Inflammatory Orofacial Pain Activates Peptidergic Neurons and Upregulates the Oxytocin Receptor Expression in Trigeminal Ganglion
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Establishment of the Animal Model
2.3. Orofacial Operant Test
2.4. Morphological Experiments
2.5. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
2.6. Statistical Analysis
3. Results
3.1. Morphological Experiments
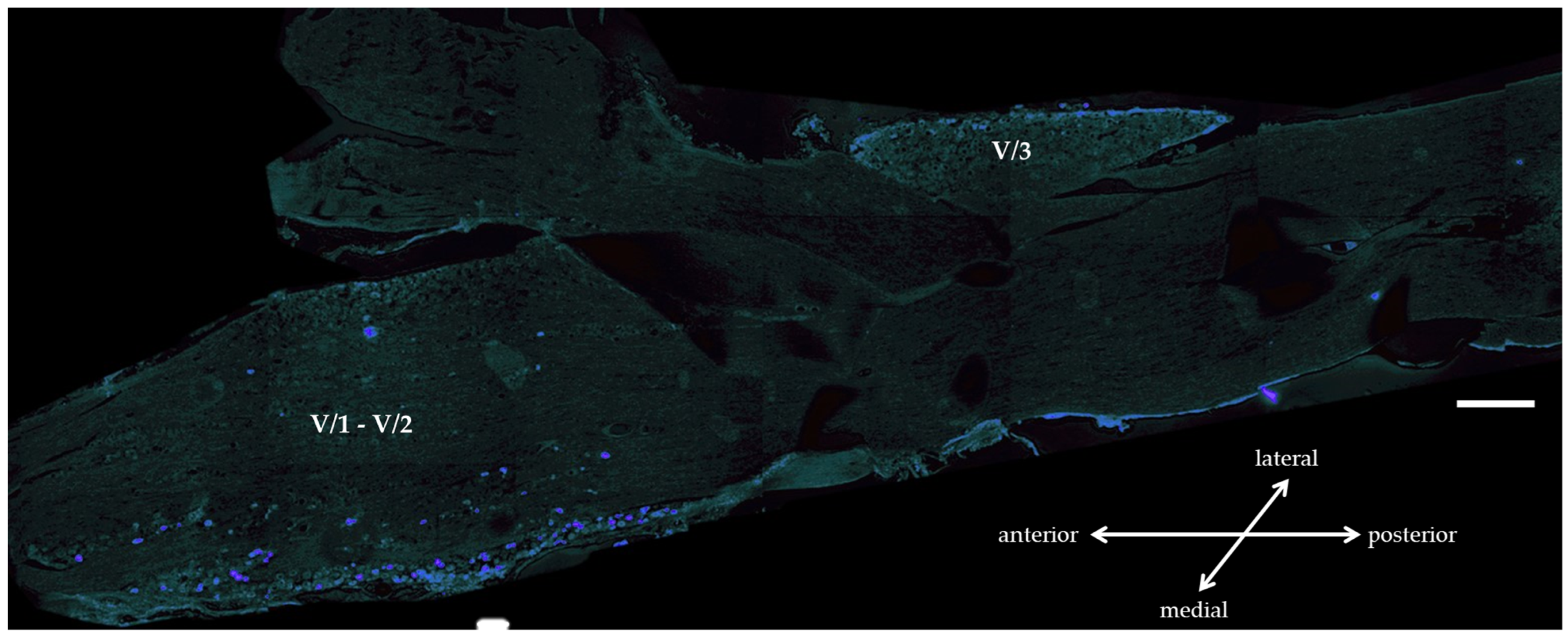
3.1.1. True Blue Tracer Injection
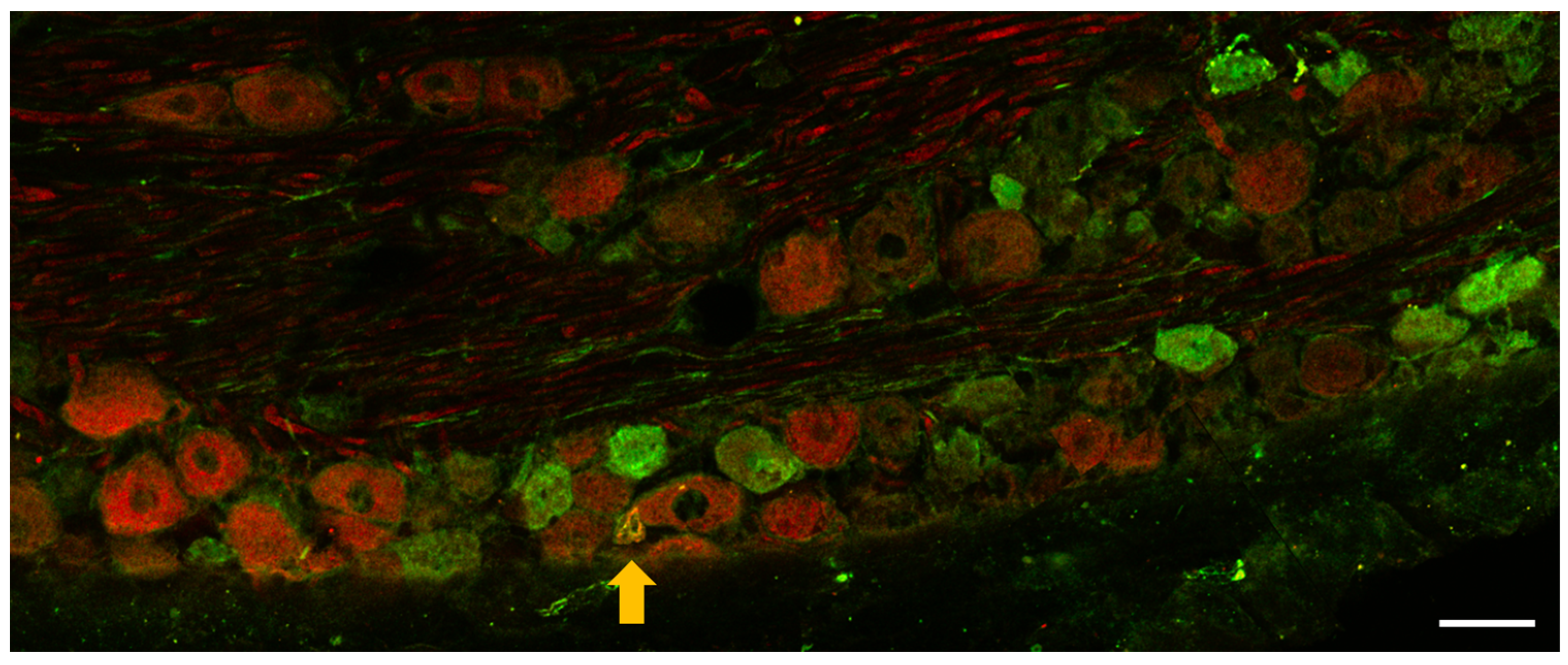
3.1.2. Immunohistochemistry of OTR and CGRP Expression in TG
3.2. Orofacial Operant Test
3.3. Transcriptional Analyses
3.3.1. Analysis of the c-Fos mRNA Expression
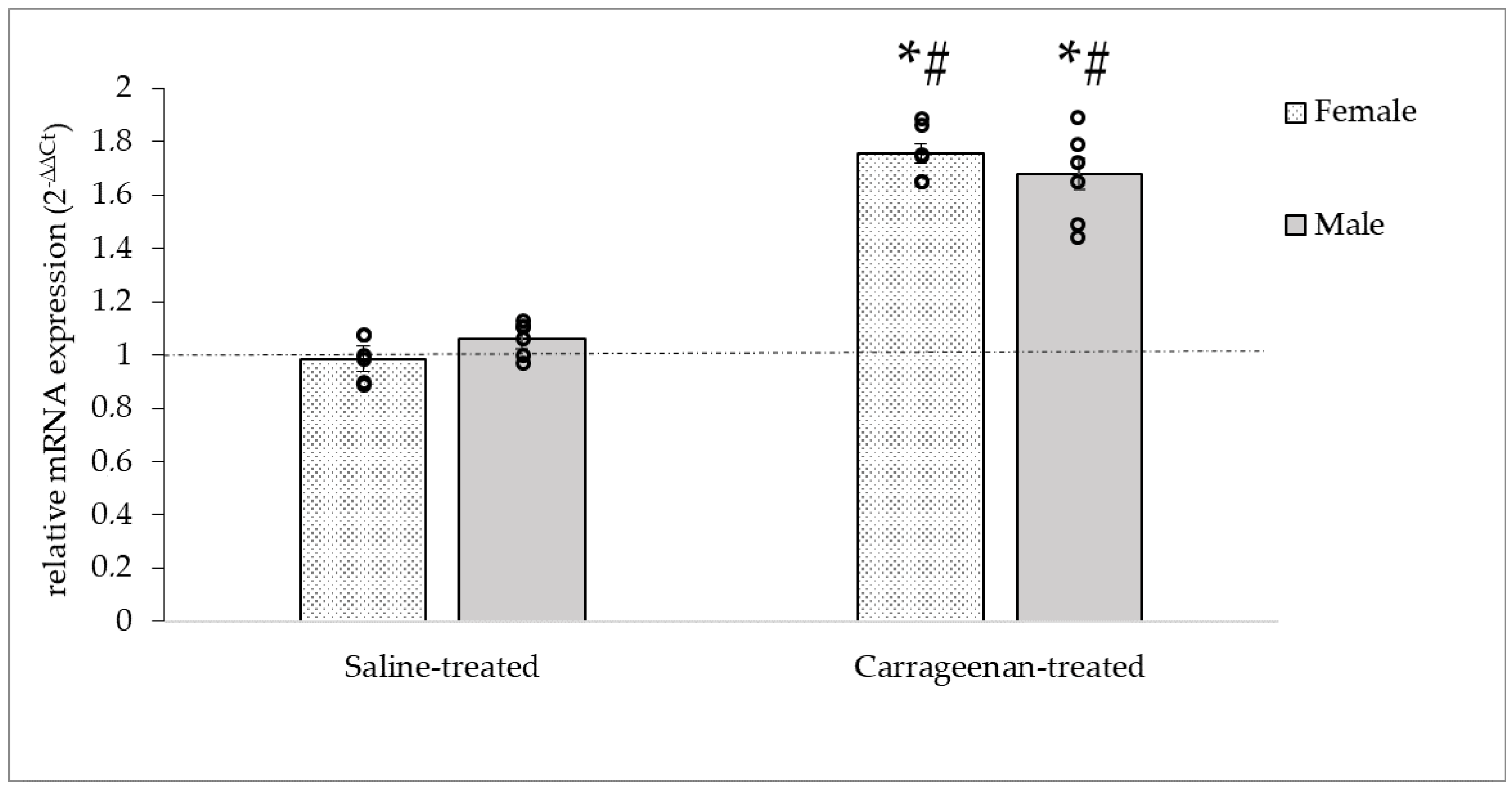
3.3.2. Analysis of the CALCA mRNA Expression
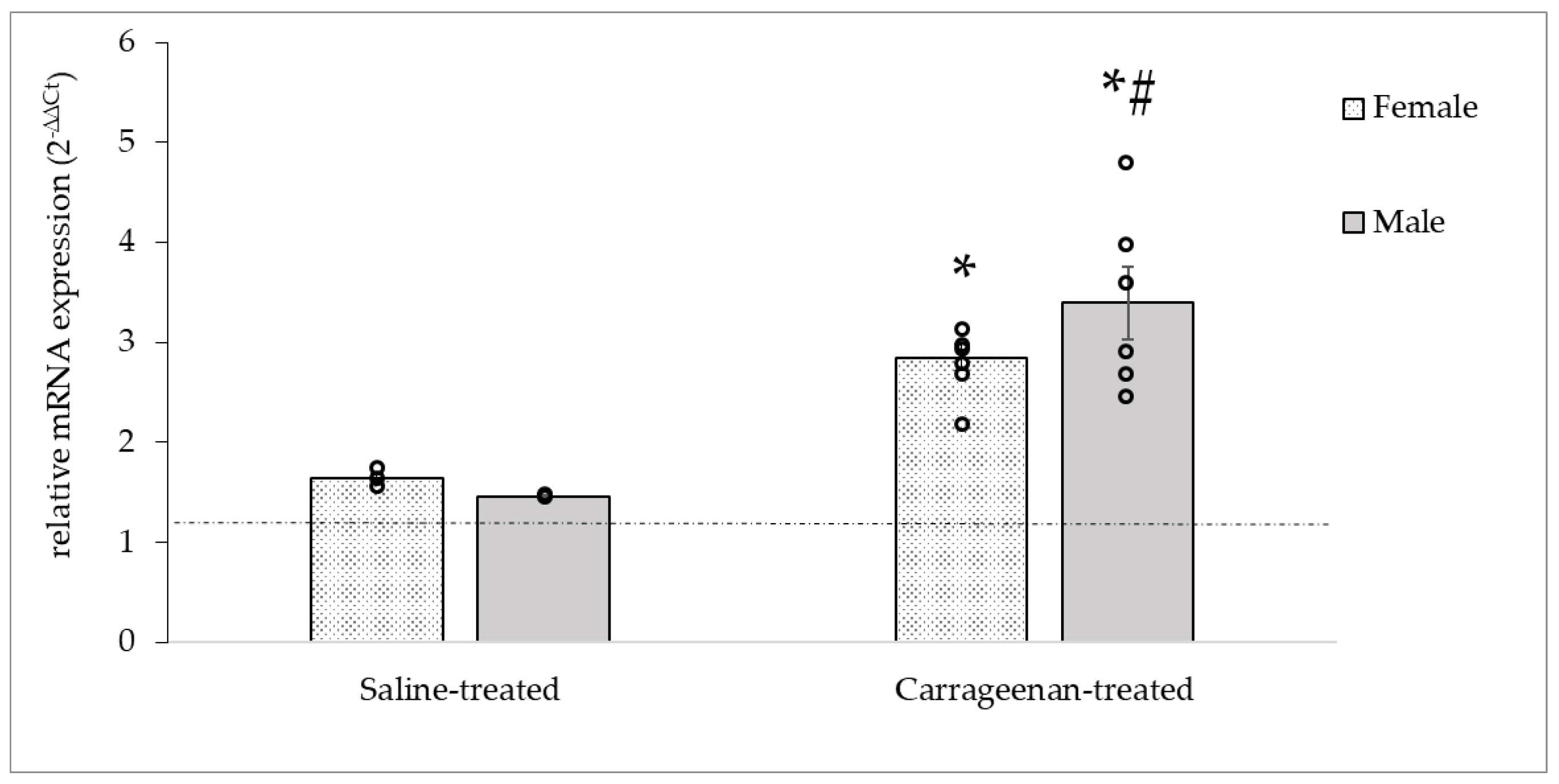
3.3.3. Analysis of the OTR mRNA Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubin, A.E.; Patapoutian, A. Nociceptors: The Sensors of the Pain Pathway. J. Clin. Investig. 2010, 120, 3760–3772. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.K.; Caterina, M.J. Functional Anatomy of the Sensory Nervous System: Updates From the Neuroscience Bench. Toxicol. Pathol. 2020, 48, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Bista, P.; Imlach, W.L. Pathological Mechanisms and Therapeutic Targets for Trigeminal Neuropathic Pain. Medicines 2019, 6, 91. [Google Scholar] [CrossRef] [PubMed]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An Overview of Animal Models of Pain: Disease Models and Outcome Measures. J. Pain 2013, 14, 1255–1269. [Google Scholar] [CrossRef]
- McCarson, K.E.; Fehrenbacher, J.C. Models of Inflammation: Carrageenan- or Complete Freund’s Adjuvant (CFA)-Induced Edema and Hypersensitivity in the Rat. Curr. Protoc. 2021, 1, e202. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Aita, M.; Chavkin, C. Partial Infraorbital Nerve Ligation as a Model of Trigeminal Nerve Injury in the Mouse: Behavioral, Neural, and Glial Reactions. J. Pain 2008, 9, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Eliava, M.; Melchior, M.; Knobloch-Bollmann, H.S.; Wahis, J.; da Silva Gouveia, M.; Tang, Y.; Ciobanu, A.C.; Triana Del Rio, R.; Roth, L.C.; Althammer, F.; et al. A New Population of Parvocellular Oxytocin Neurons Controlling Magnocellular Neuron Activity and Inflammatory Pain Processing. Neuron 2016, 89, 1291–1304. [Google Scholar] [CrossRef]
- Ayar, A.; Ozcan, M.; Alcin, E.; Serhatlioglu, I.; Ozcan, S.; Kutlu, S.; Kelestimur, H. Oxytocin Activates Calcium Signaling in Rat Sensory Neurons through a Protein Kinase C-Dependent Mechanism. J. Physiol. Biochem. 2014, 70, 43–48. [Google Scholar] [CrossRef]
- Che, T.; Sun, H.; Li, J.; Yu, X.; Zhu, D.; Xue, B.; Liu, K.; Zhang, M.; Kunze, W.; Liu, C. Oxytocin Hyperpolarizes Cultured Duodenum Myenteric Intrinsic Primary Afferent Neurons by Opening BK(Ca) Channels through IP3 Pathway. J. Neurochem. 2012, 121, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, B.; Han, T.; Huang, K.; Gong, L.; Ma, X.; Liu, K.; Cui, S.; Zhang, M.; Kunze, W.; et al. Oxytocin Down-Regulates Mesenteric Afferent Sensitivity via the Enteric OTR/NNOS/NO/K ATP Pathway in Rat. Neurogastroenterol. Motil. 2015, 27, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Gao, F.; Li, J.; Li, J.; Yu, X.; Ma, X.; Zheng, W.; Cui, S.; Liu, K.; Zhang, M.; et al. Oxytocin-Induced Membrane Hyperpolarization in Pain-Sensitive Dorsal Root Ganglia Neurons Mediated by Ca(2+)/NNOS/NO/KATP Pathway. Neuroscience 2015, 289, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.H.; Abele, H.; Plappert, C.F. The Role of Oxytocin and the Effect of Stress during Childbirth: Neurobiological Basics and Implications for Mother and Child. Front. Endocrinol. 2021, 12, 742236. [Google Scholar] [CrossRef] [PubMed]
- Tzabazis, A.; Kori, S.; Mechanic, J.; Miller, J.; Pascual, C.; Manering, N.; Carson, D.; Klukinov, M.; Spierings, E.; Jacobs, D.; et al. Oxytocin and Migraine Headache. Headache 2017, 57 (Suppl. 2), 64–75. [Google Scholar] [CrossRef]
- Tzabazis, A.; Mechanic, J.; Miller, J.; Klukinov, M.; Pascual, C.; Manering, N.; Carson, D.S.; Jacobs, A.; Qiao, Y.; Cuellar, J.; et al. Oxytocin Receptor: Expression in the Trigeminal Nociceptive System and Potential Role in the Treatment of Headache Disorders. Cephalalgia 2016, 36, 943–950. [Google Scholar] [CrossRef]
- Jójárt, J.; Jójárt, I.; Boda, K.; Gálfi, M.; Mihály, A.; B.-Baldauf, Z.; Vecsernyés, M. Distribution of Oxytocin-Immunoreactive Neuronal Elements in the Rat Spinal Cord. Acta Biol. Hung. 2009, 60, 333–346. [Google Scholar] [CrossRef]
- Eftekhari, S.; Salvatore, C.A.; Johansson, S.; Chen, T.; Zeng, Z.; Edvinsson, L. Localization of CGRP, CGRP Receptor, PACAP and Glutamate in Trigeminal Ganglion. Relation to the Blood–Brain Barrier. Brain Res. 2015, 1600, 93–109. [Google Scholar] [CrossRef]
- Krause, D.N.; Warfvinge, K.; Haanes, K.A.; Edvinsson, L. Hormonal Influences in Migraine—Interactions of Oestrogen, Oxytocin and CGRP. Nat. Rev. Neurol. 2021, 17, 621–633. [Google Scholar] [CrossRef]
- Myers, M.J.; Deaver, C.M.; Lewandowski, A.J. Molecular Mechanism of Action Responsible for Carrageenan-Induced Inflammatory Response. Mol. Immunol. 2019, 109, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Wixson, S.K.; Smiler, K.L. Chapter 9—Anesthesia and Analgesia in Rodents. In Anesthesia and Analgesia in Laboratory Animals; Kohn, D.F., Wixson, S.K., White, W.J., John Benson, G., Eds.; American College of Laboratory Animal Medicine: Schaumburg, IL, USA; Academic Press: San Diego, CA, USA, 1997; pp. 165–203. ISBN 978-0-12-417570-9. [Google Scholar]
- Cha, M.; Kohan, K.J.; Zuo, X.; Ling, J.X.; Gu, J.G. Assessment of Chronic Trigeminal Neuropathic Pain by the Orofacial Operant Test in Rats. Behav. Brain Res. 2012, 234, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Bangaru, M.L.Y.; Park, F.; Hudmon, A.; McCallum, J.B.; Hogan, Q.H. Quantification of Gene Expression after Painful Nerve Injury: Validation of Optimal Reference Genes. J. Mol. Neurosci. 2012, 46, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Legradi, A.; Dulka, K.; Jancso, G.; Gulya, K. Orofacial Skin Inflammation Increases the Number of Macrophages in the Maxillary Subregion of the Rat Trigeminal Ganglion in a Corticosteroid-Reversible Manner. Cell Tissue Res. 2020, 382, 551–561. [Google Scholar] [CrossRef]
- Schueler, M.; Neuhuber, W.L.; De Col, R.; Messlinger, K. Innervation of Rat and Human Dura Mater and Pericranial Tissues in the Parieto-Temporal Region by Meningeal Afferents. Headache 2014, 54, 996–1009. [Google Scholar] [CrossRef] [PubMed]
- Moreno-López, Y.; Martínez-Lorenzana, G.; Condés-Lara, M.; Rojas-Piloni, G. Identification of Oxytocin Receptor in the Dorsal Horn and Nociceptive Dorsal Root Ganglion Neurons. Neuropeptides 2013, 47, 117–123. [Google Scholar] [CrossRef]
- Warfvinge, K.; Krause, D.N.; Maddahi, A.; Grell, A.-S.; Edvinsson, J.C.; Haanes, K.A.; Edvinsson, L. Oxytocin as a Regulatory Neuropeptide in the Trigeminovascular System: Localization, Expression and Function of Oxytocin and Oxytocin Receptors. Cephalalgia 2020, 40, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Korczeniewska, O.A.; Husain, S.; Khan, J.; Eliav, E.; Soteropoulos, P.; Benoliel, R. Differential Gene Expression in Trigeminal Ganglia of Male and Female Rats Following Chronic Constriction of the Infraorbital Nerve. Eur. J. Pain 2018, 22, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.E.; Smith, M.S.; Verbalis, J.G. C-Fos and Related Immediate Early Gene Products as Markers of Activity in Neuroendocrine Systems. Front. Neuroendocrinol. 1993, 14, 173–213. [Google Scholar] [CrossRef] [PubMed]
- Demartini, C.; Tassorelli, C.; Zanaboni, A.M.; Tonsi, G.; Francesconi, O.; Nativi, C.; Greco, R. The Role of the Transient Receptor Potential Ankyrin Type-1 (TRPA1) Channel in Migraine Pain: Evaluation in an Animal Model. J. Headache Pain 2017, 18, 94. [Google Scholar] [CrossRef]
- Greco, R.; Demartini, C.; Zanaboni, A.; Tassorelli, C. Chronic and Intermittent Administration of Systemic Nitroglycerin in the Rat Induces an Increase in the Gene Expression of CGRP in Central Areas: Potential Contribution to Pain Processing. J. Headache Pain 2018, 19, 51. [Google Scholar] [CrossRef] [PubMed]
- Hervig, M.; Hofman-Bang, J.; Mikkelsen, J. Effect of Brain-Derived Neurotrophic Factor on Activity-Regulated Cytoskeleton-Associated Protein Gene Expression in Primary Frontal Cortical Neurons. Comparison with NMDA and AMPA. Eur. J. Pharmacol. 2011, 660, 351–357. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Ji, R.-R. C-Fos or PERK, Which Is a Better Marker for Neuronal Activation and Central Sensitization after Noxious Stimulation and Tissue Injury? TOPAINJ 2009, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the Trigeminal System in Migraine. Headache 2019, 59, 659–681. [Google Scholar] [CrossRef] [PubMed]
- Djouhri, L. Aδ-Fiber Low Threshold Mechanoreceptors Innervating Mammalian Hairy Skin: A Review of Their Receptive, Electrophysiological and Cytochemical Properties in Relation to Aδ-Fiber High Threshold Mechanoreceptors. Neurosci. Biobehav. Rev. 2016, 61, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Handler, A.; Ginty, D.D. The Mechanosensory Neurons of Touch and Their Mechanisms of Activation. Nat. Rev. Neurosci. 2021, 22, 521–537. [Google Scholar] [CrossRef]
- Ghitani, N.; Barik, A.; Szczot, M.; Thompson, J.H.; Li, C.; Le Pichon, C.E.; Krashes, M.J.; Chesler, A.T. Specialized Mechanosensory Nociceptors Mediating Rapid Responses to Hair Pull. Neuron 2017, 95, 944–954.e4. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, S.; Salvatore, C.A.; Calamari, A.; Kane, S.A.; Tajti, J.; Edvinsson, L. Differential Distribution of Calcitonin Gene-Related Peptide and Its Receptor Components in the Human Trigeminal Ganglion. Neuroscience 2010, 169, 683–696. [Google Scholar] [CrossRef]



| Target Gene | Primer Pairs (5′→3′) | Reference Sequence |
|---|---|---|
| GAPDH | 1 Fw: AGACAGCCGCATCTTCTTGT 1 Rev: TGATGGCAACAATGTCCACT | NM_017008.4 |
| CALCA | Fw: GCTGCCCAGATCAAGAGTCA Rev: ACCTGGTGAGCGATGACTTG | NM_017338.2 |
| c-Fos | Fw: GGGAGGACCTTATCTGTGCG Rev: TCTCCGGAAGAGGTGAGGAC | NM_022197 |
| OTR | Fw: TTCTTCTGCTGCTCTGCTCGT Rev: TCATGCTGAGATGGCTGAGA | NM_012871.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kemenesi-Gedei, P.B.; Csabafi, K.A.; Kis, G. Inflammatory Orofacial Pain Activates Peptidergic Neurons and Upregulates the Oxytocin Receptor Expression in Trigeminal Ganglion. Biomedicines 2023, 11, 2419. https://doi.org/10.3390/biomedicines11092419
Kemenesi-Gedei PB, Csabafi KA, Kis G. Inflammatory Orofacial Pain Activates Peptidergic Neurons and Upregulates the Oxytocin Receptor Expression in Trigeminal Ganglion. Biomedicines. 2023; 11(9):2419. https://doi.org/10.3390/biomedicines11092419
Chicago/Turabian StyleKemenesi-Gedei, Péter Bátor, Krisztina Anna Csabafi, and Gyöngyi Kis. 2023. "Inflammatory Orofacial Pain Activates Peptidergic Neurons and Upregulates the Oxytocin Receptor Expression in Trigeminal Ganglion" Biomedicines 11, no. 9: 2419. https://doi.org/10.3390/biomedicines11092419
APA StyleKemenesi-Gedei, P. B., Csabafi, K. A., & Kis, G. (2023). Inflammatory Orofacial Pain Activates Peptidergic Neurons and Upregulates the Oxytocin Receptor Expression in Trigeminal Ganglion. Biomedicines, 11(9), 2419. https://doi.org/10.3390/biomedicines11092419





