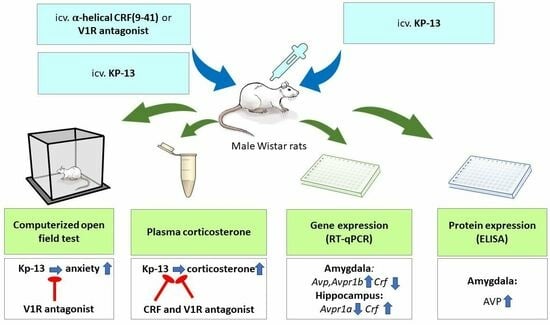
A Brain Region-Dependent Alteration in the Expression of Vasopressin, Corticotropin-Releasing Factor, and Their Receptors Might Be in the Background of Kisspeptin-13-Induced Hypothalamic-Pituitary-Adrenal Axis Activation and Anxiety in Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Housing Conditions
2.2. Surgery
2.3. Treatment
2.4. mRNA Extraction and Quantitative Real-Time PCR
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
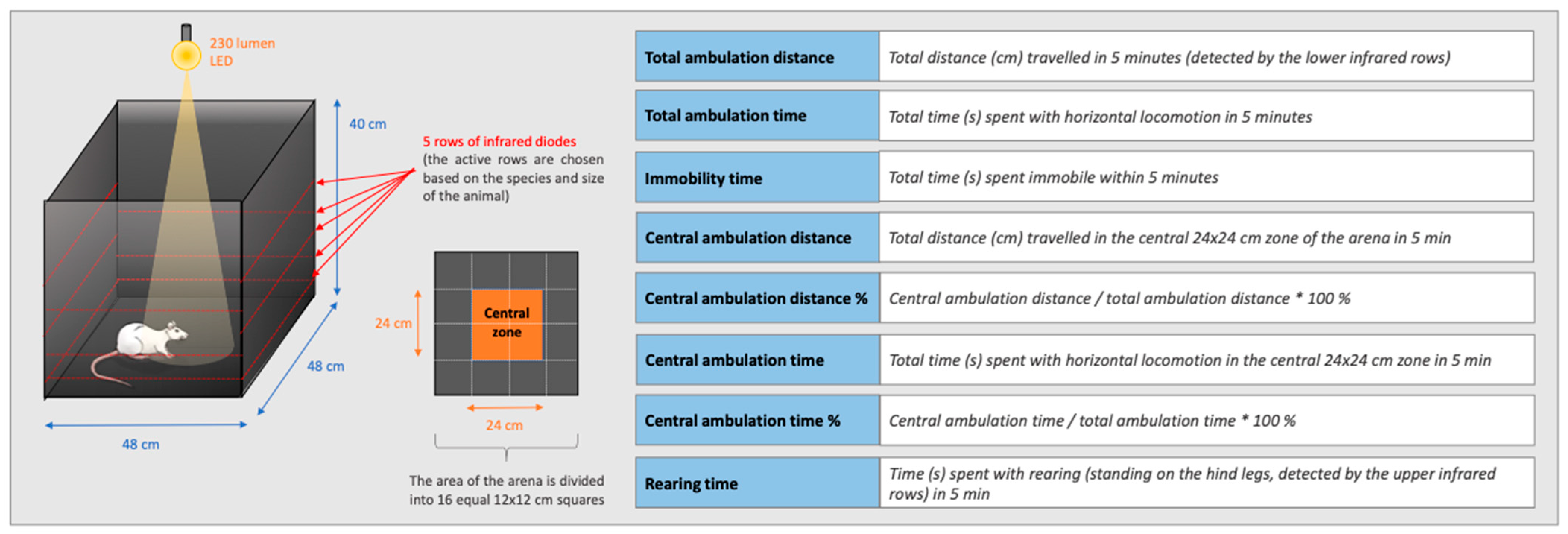
2.6. Open Field Test
2.7. Plasma Corticosterone Measurement
2.8. Statistical Analysis
3. Results
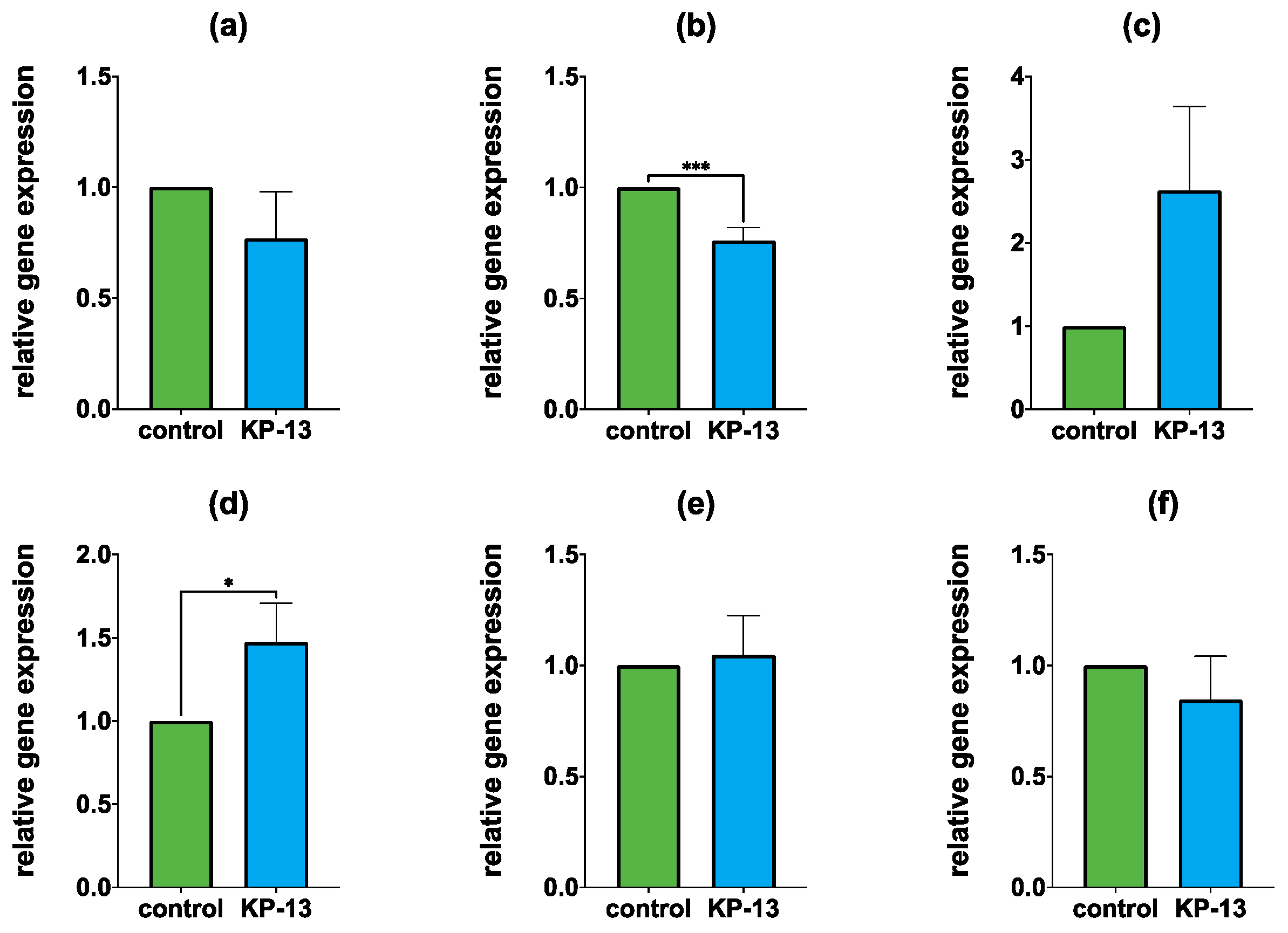
3.1. qPCR
3.1.1. Gene Expression in the Amygdala
3.1.2. Gene Expression in the Hippocampus
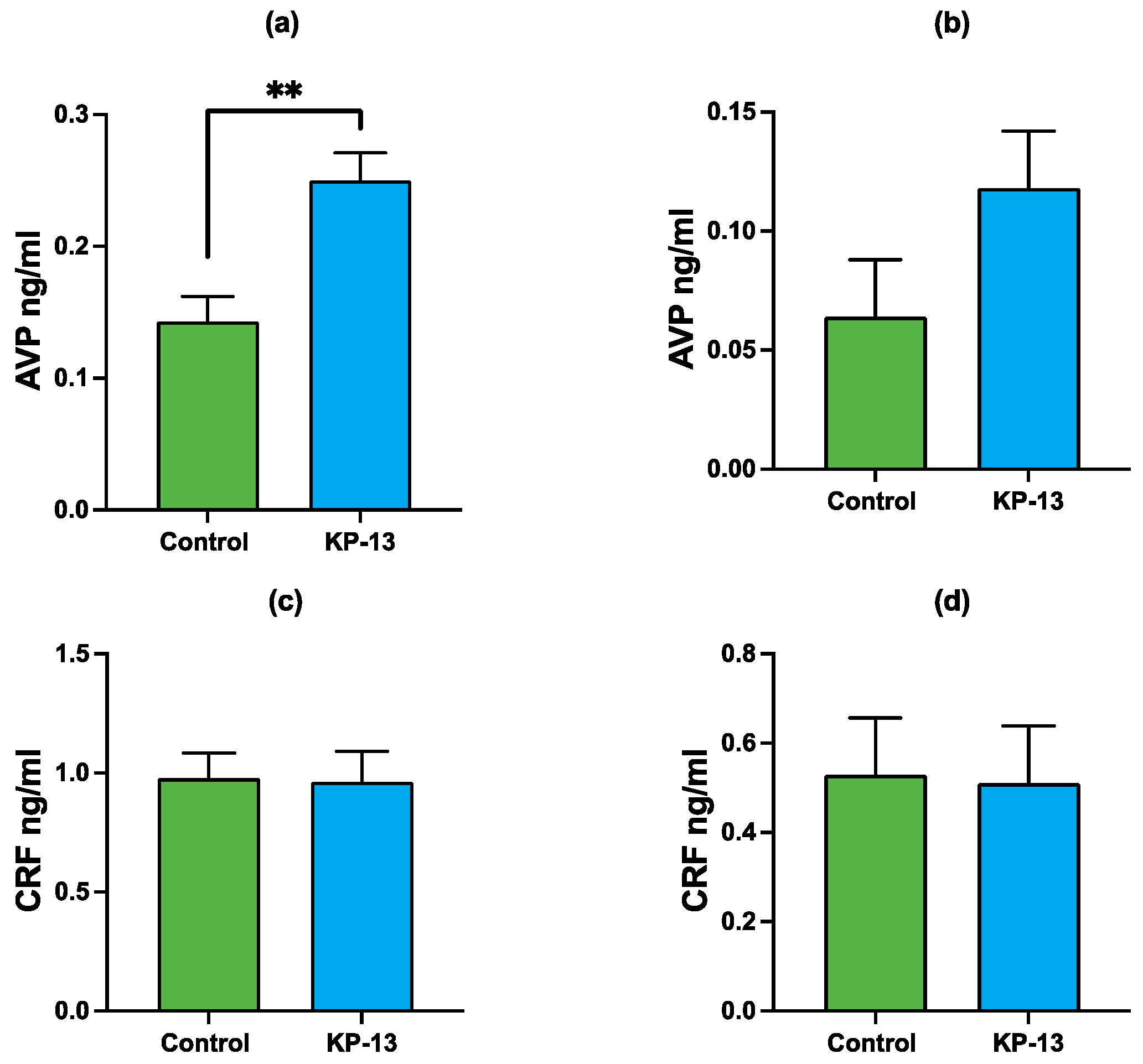
3.2. Enzyme-Linked Immunosorbent Assay
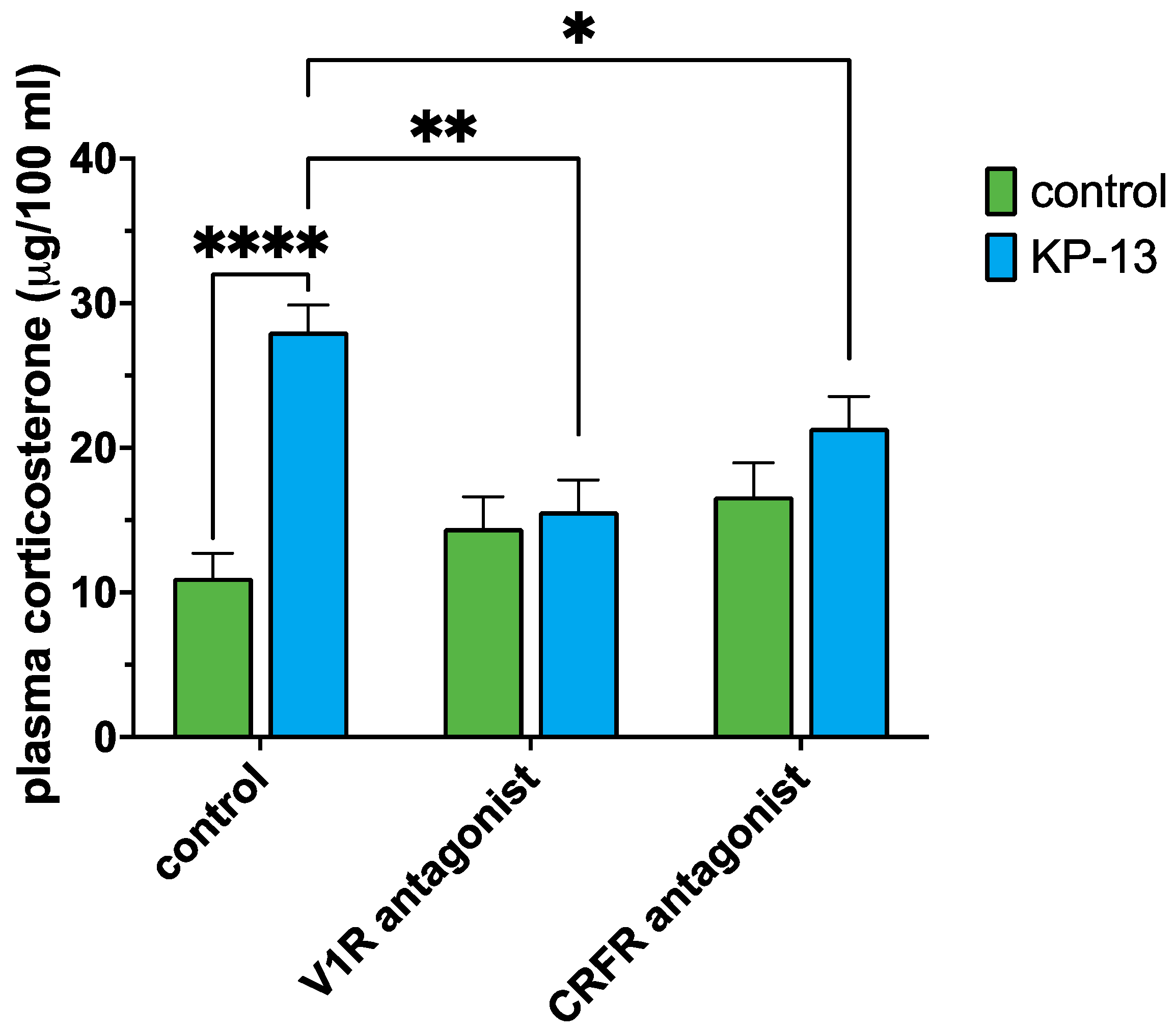
3.3. Plasma Corticosterone Level
3.4. Open Field Test
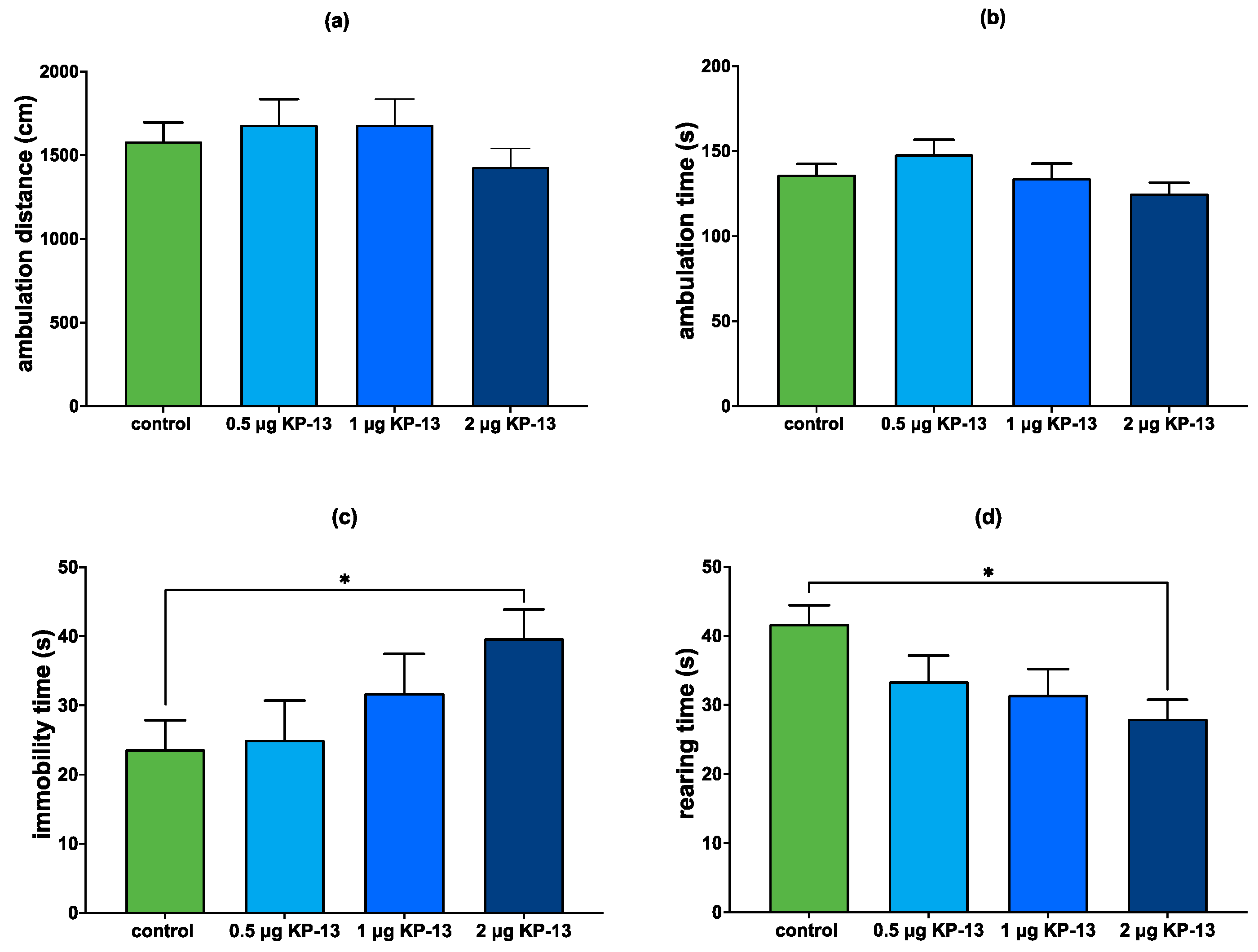
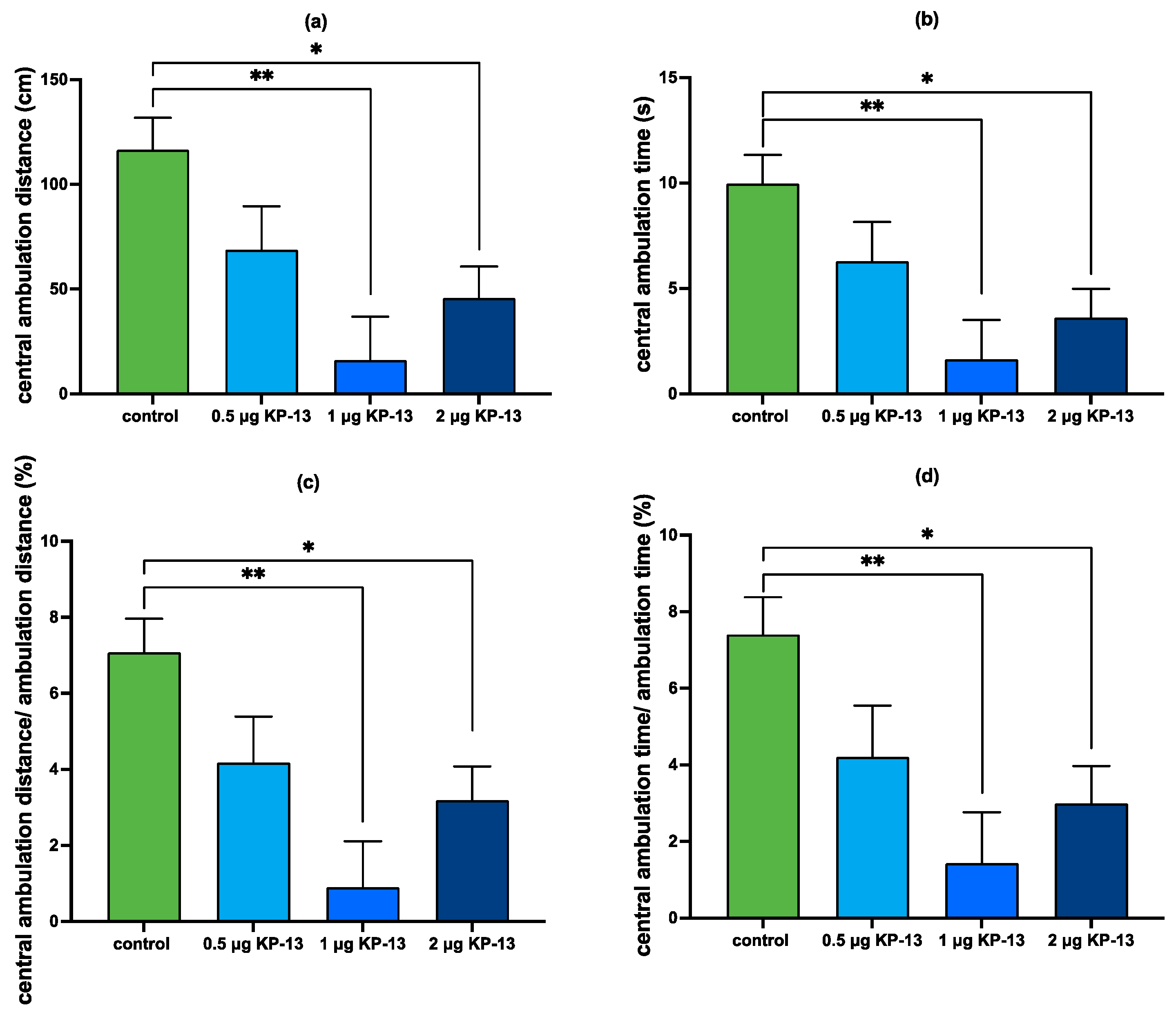
3.4.1. The Effect of KP-13 on Open-Field Behavior
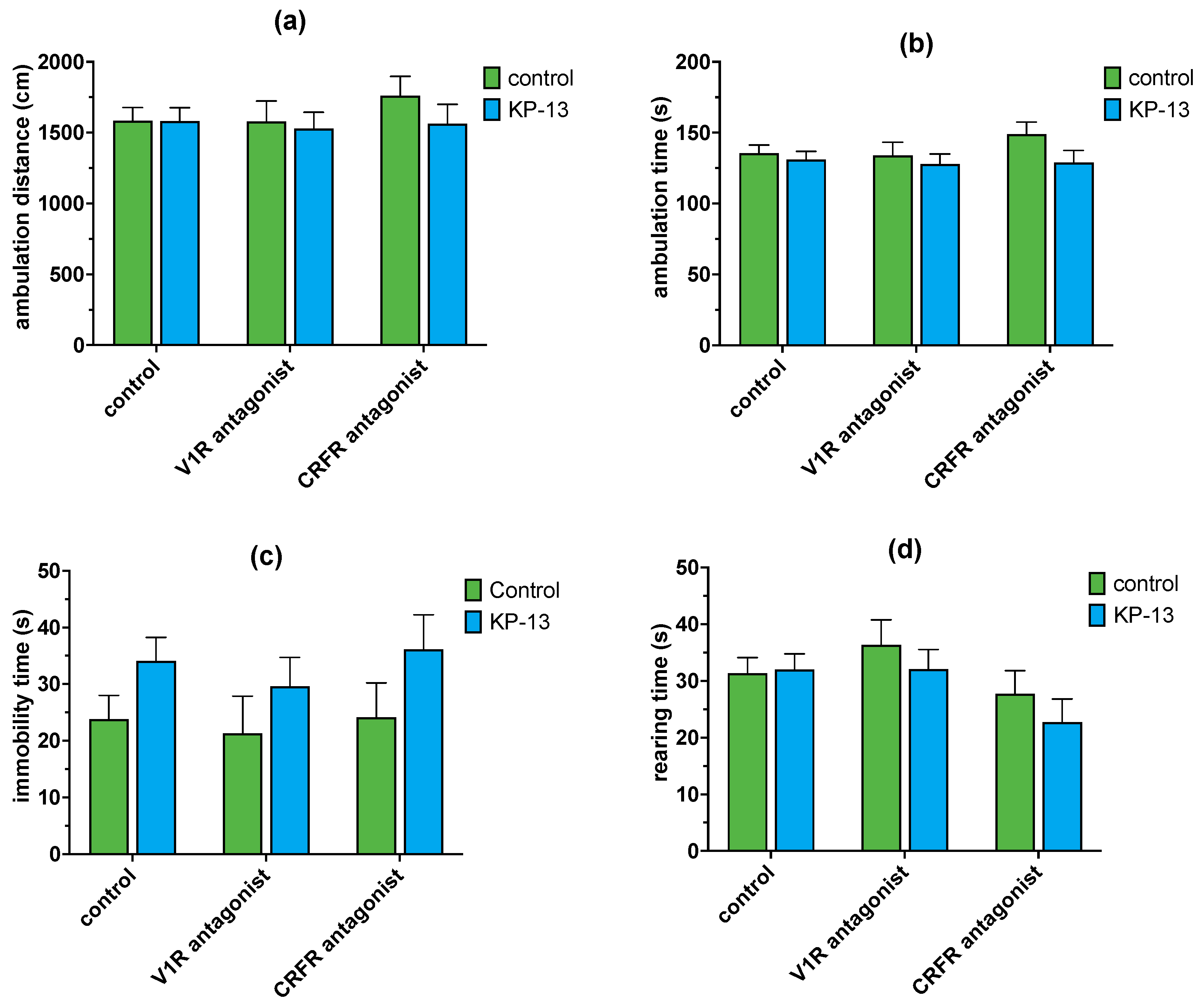
3.4.2. The Effect of V1R and CRFR Antagonists on KP-13-Induced Open-Field Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fukusumi, S.; Fujii, R.; Hinuma, S. Recent Advances in Mammalian RFamide Peptides: The Discovery and Functional Analyses of PrRP, RFRPs and QRFP. Peptides 2006, 27, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Sobrino, V.; Avendaño, M.S.; Perdices-López, C.; Jimenez-Puyer, M.; Tena-Sempere, M. Kisspeptins and the Neuroendocrine Control of Reproduction: Recent Progress and New Frontiers in Kisspeptin Research. Front. Neuroendocrinol. 2022, 65, 100977. [Google Scholar] [CrossRef] [PubMed]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.M.; Le Poul, E.; Brézillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The Metastasis Suppressor Gene KiSS-1 Encodes Kisspeptins, the Natural Ligands of the Orphan G Protein-Coupled Receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef] [PubMed]
- Ohtaki, T.; Shintani, Y.; Honda, S.I.; Matsumoto, H.; Hori, A.; Kanehashi, K.; Terao, Y.; Kumano, S.; Takatsu, Y.; Masuda, Y.; et al. Metastasis Suppressor Gene KiSS-1 Encodes Peptide Ligand of a G-Protein-Coupled Receptor. Nature 2001, 411, 613–617. [Google Scholar] [CrossRef]
- Kirby, H.R.; Maguire, J.J.; Colledge, W.H.; Davenport, A.P. International Union of Basic and Clinical Pharmacology. LXXVII. Kisspeptin Receptor Nomenclature, Distribution, and Function. Pharmacol. Rev. 2010, 62, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, G.C.; Dun, S.L.; Ohsawa, M.; Yin, D.; Yang, J.; Jaw, K.C.; Brailoiu, E.; Dun, N.J. KiSS-1 Expression and Metastin-like Immunoreactivity in the Rat Brain. J. Comp. Neurol. 2005, 481, 314–329. [Google Scholar] [CrossRef]
- Overgaard, A.; Tena-Sempere, M.; Franceschini, I.; Desroziers, E.; Simonneaux, V.; Mikkelsen, J.D. Comparative Analysis of Kisspeptin-Immunoreactivity Reveals Genuine Differences in the Hypothalamic Kiss1 Systems between Rats and Mice. Peptides 2013, 45, 85–90. [Google Scholar] [CrossRef]
- Lee, J.; Miele, M.E.; Hicks, D.J.; Karen, K.; Trent, J.; Weissman, B.; Welch, D.R. KiSS-1, a Novel Human Malignant Melanoma Metastasis-Suppressor Gene. J. Natl. Cancer Inst. 1996, 88, 1731–1737. [Google Scholar] [CrossRef]
- Lee, D.K.; Nguyen, T.; O’Neill, G.P.; Cheng, R.; Liu, Y.; Howard, A.D.; Coulombe, N.; Tan, C.P.; Tang-Nguyen, A.-T.; George, S.R.; et al. Discovery of a Receptor Related to the Galanin Receptors. FEBS Lett. 1999, 446, 103–107. [Google Scholar] [CrossRef]
- Higo, S.; Honda, S.; Iijima, N.; Ozawa, H. Mapping of Kisspeptin Receptor MRNA in the Whole Rat Brain and Its Co-Localisation with Oxytocin in the Paraventricular Nucleus. J. Neuroendocrinol. 2016, 28, 1–8. [Google Scholar] [CrossRef]
- Elhabazi, K.; Humbert, J.P.; Bertin, I.; Schmitt, M.; Bihel, F.; Bourguignon, J.J.; Bucher, B.; Becker, J.A.J.; Sorg, T.; Meziane, H.; et al. Endogenous Mammalian RF-Amide Peptides, Including PrRP, Kisspeptin and 26RFa, Modulate Nociception and Morphine Analgesia via NPFF Receptors. Neuropharmacology 2013, 75, 164–171. [Google Scholar] [CrossRef]
- Lyubimov, Y.; Engstrom, M.; Wurster, S.; Savola, J.M.; Korpi, E.R.; Panula, P. Human Kisspeptins Activate Neuropeptide FF2 Receptor. Neuroscience 2010, 170, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Bonini, J.A.; Jones, K.A.; Adham, N.; Forray, C.; Artymyshyn, R.; Durkin, M.M.; Smith, K.E.; Tamm, J.A.; Boteju, L.W.; Lakhlani, P.P.; et al. Identification and Characterization of Two G Protein-Coupled Receptors for Neuropeptide FF. J. Biol. Chem. 2000, 275, 39324–39331. [Google Scholar] [CrossRef]
- Wu, C.H.; Tao, P.L.; Huang, E.Y.K. Distribution of Neuropeptide FF (NPFF) Receptors in Correlation with Morphine-Induced Reward in the Rat Brain. Peptides 2010, 31, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Higo, S.; Kanaya, M.; Ozawa, H. Expression Analysis of Neuropeptide FF Receptors on Neuroendocrine-Related Neurons in the Rat Brain Using Highly Sensitive in Situ Hybridization. Histochem. Cell Biol. 2021, 155, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Seminara, S.B.; Messager, S.; Dixon, J.; Slaugenhaupt, S.A.; O’Rahilly, S.; Carlton, M.B.L.; Bo-Abbas, Y.; Schwinof, K.M.; Chatzidaki, E.E.; Gusella, J.F.; et al. The GPR54 Gene as a Regulator of Puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.G.A.; Dhillo, W.S.; Comninos, A.N. Kisspeptin and the Control of Emotions, Mood and Reproductive Behaviour. J. Endocrinol. 2018, 239, R1–R12. [Google Scholar] [CrossRef] [PubMed]
- Csabafi, K.; Bagosi, Z.; Dobó, É.; Szakács, J.; Telegdy, G.; Szabó, G. Kisspeptin Modulates Pain Sensitivity of CFLP Mice. Peptides 2018, 105, 21–27. [Google Scholar] [CrossRef]
- Csabafi, K.; Jászberényi, M.; Bagosi, Z.; Lipták, N.; Telegdy, G. Effects of Kisspeptin-13 on the Hypothalamic-Pituitary-Adrenal Axis, Thermoregulation, Anxiety and Locomotor Activity in Rats. Behav. Brain Res. 2013, 241, 56–61. [Google Scholar] [CrossRef]
- Ibos, K.E.; Bodnár, É.; Bagosi, Z.; Bozsó, Z.; Tóth, G.; Szabó, G.; Csabafi, K. Kisspeptin-8 Induces Anxiety-like Behavior and Hypolocomotion by Activating the Hpa Axis and Increasing Gaba Release in the Nucleus Accumbens in Rats. Biomedicines 2021, 9, 112. [Google Scholar] [CrossRef]
- Aguilera, G. The Hypothalamic–Pituitary–Adrenal Axis and Neuroendocrine Responses to Stress. In Handbook of Neuroendocrinology; Elsevier: London, UK, 2012; pp. 175–196. [Google Scholar]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. In Comprehensive Physiology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; Volume 6, pp. 603–621. [Google Scholar]
- Russell, G.; Lightman, S. The Human Stress Response. Nat. Rev. Endocrinol. 2019, 15, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.B.; Tschetter, K.E.; Ronan, P.J. Inhibition of Corticotropin Releasing Factor Expression in the Central Nucleus of the Amygdala Attenuates Stress-Induced Behavioral and Endocrine Responses. Front. Neurosci. 2013, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Paretkar, T.; Dimitrov, E. The Central Amygdala Corticotropin-Releasing Hormone (CRH) Neurons Modulation of Anxiety-like Behavior and Hippocampus-Dependent Memory in Mice. Neuroscience 2018, 390, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Gunn, B.G.; Sanchez, G.A.; Lynch, G.; Baram, T.Z.; Chen, Y. Hyper-Diversity of CRH Interneurons in Mouse Hippocampus. Brain Struct. Funct. 2019, 224, 583–598. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Brunson, K.L.; Adelmann, G.; Bender, R.A.; Frotscher, M.; Baram, T.Z. Hippocampal Corticotropin Releasing Hormone: Pre- and Postsynaptic Location and Release by Stress. Neuroscience 2004, 126, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, V.; Mazzitelli, M.; Cragg, B.; Ji, G.; Navratilova, E.; Porreca, F. Amygdala, Neuropeptides, and Chronic Pain-Related Affective Behaviors. Neuropharmacology 2020, 170, 108052. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.H.; Abdulai-Saiku, S.; Vyas, A. Arginine Vasopressin in the Medial Amygdala Causes Greater Post-Stress Recruitment of Hypothalamic Vasopressin Neurons. Mol. Brain 2021, 14, 141. [Google Scholar] [CrossRef]
- Cilz, N.I.; Cymerblit-Sabba, A.; Young, W.S. Oxytocin and Vasopressin in the Rodent Hippocampus. Genes Brain Behav. 2019, 18, e12535. [Google Scholar] [CrossRef]
- Zagrean, A.-M.; Georgescu, I.-A.; Iesanu, M.I.; Ionescu, R.-B.; Haret, R.M.; Panaitescu, A.M.; Zagrean, L. Oxytocin and Vasopressin in the Hippocampus. Vitam. Horm. 2022, 118, 83–127. [Google Scholar] [CrossRef]
- Dedic, N.; Chen, A.; Deussing, J.M. The CRF Family of Neuropeptides and Their Receptors—Mediators of the Central Stress Response. Curr. Mol. Pharmacol. 2017, 11, 4–31. [Google Scholar] [CrossRef]
- Sztainberg, Y.; Kuperman, Y.; Tsoory, M.; Lebow, M.; Chen, A. The Anxiolytic Effect of Environmental Enrichment Is Mediated via Amygdalar CRF Receptor Type 1. Mol. Psychiatry 2010, 15, 905–917. [Google Scholar] [CrossRef]
- Pałasz, A.; Janas-Kozik, M.; Borrow, A.; Arias-Carrión, O.; Worthington, J.J. The Potential Role of the Novel Hypothalamic Neuropeptides Nesfatin-1, Phoenixin, Spexin and Kisspeptin in the Pathogenesis of Anxiety and Anorexia Nervosa. Neurochem. Int. 2018, 113, 120–136. [Google Scholar] [CrossRef]
- Bale, T.L.; Contarino, A.; Smith, G.W.; Chan, R.; Gold, L.H.; Sawchenko, P.E.; Koob, G.F.; Vale, W.W.; Lee, K.F. Mice Deficient for Corticotropin-Releasing Hormone Receptor-2 Display Anxiety-like Behaviour and Are Hypersensitive to Stress. Nat. Genet. 2000, 24, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.W.E.; Portela, N.C.; Silva, M.S.; Céspedes, I.C.; Bittencourt, J.C.; Viana, M.B. The Activation and Blockage of CRF Type 2 Receptors of the Medial Amygdala Alter Elevated T-Maze Inhibitory Avoidance, an Anxiety-Related Response. Behav. Brain Res. 2016, 305, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.S.C.F.; Souza, T.M.O.; Pereira, B.A.; Ribeiro, D.A.; Céspedes, I.C.; Bittencourt, J.C.; Viana, M.B. The Blockage of Ventromedial Hypothalamus CRF Type 2 Receptors Impairs Escape Responses in the Elevated T-Maze. Behav. Brain Res. 2017, 329, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Rotondo, F.; Butz, H.; Syro, L.V.; Yousef, G.M.; Di Ieva, A.; Restrepo, L.M.; Quintanar-Stephano, A.; Berczi, I.; Kovacs, K. Arginine Vasopressin (AVP): A Review of Its Historical Perspectives, Current Research and Multifunctional Role in the Hypothalamo-Hypophysial System. Pituitary 2016, 19, 345–355. [Google Scholar] [CrossRef]
- Aguilera, G.; Subburaju, S.; Young, S.; Chen, J. The Parvocellular Vasopressinergic System and Responsiveness of the Hypothalamic Pituitary Adrenal Axis during Chronic Stress. Prog. Brain Res. 2008, 170, 29–39. [Google Scholar] [CrossRef]
- Csikota, P.; Fodor, A.; Balázsfi, D.; Pintér, O.; Mizukami, H.; Weger, S.; Heilbronn, R.; Engelmann, M.; Zelena, D. Vasopressinergic Control of Stress-Related Behavior: Studies in Brattleboro Rats. Stress 2016, 19, 349–361. [Google Scholar] [CrossRef]
- Hernández, V.S.; Hernández, O.R.; de La Mora, M.P.; Gómora, M.J.; Fuxe, K.; Eiden, L.E.; Zhang, L. Hypothalamic Vasopressinergic Projections Innervate Central Amygdala GABAergic Neurons: Implications for Anxiety and Stress Coping. Front. Neural Circuits 2016, 10, 92. [Google Scholar] [CrossRef]
- Bhattacharya, S.K.; Chakrabarti, A.; Glover, V. Stress and Water Balance: The Roles of ANP, AVP and Isatin. Indian J. Exp. Biol. 1998, 36, 1195–1200. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-12-374121-9. [Google Scholar]
- Telegdy, G.; Adamik, A. Mediators Involved in the Hyperthermic Action of Neuromedin U in Rats. Regul. Pept. 2014, 192–193, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Jászberényi, M.; Bujdosó, E.; Telegdy, G. Behavioral, Neuroendocrine and Thermoregulatory Actions of Apelin-13. Neuroscience 2004, 129, 811–816. [Google Scholar] [CrossRef]
- Bagosi, Z.; Csabafi, K.; Palotai, M.; Jászberényi, M.; Földesi, I.; Gardi, J.; Szabó, G.; Telegdy, G. The Effect of Urocortin I on the Hypothalamic ACTH Secretagogues and Its Impact on the Hypothalamic-Pituitary-Adrenal Axis. Neuropeptides 2014, 48, 15–20. [Google Scholar] [CrossRef]
- Zenker, N.; Bernstein, D.E. The Estimation of Small Amounts of Corticosterone in Rat Plasma. J. Biol. Chem. 1958, 231, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Purves, H.D.; Sirett, N.E. Assay of Corticotrophin in Dexamethasone-Treated Rats. Endocrinology 1965, 77, 366–374. [Google Scholar] [CrossRef]
- Pineda, R.; Plaisier, F.; Millar, R.P.; Ludwig, M. Amygdala Kisspeptin Neurons: Putative Mediators of Olfactory Control of the Gonadotropic Axis. Neuroendocrinology 2017, 104, 223–238. [Google Scholar] [CrossRef]
- Lass, G.; Li, X.F.; Voliotis, M.; Wall, E.; de Burgh, R.A.; Ivanova, D.; McIntyre, C.; Lin, X.-H.; Colledge, W.H.; Lightman, S.L.; et al. GnRH Pulse Generator Frequency Is Modulated by Kisspeptin and GABA-Glutamate Interactions in the Posterodorsal Medial Amygdala in Female Mice. J. Neuroendocrinol. 2022, 34, e13207. [Google Scholar] [CrossRef]
- Vinkers, C.H.; Bijlsma, E.Y.; Houtepen, L.C.; Westphal, K.G.C.; Veening, J.G.; Groenink, L.; Olivier, B. Medial Amygdala Lesions Differentially Influence Stress Responsivity and Sensorimotor Gating in Rats. Physiol. Behav. 2010, 99, 395–401. [Google Scholar] [CrossRef]
- Tong, W.H.; Abdulai-Saiku, S.; Vyas, A. Testosterone Reduces Fear and Causes Drastic Hypomethylation of Arginine Vasopressin Promoter in Medial Extended Amygdala of Male Mice. Front. Behav. Neurosci. 2019, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Hari Dass, S.A.; Vyas, A. Copulation or Sensory Cues from the Female Augment Fos Expression in Arginine Vasopressin Neurons of the Posterodorsal Medial Amygdala of Male Rats. Front. Zool. 2014, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Semaan, S.J.; Clifton, D.K.; Steiner, R.A.; Dhamija, S.; Kauffman, A.S. Regulation of Kiss1 Expression by Sex Steroids in the Amygdala of the Rat and Mouse. Endocrinology 2011, 152, 2020–2030. [Google Scholar] [CrossRef]
- Di Giorgio, N.P.; Semaan, S.J.; Kim, J.; López, P.V.; Bettler, B.; Libertun, C.; Lux-Lantos, V.A.; Kauffman, A.S. Impaired GABAB Receptor Signaling Dramatically Up-Regulates Kiss1 Expression Selectively in Nonhypothalamic Brain Regions of Adult but Not Prepubertal Mice. Endocrinology 2014, 155, 1033–1044. [Google Scholar] [CrossRef]
- Griebel, G.; Simiand, J.; Serradeil-Le Gal, C.; Wagnon, J.; Pascal, M.; Scatton, B.; Maffrand, J.-P.; Soubrié, P. Anxiolytic- and Antidepressant-like Effects of the Non-Peptide Vasopressin V1b Receptor Antagonist, SSR149415, Suggest an Innovative Approach for the Treatment of Stress-Related Disorders. Proc. Natl. Acad. Sci. USA 2002, 99, 6370–6375. [Google Scholar] [CrossRef] [PubMed]
- Salomé, N.; Stemmelin, J.; Cohen, C.; Griebel, G. Differential Roles of Amygdaloid Nuclei in the Anxiolytic- and Antidepressant-like Effects of the V1b Receptor Antagonist, SSR149415, in Rats. Psychopharmacology 2006, 187, 237–244. [Google Scholar] [CrossRef]
- Refojo, D.; Schweizer, M.; Kuehne, C.; Ehrenberg, S.; Thoeringer, C.; Vogl, A.M.; Dedic, N.; Schumacher, M.; von Wolff, G.; Avrabos, C.; et al. Glutamatergic and Dopaminergic Neurons Mediate Anxiogenic and Anxiolytic Effects of CRHR1. Science 2011, 333, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Dedic, N.; Kühne, C.; Jakovcevski, M.; Hartmann, J.; Genewsky, A.J.; Gomes, K.S.; Anderzhanova, E.; Pöhlmann, M.L.; Chang, S.; Kolarz, A.; et al. Chronic CRH Depletion from GABAergic, Long-Range Projection Neurons in the Extended Amygdala Reduces Dopamine Release and Increases Anxiety. Nat. Neurosci. 2018, 21, 803–807. [Google Scholar] [CrossRef]
- Chen, Y.; Bender, R.A.; Frotscher, M.; Baram, T.Z. Novel and Transient Populations of Corticotropin-Releasing Hormone-Expressing Neurons in Developing Hippocampus Suggest Unique Functional Roles: A Quantitative Spatiotemporal Analysis. J. Neurosci. 2001, 21, 7171–7181. [Google Scholar] [CrossRef] [PubMed]
- Telegdy, G.; Adamik, Á. The Action of Kisspeptin-13 on Passive Avoidance Learning in Mice. Involvement of Transmitters. Behav. Brain Res. 2013, 243, 300–305. [Google Scholar] [CrossRef]
- Jiang, J.H.; He, Z.; Peng, Y.L.; Jin, W.D.; Wang, Z.; Han, R.W.; Chang, M.; Wang, R. Kisspeptin-13 Enhances Memory and Mitigates Memory Impairment Induced by Aβ1-42 in Mice Novel Object and Object Location Recognition Tasks. Neurobiol. Learn. Mem. 2015, 123, 187–195. [Google Scholar] [CrossRef]
- Milton, N.G.N.; Chilumuri, A.; Rocha-Ferreira, E.; Nercessian, A.N.; Ashioti, M. Kisspeptin Prevention of Amyloid-β Peptide Neurotoxicity in Vitro. ACS Chem. Neurosci. 2012, 3, 706–719. [Google Scholar] [CrossRef]
- Arai, A.C.; Orwig, N. Factors That Regulate KiSS1 Gene Expression in the Hippocampus. Brain Res. 2008, 1243, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Arai, A.C. The Role of Kisspeptin and GPR54 in the Hippocampus. Peptides 2009, 30, 16–25. [Google Scholar] [CrossRef]
- Chen, Y.; Fenoglio, K.A.; Dubé, C.M.; Grigoriadis, D.E.; Baram, T.Z. Cellular and Molecular Mechanisms of Hippocampal Activation by Acute Stress Are Age-Dependent. Mol. Psychiatry 2006, 11, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Kozlovsky, N.; Zohar, J.; Kaplan, Z.; Cohen, H. Microinfusion of a Corticotrophin-Releasing Hormone Receptor 1 Antisense Oligodeoxynucleotide into the Dorsal Hippocampus Attenuates Stress Responses at Specific Times After Stress Exposure. J. Neuroendocrinol. 2012, 24, 489–503. [Google Scholar] [CrossRef]
- Szot, P.; Bale, T.L.; Dorsa, D.M. Distribution of Messenger RNA for the Vasopressin V1a Receptor in the CNS of Male and Female Rats. Mol. Brain Res. 1994, 24, 1–10. [Google Scholar] [CrossRef]
- Gong, S.; Zheng, C.; Doughty, M.L.; Losos, K.; Didkovsky, N.; Schambra, U.B.; Nowak, N.J.; Joyner, A.; Leblanc, G.; Hatten, M.E.; et al. A Gene Expression Atlas of the Central Nervous System Based on Bacterial Artificial Chromosomes. Nature 2003, 425, 917–925. [Google Scholar] [CrossRef]
- Ramanathan, G.; Cilz, N.I.; Kurada, L.; Hu, B.; Wang, X.; Lei, S. Vasopressin Facilitates GABAergic Transmission in Rat Hippocampus via Activation of V1A Receptors. Neuropharmacology 2012, 63, 1218–1226. [Google Scholar] [CrossRef]
- Bielsky, I.F.; Hu, S.B.; Szegda, K.L.; Westphal, H.; Young, L.J. Profound Impairment in Social Recognition and Reduction in Anxiety-like Behavior in Vasopressin V1a Receptor Knockout Mice. Neuropsychopharmacology 2004, 29, 483–493. [Google Scholar] [CrossRef]
- Bleickardt, C.J.; Mullins, D.E.; Macsweeney, C.P.; Werner, B.J.; Pond, A.J.; Guzzi, M.F.; Martin, F.D.C.; Varty, G.B.; Hodgson, R.A. Characterization of the V1a Antagonist, JNJ-17308616, in Rodent Models of Anxiety-like Behavior. Psychopharmacology 2009, 202, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Pliska, V. Models to Explain Dose-Response Relationships That Exhibit a Downturn Phase. Trends Pharmacol. Sci. 1994, 15, 178–181. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic Mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef]
- Himanshu; Dharmila; Sarkar, D.; Nutan. A Review of Behavioral Tests to Evaluate Different Types of Anxiety and Anti-Anxiety Effects. Clin. Psychopharmacol. Neurosci. 2020, 18, 341–351. [Google Scholar] [CrossRef]
- Ogawa, S.; Parhar, I.S. Biological Significance of Kisspeptin-Kiss 1 Receptor Signaling in the Habenula of Teleost Species. Front. Endocrinol. 2018, 9, 222. [Google Scholar] [CrossRef] [PubMed]
- Adekunbi, D.A.; Li, X.F.; Lass, G.; Shetty, K.; Adegoke, O.A.; Yeo, S.H.; Colledge, W.H.; Lightman, S.L.; O’Byrne, K.T. Kisspeptin Neurones in the Posterodorsal Medial Amygdala Modulate Sexual Partner Preference and Anxiety in Male Mice. J. Neuroendocrinol. 2018, 30, e12572. [Google Scholar] [CrossRef]
- Rao, Y.S.; Mott, N.N.; Pak, T.R. Effects of Kisspeptin on Parameters of the HPA Axis. Endocrine 2011, 39, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Comninos, A.N.; Wall, M.B.; Demetriou, L.; Thomas, S.A.; Wilson, S.R.; Jayasena, C.N.; Bloom, S.R.; Bassett, P.; Hönigsperger, C.; Mehta, A.; et al. Kisspeptin Modulates Sexual and Emotional Brain Processing in Humans. J. Clin. Investig. 2017, 127, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Delmas, S.; Porteous, R.; Bergin, D.H.; Herbison, A.E. Altered Aspects of Anxiety-Related Behavior in Kisspeptin Receptor-Deleted Male Mice. Sci. Rep. 2018, 8, 2–8. [Google Scholar] [CrossRef]
- Rana, T.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Abdeen, A.; Ibrahim, S.F.; Mani, V.; Iqbal, M.S.; Bhatia, S.; et al. Exploring the Role of Neuropeptides in Depression and Anxiety. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 114, 110478. [Google Scholar] [CrossRef]
- Holsboer, F.; Ising, M. Hypothalamic Stress Systems in Mood Disorders. Handb. Clin. Neurol. 2021, 182, 33–48. [Google Scholar] [CrossRef]
- Hu, K.-L.; Chen, Z.; Li, X.; Cai, E.; Yang, H.; Chen, Y.; Wang, C.; Ju, L.; Deng, W.; Mu, L. Advances in Clinical Applications of Kisspeptin-GnRH Pathway in Female Reproduction. Reprod Biol. Endocrinol. 2022, 20, 81. [Google Scholar] [CrossRef]
- Oyola, M.G.; Handa, R.J. Hypothalamic–Pituitary–Adrenal and Hypothalamic–Pituitary–Gonadal Axes: Sex Differences in Regulation of Stress Responsivity. Stress 2017, 20, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Schwanhüusser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global Quantification of Mammalian Gene Expression Control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, F.; Hentze, M.W. Molecular Mechanisms of Translational Control. Nat. Rev. Mol. Cell Biol. 2004, 5, 827–835. [Google Scholar] [CrossRef] [PubMed]



| Genes | Forward (5′ → 3′) | Reverse (5′ → 3′) |
|---|---|---|
| Avp | CTG ACA TGG AGC TGA GAC AGT | CGC AGC TCT CGT CGC T |
| Avpr1a | TGG ACC GAT TCA GAA AAC CCT | GTT GGG CTC CGG TTG TTA GA |
| Avpr1b | CAG CAT AGG AGC CAA CCA TCA A | GAA AGC CCA GCT AAG CCG T |
| Crf | TGG TGT GGA GAA ACT CAG AGC | CAT GTT AGG GGC GCT CTC TTC |
| Crfr1 | CGA AGA GAA GAA GAG CAA AGT ACA C | GCG TAG GAT GAA AGC CGA GA |
| Crfr2 | CCC GAA GGT CCC TAC TCC TA | CTG CTT GTC ATC CAA AAT GGG T |
| Gapdh | CGG CCA AAT CTG AGG CAA GA | TTT TGT GAT GCG TGT GTA GCG |
| Steps | Temperature °C | Time | Number of Cycles |
|---|---|---|---|
| Uracil DNA glycosylase pretreatment | 50 | 2 min | 1 |
| Initial denaturation | 95 | 10 min | 1 |
| Denaturation | 95 | 15 s | 40 |
| Annealing | 60 | 30 s | |
| Extension | 72 | 30 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Csabafi, K.; Ibos, K.E.; Bodnár, É.; Filkor, K.; Szakács, J.; Bagosi, Z. A Brain Region-Dependent Alteration in the Expression of Vasopressin, Corticotropin-Releasing Factor, and Their Receptors Might Be in the Background of Kisspeptin-13-Induced Hypothalamic-Pituitary-Adrenal Axis Activation and Anxiety in Rats. Biomedicines 2023, 11, 2446. https://doi.org/10.3390/biomedicines11092446
Csabafi K, Ibos KE, Bodnár É, Filkor K, Szakács J, Bagosi Z. A Brain Region-Dependent Alteration in the Expression of Vasopressin, Corticotropin-Releasing Factor, and Their Receptors Might Be in the Background of Kisspeptin-13-Induced Hypothalamic-Pituitary-Adrenal Axis Activation and Anxiety in Rats. Biomedicines. 2023; 11(9):2446. https://doi.org/10.3390/biomedicines11092446
Chicago/Turabian StyleCsabafi, Krisztina, Katalin Eszter Ibos, Éva Bodnár, Kata Filkor, Júlia Szakács, and Zsolt Bagosi. 2023. "A Brain Region-Dependent Alteration in the Expression of Vasopressin, Corticotropin-Releasing Factor, and Their Receptors Might Be in the Background of Kisspeptin-13-Induced Hypothalamic-Pituitary-Adrenal Axis Activation and Anxiety in Rats" Biomedicines 11, no. 9: 2446. https://doi.org/10.3390/biomedicines11092446
APA StyleCsabafi, K., Ibos, K. E., Bodnár, É., Filkor, K., Szakács, J., & Bagosi, Z. (2023). A Brain Region-Dependent Alteration in the Expression of Vasopressin, Corticotropin-Releasing Factor, and Their Receptors Might Be in the Background of Kisspeptin-13-Induced Hypothalamic-Pituitary-Adrenal Axis Activation and Anxiety in Rats. Biomedicines, 11(9), 2446. https://doi.org/10.3390/biomedicines11092446