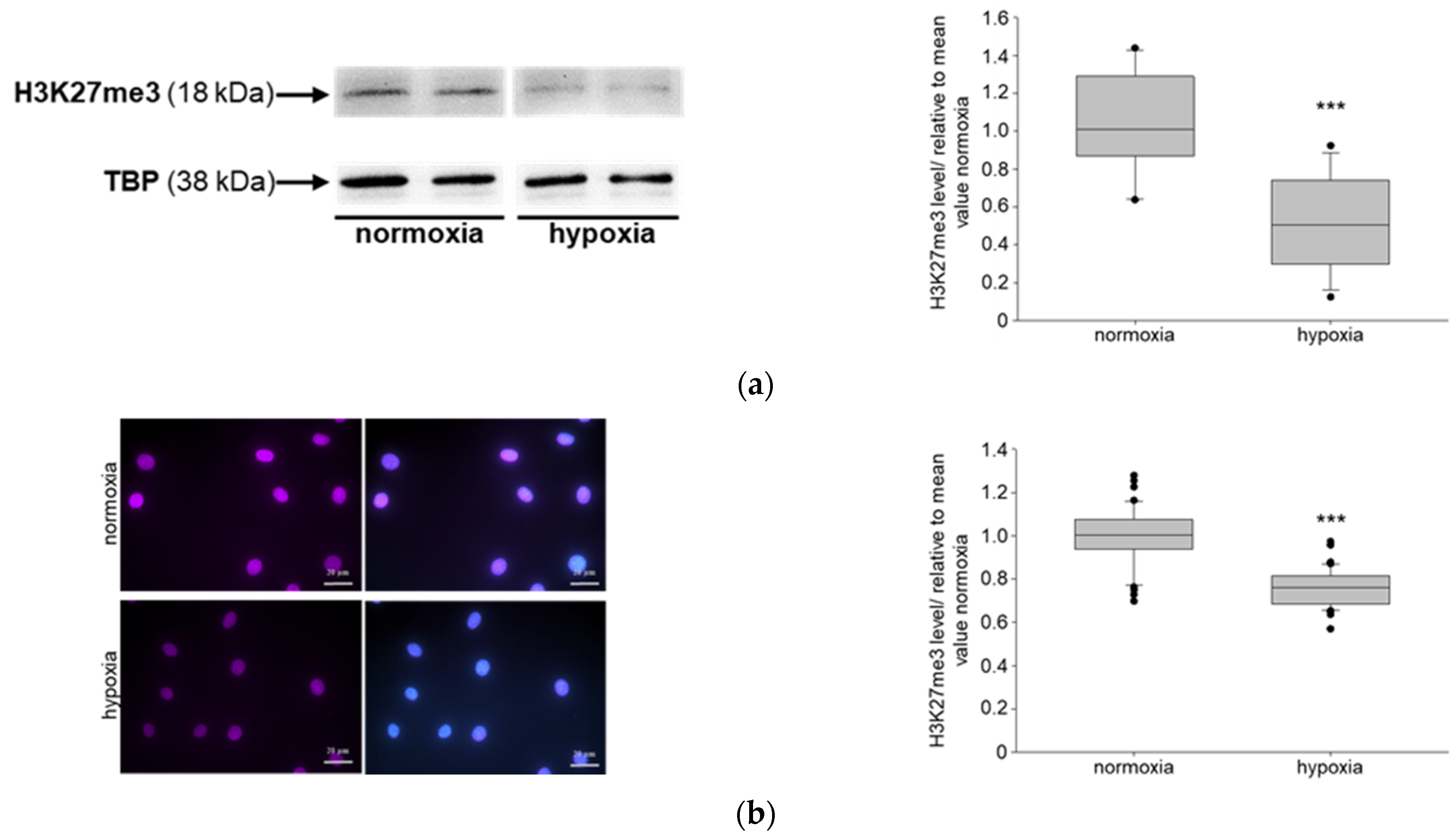
3.1. Hypoxia Affects NIPP1, EZH2 and H3K27me3 in Podocytes In Vitro
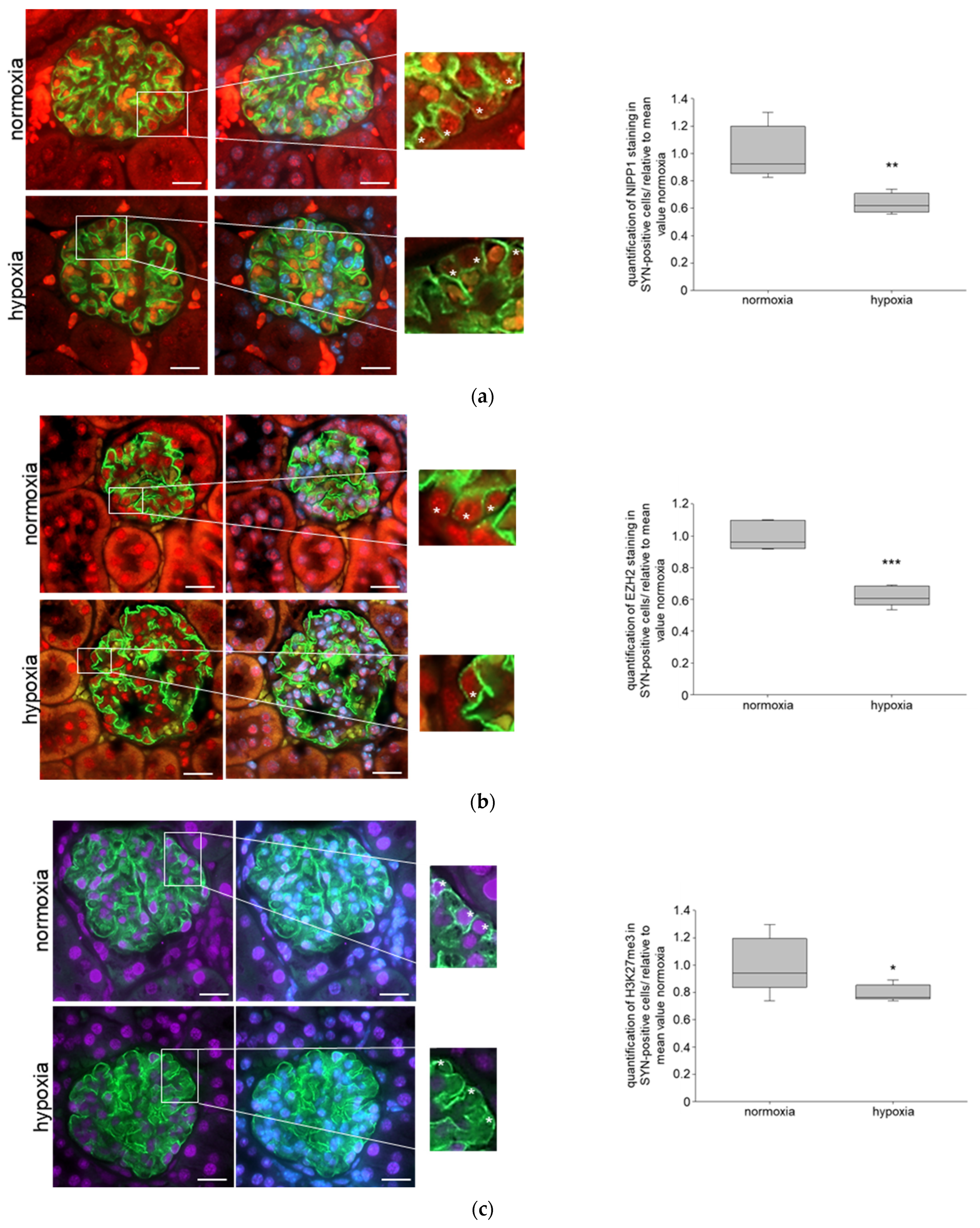
3.2. Systemic Hypoxia Influences NIPP1 and EZH2 Protein Expression and Trimethylation of H3K27
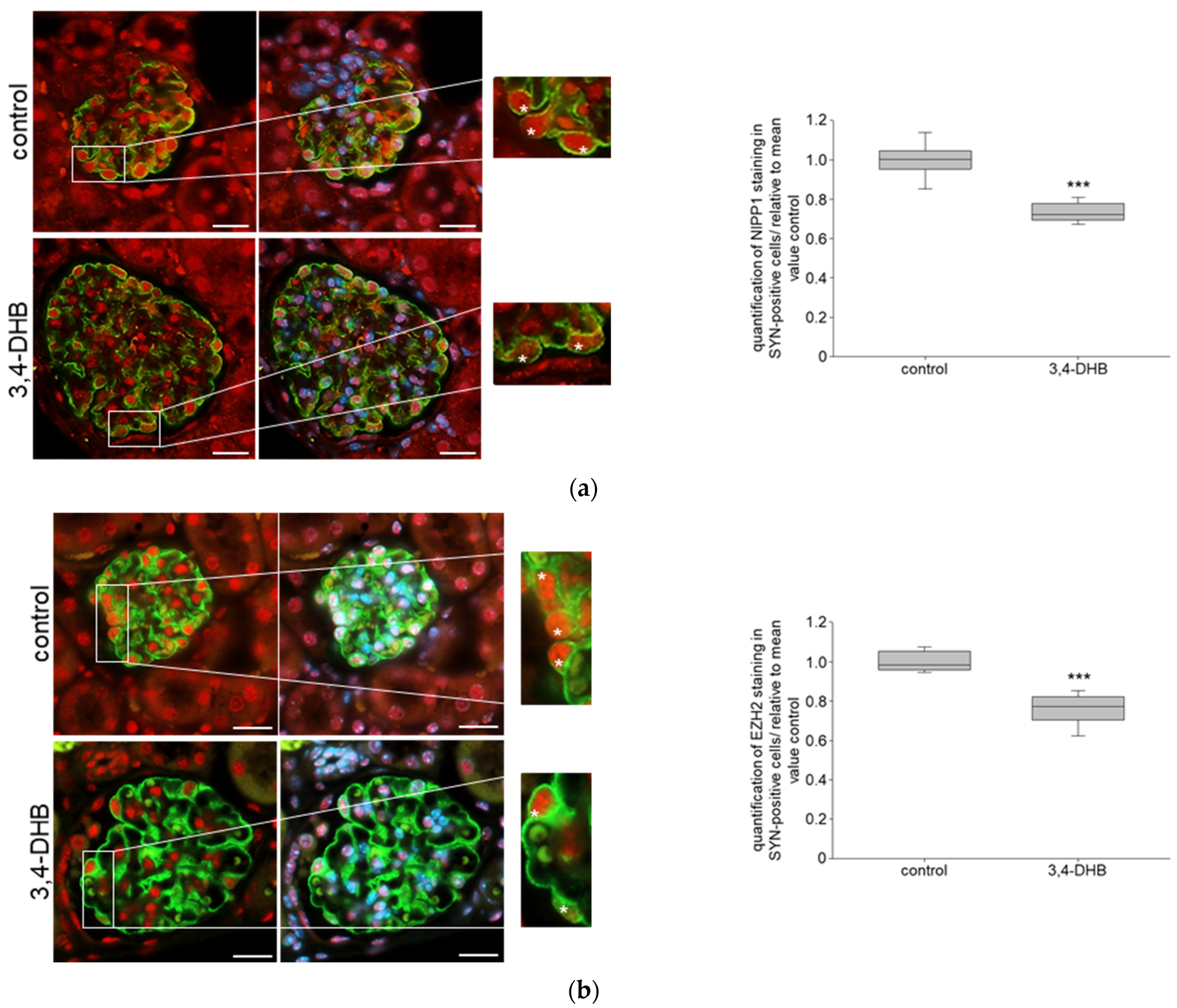
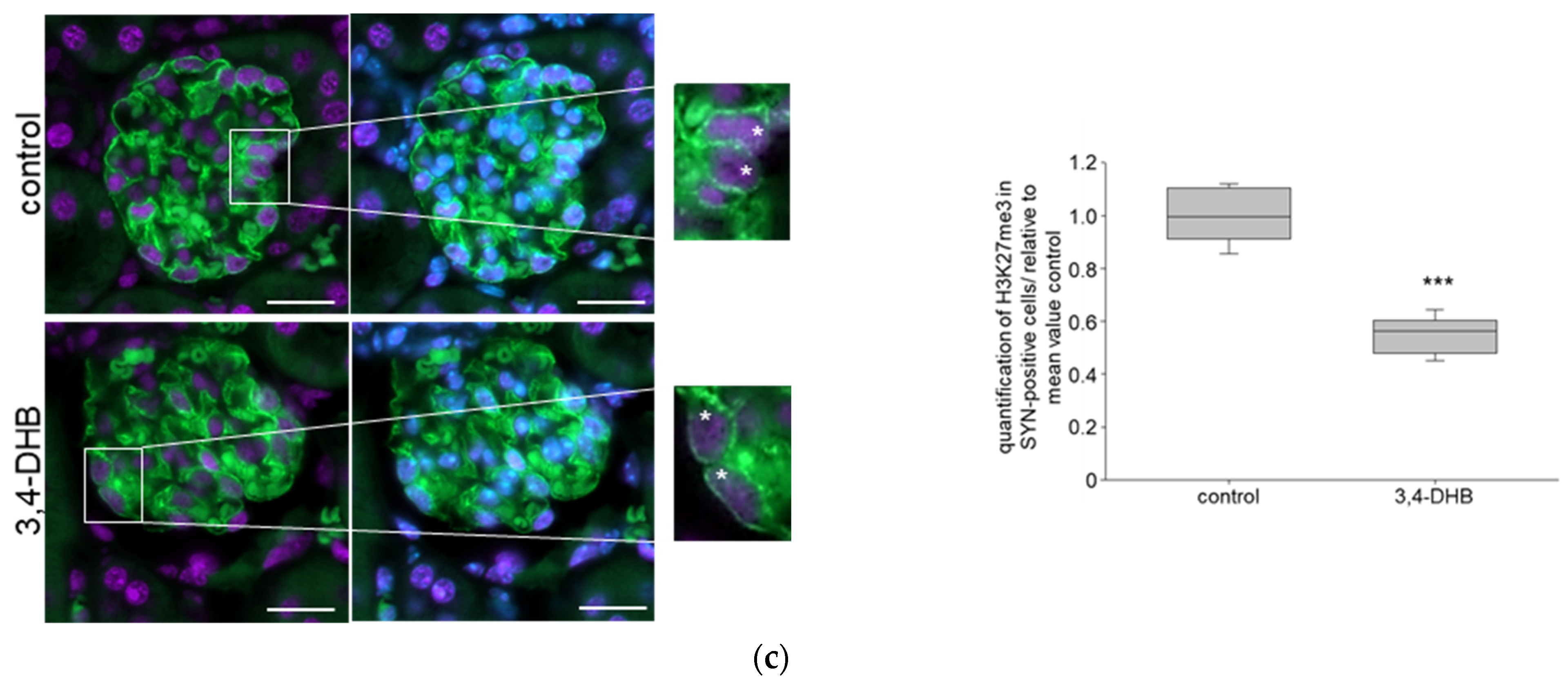
3.3. Pharmacological Activation of HIFs Reduces the Expression of NIPP1 and EZH2 as Well as H3K27 Trimethlyation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. (2011) 2022, 12, 7–11. [Google Scholar] [CrossRef]
- Reidy, K.; Kang, H.M.; Hostetter, T.; Susztak, K. Molecular mechanisms of diabetic kidney disease. J. Clin. Investig. 2014, 124, 2333–2340. [Google Scholar] [CrossRef] [PubMed]
- Conti, S.; Perico, N.; Novelli, R.; Carrara, C.; Benigni, A.; Remuzzi, G. Early and late scanning electron microscopy findings in diabetic kidney disease. Sci. Rep. 2018, 8, 4909. [Google Scholar] [CrossRef] [PubMed]
- Rutkowski, J.M.; Wang, Z.V.; Park, A.S.; Zhang, J.; Zhang, D.; Hu, M.C.; Moe, O.W.; Susztak, K.; Scherer, P.E. Adiponectin promotes functional recovery after podocyte ablation. J. Am. Soc. Nephrol. 2013, 24, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Wharram, B.L.; Goyal, M.; Wiggins, J.E.; Sanden, S.K.; Hussain, S.; Filipiak, W.E.; Saunders, T.L.; Dysko, R.C.; Kohno, K.; Holzman, L.B.; et al. Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J. Am. Soc. Nephrol. 2005, 16, 2941–2952. [Google Scholar] [CrossRef]
- Matsusaka, T.; Xin, J.; Niwa, S.; Kobayashi, K.; Akatsuka, A.; Hashizume, H.; Wang, Q.C.; Pastan, I.; Fogo, A.B.; Ichikawa, I. Genetic engineering of glomerular sclerosis in the mouse via control of onset and severity of podocyte-specific injury. J. Am. Soc. Nephrol. 2005, 16, 1013–1023. [Google Scholar] [CrossRef]
- Margueron, R.; Reinberg, D. The Polycomb complex PRC2 and its mark in life. Nature 2011, 469, 343–349. [Google Scholar] [CrossRef]
- Beisswenger, P.; Ruggiero-Lopez, D. Metformin inhibition of glycation processes. Diabetes Metab. 2003, 29 Pt 2, 6S95–6S103. [Google Scholar] [CrossRef]
- Liebisch, M.; Bondeva, T.; Franke, S.; Daniel, C.; Amann, K.; Wolf, G. Activation of the receptor for advanced glycation end products induces nuclear inhibitor of protein phosphatase-1 suppression. Kidney Int. 2014, 86, 103–117. [Google Scholar] [CrossRef]
- Liebisch, M.; Wolf, G. AGE-Induced Suppression of EZH2 Mediates Injury of Podocytes by Reducing H3K27me3. Am. J. Nephrol. 2020, 51, 676–692. [Google Scholar] [CrossRef]
- Kushwaha, K.; Sharma, S.; Gupta, J. Metabolic memory and diabetic nephropathy: Beneficial effects of natural epigenetic modifiers. Biochimie 2020, 170, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, L.; Hou, W.; Huang, T.; Chen, X.; Qi, J.; Zhao, Y.; Zhu, M. Epigenetics in the pathogenesis of diabetic nephropathy. Acta Biochim. Biophys. Sin. 2022, 54, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Zhen, J.; Zhang, C.; Wan, Q.; Liu, G.; Wei, X.; Zhang, Y.; Wang, Z.; Han, H.; et al. Histone deacetylase 4 selectively contributes to podocyte injury in diabetic nephropathy. Kidney Int. 2014, 86, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Medina Rangel, P.X.; Cross, E.; Liu, C.; Pedigo, C.E.; Tian, X.; Gutierrez-Calabres, E.; Nagata, S.; Priyadarshini, A.; Lerner, G.; Bunda, P.; et al. Cell Cycle and Senescence Regulation by Podocyte Histone Deacetylase 1 and 2. J. Am. Soc. Nephrol. 2023, 34, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Inagi, R. Epigenetic Regulation Through SIRT1 in Podocytes. Curr. Hypertens. Rev. 2016, 12, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liang, K.; Zhen, J.; Zhou, M.; Wang, X.; Wang, Z.; Wei, X.; Zhang, Y.; Sun, Y.; Zhou, Z.; et al. Sirt6 deficiency exacerbates podocyte injury and proteinuria through targeting Notch signaling. Nat. Commun. 2017, 8, 413. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Sasamura, H.; Nakamura, M.; Sakamaki, Y.; Azegami, T.; Oguchi, H.; Tokuyama, H.; Wakino, S.; Hayashi, K.; Itoh, H. Renin-angiotensin blockade resets podocyte epigenome through Kruppel-like Factor 4 and attenuates proteinuria. Kidney Int. 2015, 88, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Sasamura, H.; Nakamura, M.; Azegami, T.; Oguchi, H.; Sakamaki, Y.; Itoh, H. KLF4-dependent epigenetic remodeling modulates podocyte phenotypes and attenuates proteinuria. J. Clin. Investig. 2014, 124, 2523–2537. [Google Scholar] [CrossRef]
- Ettou, S.; Jung, Y.L.; Miyoshi, T.; Jain, D.; Hiratsuka, K.; Schumacher, V.; Taglienti, M.E.; Morizane, R.; Park, P.J.; Kreidberg, J.A. Epigenetic transcriptional reprogramming by WT1 mediates a repair response during podocyte injury. Sci. Adv. 2020, 6, eabb5460. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.L.; Zhang, Y.L.; Wen, Y.; Gao, Y.M.; Liu, B.C. Hypoxia and chronic kidney disease. EBioMedicine 2022, 77, 103942. [Google Scholar] [CrossRef]
- Sugahara, M.; Pak, W.L.W.; Tanaka, T.; Tang, S.C.W.; Nangaku, M. Update on diagnosis, pathophysiology, and management of diabetic kidney disease. Nephrology 2021, 26, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Hesp, A.C.; Schaub, J.A.; Prasad, P.V.; Vallon, V.; Laverman, G.D.; Bjornstad, P.; van Raalte, D.H. The role of renal hypoxia in the pathogenesis of diabetic kidney disease: A promising target for newer renoprotective agents including SGLT2 inhibitors? Kidney Int. 2020, 98, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, I.; Wolf, G. The role of hypoxia and Morg1 in renal injury. Eur. J. Clin. Investig. 2015, 45, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Stanigut, A.M.; Pana, C.; Enciu, M.; Deacu, M.; Cimpineanu, B.; Tuta, L.A. Hypoxia-Inducible Factors and Diabetic Kidney Disease-How Deep Can We Go? Int. J. Mol. Sci. 2022, 23, 413. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Kolligundla, L.P.; Francis, J.; Pasupulati, A.K. Detrimental effects of hypoxia on glomerular podocytes. J. Physiol. Biochem. 2021, 77, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.M.; Hwang, D.Y.; Chen, S.C.; Kuo, M.C.; Hung, C.C.; Hwang, S.J.; Tsai, J.C.; Chen, H.C. B7-1 expression regulates the hypoxia-driven cytoskeleton rearrangement in glomerular podocytes. Am. J. Physiol. Renal Physiol. 2013, 304, F127–F136. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Kapur, G.; Mattoo, T.K.; Lyman, W.D. Hypoxia decreases podocyte expression of slit diaphragm proteins. Int. J. Nephrol. Renovasc. Dis. 2012, 5, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Nakuluri, K.; Mukhi, D.; Mungamuri, S.K.; Pasupulati, A.K. Stabilization of hypoxia-inducible factor 1alpha by cobalt chloride impairs podocyte morphology and slit-diaphragm function. J. Cell. Biochem. 2019, 120, 7667–7678. [Google Scholar] [CrossRef]
- Nakuluri, K.; Mukhi, D.; Nishad, R.; Saleem, M.A.; Mungamuri, S.K.; Menon, R.K.; Pasupulati, A.K. Hypoxia induces ZEB2 in podocytes: Implications in the pathogenesis of proteinuria. J. Cell. Physiol. 2019, 234, 6503–6518. [Google Scholar] [CrossRef]
- Nakuluri, K.; Nishad, R.; Mukhi, D.; Kumar, S.; Nakka, V.P.; Kolligundla, L.P.; Narne, P.; Natuva, S.S.K.; Phanithi, P.B.; Pasupulati, A.K. Cerebral ischemia induces TRPC6 via HIF1alpha/ZEB2 axis in the glomerular podocytes and contributes to proteinuria. Sci. Rep. 2019, 9, 17897. [Google Scholar] [CrossRef]
- Takahashi, N.; Yoshida, H.; Kimura, H.; Kamiyama, K.; Kurose, T.; Sugimoto, H.; Imura, T.; Yokoi, S.; Mikami, D.; Kasuno, K.; et al. Chronic hypoxia exacerbates diabetic glomerulosclerosis through mesangiolysis and podocyte injury in db/db mice. Nephrol. Dial. Transplant. 2020, 35, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhang, X.; Xue, C.; Zhang, H.; Shashaty, M.G.; Gosai, S.J.; Meyer, N.; Grazioli, A.; Hinkle, C.; Caughey, J.; et al. The long noncoding RNA landscape in hypoxic and inflammatory renal epithelial injury. Am. J. Physiol. Renal Physiol. 2015, 309, F901–F913. [Google Scholar] [CrossRef] [PubMed]
- Kroening, S.; Neubauer, E.; Wullich, B.; Aten, J.; Goppelt-Struebe, M. Characterization of connective tissue growth factor expression in primary cultures of human tubular epithelial cells: Modulation by hypoxia. Am. J. Physiol. Renal Physiol. 2010, 298, F796–F806. [Google Scholar] [CrossRef] [PubMed]
- Nangaku, M.; Hirakawa, Y.; Mimura, I.; Inagi, R.; Tanaka, T. Epigenetic Changes in the Acute Kidney Injury-to-Chronic Kidney Disease Transition. Nephron 2017, 137, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Tanemoto, F.; Nangaku, M.; Mimura, I. Epigenetic memory contributing to the pathogenesis of AKI-to-CKD transition. Front. Mol. Biosci. 2022, 9, 1003227. [Google Scholar] [CrossRef] [PubMed]
- Schiwek, D.; Endlich, N.; Holzman, L.; Holthofer, H.; Kriz, W.; Endlich, K. Stable expression of nephrin and localization to cell-cell contacts in novel murine podocyte cell lines. Kidney Int. 2004, 66, 91–101. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Loeffler, I.; Wolf, G. Morg1 heterozygous deficiency ameliorates hypoxia-induced acute renal injury. Am. J. Physiol. Renal Physiol. 2015, 308, F511–F521. [Google Scholar] [CrossRef][Green Version]
- Schindler, K.; Bondeva, T.; Schindler, C.; Claus, R.A.; Franke, S.; Wolf, G. Preconditioned suppression of prolyl-hydroxylases attenuates renal injury but increases mortality in septic murine models. Nephrol. Dial. Transplant. 2016, 31, 1100–1113. [Google Scholar] [CrossRef]
- Cockwell, P.; Fisher, L.A. The global burden of chronic kidney disease. Lancet 2020, 395, 662–664. [Google Scholar] [CrossRef]
- Majumder, S.; Thieme, K.; Batchu, S.N.; Alghamdi, T.A.; Bowskill, B.B.; Kabir, M.G.; Liu, Y.; Advani, S.L.; White, K.E.; Geldenhuys, L.; et al. Shifts in podocyte histone H3K27me3 regulate mouse and human glomerular disease. J. Clin. Investig. 2018, 128, 483–499. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, F.S.; Majumder, S.; Thai, K.; Abdalla, M.; Hu, P.; Advani, S.L.; White, K.E.; Bowskill, B.B.; Guarna, G.; Dos Santos, C.C.; et al. The Histone Methyltransferase Enzyme Enhancer of Zeste Homolog 2 Protects against Podocyte Oxidative Stress and Renal Injury in Diabetes. J. Am. Soc. Nephrol. 2016, 27, 2021–2034. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pamulapati, H.; Tikoo, K. Fatty acid induced metabolic memory involves alterations in renal histone H3K36me2 and H3K27me3. Mol. Cell. Endocrinol. 2016, 422, 233–242. [Google Scholar] [CrossRef]
- Komers, R.; Mar, D.; Denisenko, O.; Xu, B.; Oyama, T.T.; Bomsztyk, K. Epigenetic changes in renal genes dysregulated in mouse and rat models of type 1 diabetes. Lab. Investig. 2013, 93, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Reddy, M.A.; Das, S.; Oh, H.J.; Abdollahi, M.; Yuan, H.; Zhang, E.; Lanting, L.; Wang, M.; Natarajan, R. Dysregulation of histone H3 lysine 27 trimethylation in transforming growth factor-beta1-induced gene expression in mesangial cells and diabetic kidney. J. Biol. Chem. 2019, 294, 12695–12707. [Google Scholar] [CrossRef] [PubMed]
- Thieme, K.; Pereira, B.M.V.; da Silva, K.S.; Fabre, N.T.; Catanozi, S.; Passarelli, M.; Correa-Giannella, M.L. Chronic advanced-glycation end products treatment induces TXNIP expression and epigenetic changes in glomerular podocytes in vivo and in vitro. Life Sci. 2021, 270, 118997. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Li, P. O-linked N-acetylglucosaminyltransferase OGT inhibits diabetic nephropathy by stabilizing histone methyltransferases EZH2 via the HES1/PTEN axis. Life Sci. 2021, 274, 119226. [Google Scholar] [CrossRef]
- Yang, Y.; Luan, Y.; Feng, Q.; Chen, X.; Qin, B.; Ren, K.D.; Luan, Y. Epigenetics and Beyond: Targeting Histone Methylation to Treat Type 2 Diabetes Mellitus. Front. Pharmacol. 2021, 12, 807413. [Google Scholar] [CrossRef]
- Guo, Y.; Xiong, Z.; Guo, X. Histone demethylase KDM6B regulates human podocyte differentiation in vitro. Biochem. J. 2019, 476, 1741–1751. [Google Scholar] [CrossRef]
- Huang, Y.S.; Hsieh, H.Y.; Shih, H.M.; Sytwu, H.K.; Wu, C.C. Urinary Xist is a potential biomarker for membranous nephropathy. Biochem. Biophys. Res. Commun. 2014, 452, 415–421. [Google Scholar] [CrossRef]
- Wan, J.; Hou, X.; Zhou, Z.; Geng, J.; Tian, J.; Bai, X.; Nie, J. WT1 ameliorates podocyte injury via repression of EZH2/beta-catenin pathway in diabetic nephropathy. Free Radic. Biol. Med. 2017, 108, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.W.; Zhang, J.H.; Liu, F.X.; Wang, X.T.; Pan, S.K.; Jiang, D.K.; Zhao, Z.H.; Liu, Z.S. Silencing of long noncoding RNA PVT1 inhibits podocyte damage and apoptosis in diabetic nephropathy by upregulating FOXA1. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zang, X.; Ponnusamy, M.; Masucci, M.V.; Tolbert, E.; Gong, R.; Zhao, T.C.; Liu, N.; Bayliss, G.; Dworkin, L.D.; et al. Enhancer of Zeste Homolog 2 Inhibition Attenuates Renal Fibrosis by Maintaining Smad7 and Phosphatase and Tensin Homolog Expression. J. Am. Soc. Nephrol. 2016, 27, 2092–2108. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Li, X.; Chen, X.; Xiong, Y.; Cao, Y.; Wang, Z. Drp1 activates ROS/HIF-1alpha/EZH2 and triggers mitochondrial fragmentation to deteriorate hypercalcemia-associated neuronal injury in mouse model of chronic kidney disease. J. Neuroinflammation 2022, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xing, J.; Wang, H.; Yu, E. The SLC34A2-ROS-HIF-1-induced up-regulation of EZH2 expression promotes proliferation and chemo-resistance to apoptosis in colorectal cancer. Biosci. Rep. 2019, 39, BSR20180268. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Z.; Weng, X.; Chen, H.; Du, Y.; Diao, C.; Liu, X.; Wang, L. Enhancer of zeste homolog 2 modulates oxidative stress-mediated pyroptosis in vitro and in a mouse kidney ischemia-reperfusion injury model. FASEB J. 2020, 34, 835–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Li, L.; Xue, X.; Xie, H.; Shi, H.; Hu, Y. A lncRNA coordinates with Ezh2 to inhibit HIF-1alpha transcription and suppress cancer cell adaption to hypoxia. Oncogene 2020, 39, 1860–1874. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Prabhakar, N.R.; Nanduri, J. Protein phosphatase 1 regulates reactive oxygen species-dependent degradation of histone deacetylase 5 by intermittent hypoxia. Am. J. Physiol. Cell Physiol. 2022, 323, C423–C431. [Google Scholar] [CrossRef]
- Huang, Y.; Tao, Y.; Hu, K.; Lin, F.; Li, X.; Feng, T.; Wang, Z.M. Hypoxia-induced NIPP1 activation enhances metastatic potential and predicts poor prognosis in hepatocellular carcinoma. Tumour Biol. 2016, 37, 14903–14914. [Google Scholar] [CrossRef]
- Comerford, K.M.; Leonard, M.O.; Cummins, E.P.; Fitzgerald, K.T.; Beullens, M.; Bollen, M.; Taylor, C.T. Regulation of protein phosphatase 1gamma activity in hypoxia through increased interaction with NIPP1: Implications for cellular metabolism. J. Cell. Physiol. 2006, 209, 211–218. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barth, J.; Loeffler, I.; Bondeva, T.; Liebisch, M.; Wolf, G. The Role of Hypoxia on the Trimethylation of H3K27 in Podocytes. Biomedicines 2023, 11, 2475. https://doi.org/10.3390/biomedicines11092475
Barth J, Loeffler I, Bondeva T, Liebisch M, Wolf G. The Role of Hypoxia on the Trimethylation of H3K27 in Podocytes. Biomedicines. 2023; 11(9):2475. https://doi.org/10.3390/biomedicines11092475
Chicago/Turabian StyleBarth, Johanna, Ivonne Loeffler, Tzvetanka Bondeva, Marita Liebisch, and Gunter Wolf. 2023. "The Role of Hypoxia on the Trimethylation of H3K27 in Podocytes" Biomedicines 11, no. 9: 2475. https://doi.org/10.3390/biomedicines11092475
APA StyleBarth, J., Loeffler, I., Bondeva, T., Liebisch, M., & Wolf, G. (2023). The Role of Hypoxia on the Trimethylation of H3K27 in Podocytes. Biomedicines, 11(9), 2475. https://doi.org/10.3390/biomedicines11092475





