Abstract
Autism spectrum disorder (ASD) is rather common, presenting with prevalent early problems in social communication and accompanied by repetitive behavior. As vasopressin was implicated not only in salt-water homeostasis and stress-axis regulation, but also in social behavior, its role in the development of ASD might be suggested. In this review, we summarized a wide range of problems associated with ASD to which vasopressin might contribute, from social skills to communication, motor function problems, autonomous nervous system alterations as well as sleep disturbances, and altered sensory information processing. Beside functional connections between vasopressin and ASD, we draw attention to the anatomical background, highlighting several brain areas, including the paraventricular nucleus of the hypothalamus, medial preoptic area, lateral septum, bed nucleus of stria terminalis, amygdala, hippocampus, olfactory bulb and even the cerebellum, either producing vasopressin or containing vasopressinergic receptors (presumably V1a). Sex differences in the vasopressinergic system might underline the male prevalence of ASD. Moreover, vasopressin might contribute to the effectiveness of available off-label therapies as well as serve as a possible target for intervention. In this sense, vasopressin, but paradoxically also V1a receptor antagonist, were found to be effective in some clinical trials. We concluded that although vasopressin might be an effective candidate for ASD treatment, we might assume that only a subgroup (e.g., with stress-axis disturbances), a certain sex (most probably males) and a certain brain area (targeting by means of virus vectors) would benefit from this therapy.
1. Introduction
The prevalence of autism spectrum disorder (ASD) is unfortunately rather high and increasing [1], with a 4.3:1 boy-to-girl ratio [2] (Figure S1a). Different countries may report rather different data, from 0.02% in China to 3.66% in Sweden [3]. Some publications suggest that the main reason for the uptrend is the change in diagnostic criteria and the rise in awareness [4,5]. However, the role of other factors in this increase cannot be ruled out [6]. For example, both the maternal and paternal age—which is constantly increasing [7]—and the higher socioeconomic status can increase the risk for ASD [8]. The total lifetime estimated cost of ASD increased almost four times over the last 20 years [1] (Figure S1b,d). Accordingly, the interest in ASD is also constantly increasing, with an almost exponential growth in the number of publications from 2000 (Figure S1c).
Despite intensive ongoing research, the cause of ASD is still unknown. As most disorders, ASD might also have multiple causes, indicated by the three-hit theory [9] (Figure 1). As the first “hit”, ASD is associated with many genes, called high-confidence neurodevelopmental disorder genes [10]. From the more than 1500 genes participating in neurodevelopment, 1452 genes are located on the autosomes, 129 on the X chromosome, and 5 on the mitochondrial genome. These genes encode key regulators of synaptogenesis, synaptic plasticity, cytoskeleton dynamics, protein synthesis and degradation, chromatin remodeling, transcription, and lipid homeostasis [11]. The higher prevalence of ASD in boys might be due to X chromosome implication. Indeed, two mutations (neuroligin 3 and 4 (NLGN3 and NLGN4)) of the X chromosome may predispose males to ASD [12]. Interestingly, the number of X chromosomes influenced social behavior with parallel changes in the vasopressin (VP) content of amygdala in mice and VP plasma levels in patients [13]. Moreover, twin studies also supported strong genetic effects in ASD [14]. Interestingly, a recent study found common genetic variants in angiotensin II receptor type 2 in the maternal and infant DNA samples associated with risk of ASD, presumably through its involvement in the maturation of the VP–oxytocin (OT) pathway [15]. Additionally, endocrine (among others VP) and environmental factors might also be responsible for the sex difference (see later) [16].
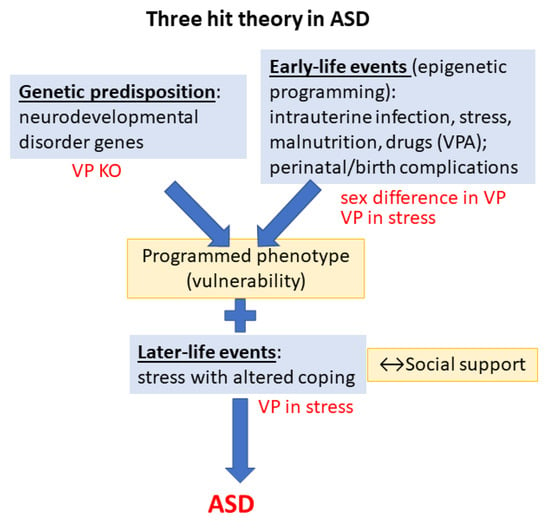
Figure 1.
The three-hit theory of autism spectrum disorder. Vasopressin may contribute to the development of symptoms at all levels. Abbreviations: ASD: autism spectrum disorder; VP: vasopressin; KO: knockout; VPA: valproate.
As the second “hit”, numerous early developmental risk factors were identified. A meta-analysis pointed out the fetus’s abnormal presentation, umbilical cord complications, fetal distress, birth injury or trauma, multiple births, maternal hemorrhage, summer birth, low birth weight, small for gestational age, congenital malformation, low 5 min Apgar score, feeding difficulties, meconium aspiration, neonatal anemia, ABO or Rh incompatibility, and hyperbilirubinemia as possible harmful perinatal events [17]. However, earlier intrauterine events might be similarly important, such as infections [18], and other maternal immune dysregulations [19], prenatal stress [19], malnutrition [20] or different drugs, like the antiepileptic valproate (VPA) [21]. The administration of VPA, a known histone deacetylase inhibitor, into the 11.5–13.5-day pregnant rodent mother influences the epigenetic machinery and induces ASD-like changes in the offspring, being a widely used animal model of ASD [22,23]. Interestingly, when acute VPA injection was used as gamma-aminobutyric acid (GABA) agonist, it was able to diminish angiotensin II- as well as hyperosmotic stimulus-induced VP rise in normal men [24,25].
As for the third “hit” (exacerbating acute stress), the measles, mumps, and rubella (MMR) vaccine was accused by the Wakefield report to increase the chance of developing ASD [26]. However, further studies contradicted this hypothesis [27,28]. Nevertheless, ASD patients may be more vulnerable to stress, with higher prevalence of, e.g., post-traumatic stress disorder [29]. In support, their hypothalamic–pituitary–adrenal (HPA) axis and autonomic nervous system (ANS) exhibit atypical functions both at resting state and during the presence of social and/or non-social stressors [30]. Higher levels of perceived stress and difficulties with coping have been reported in ASD children and adults [31].
It is more of a rule than an exception that people with ASD have at least one comorbid psychiatric disorder (80.9% of patient), with anxiety disorder being especially frequent (55.3%) [32,33,34]. Along the comorbidities, the prevalence of epilepsy [35], anxiety disorder [32], depression, obsessive compulsive disorder (OCD), specific phobias, and attention deficit hyperactivity disorder (ADHD) is higher than in the normal population [36,37,38]. For example, 33–37% of children with ASD present ADHD symptoms [39]. This makes the pathomechanism and therapies even more difficult.
Our aim was to summarize the literature exploring the possible symptoms of ASD and frequent comorbidities with a known or suggested contribution of VP and the possible therapeutic considerations. Previous studies focused either on the role of OT [40], on social skills [41] or on treatment options [42], without attempting to present a comprehensive view on VP–ASD interaction.
2. Major Symptoms of Autism Spectrum Disorder
According to the Diagnostic and Statistical Manual of Mental Disorders Fifth Edition Text Revision (DMS-5-TR) released in 2022, ASD is a pervasive neurodevelopmental disorder (NDD). Differences in behavior and developmental milestones appear in affected children by the first year of life [43,44]. The core symptoms are (1) deficits in social communication (e.g., disrupted language skills) together with anomalous socioemotional responses in several contexts; and (2) restricted, repetitive patterns of behavior [45,46] (Figure 2). Inaccurate parental recall can hinder the diagnosis [47,48]. The transition to prospective studies of high-risk infants has enabled a more comprehensive identification of early characteristics.
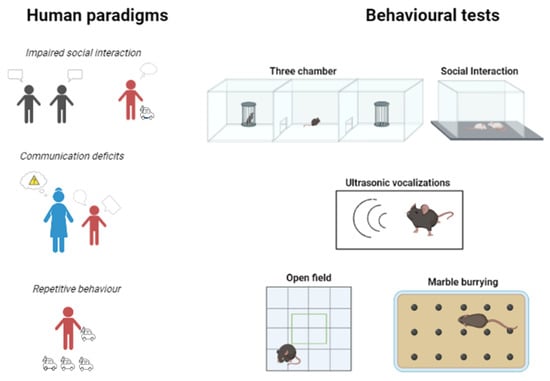
Figure 2.
Comparison of core human symptoms with animal tests modeling them. Impaired social interaction can be modeled via the three-chamber sociability test or anxiogenic social interaction in a new cage. Communication can be measured using ultrasound vocalization, while enhanced allo-grooming in rodents—observable in an open-field—is a sign of stereotyped, repetitive behavior together with the increased number of buried marbles during the marble burying test.
The large variation in the severity of symptoms within and across different groups hampered the ability to distinguish one disorder from another [49]. Of note, ASD and schizophrenia (SCZ) seem to overlap on many levels. Indeed, before the publication of the DSM-III [50], when ASD was first introduced as a different clinical diagnosis, autistic children were frequently diagnosed with childhood SCZ, which is characterized by abnormal perceptions of reality as well as deficits in social functioning [51]. Though ASD and SCZ are now classified as distinct disorders, they frequently co-occur and share common genetic risk factors and symptom presentations [52] (Figure 3).
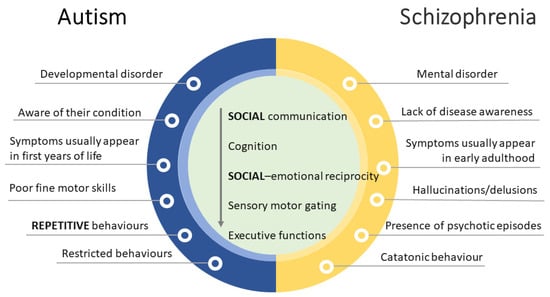
Figure 3.
Comparison of autism spectrum disorder (ASD) and schizophrenia (SCZ). Despite core similarities in social and cognitive domains, several unique features can be observed making the differential diagnosis easier.
In 1994, the categorical diagnoses of Asperger’s disorder, childhood disintegrative disorder, Rett’s disorder, and Pervasive Developmental Disorder not otherwise specified were introduced [53]. These disorders are distinguished by three core deficits: (1) impaired reciprocal social interaction, (2) deficient communication, and (3) restricted/repetitive behavioral or interest patterns [54] (Table 1, Figure 2). The range and severity of these impairments vary, and they frequently change with the acquisition of other developmental skills [55].
The 5th edition of the DSM altered the diagnostic criteria and put all the previous mentioned disorders (excluding SCZ) under the ASD category. The number of deficits in the core domains has been reduced to two ((1) social communication and (2) repetitive behavior; see earlier). ASD was diagnosed if a patient showed at least three social communication symptoms (e.g., reduced eye contact, lack of facial expressions, and impaired ability to start or maintain a conversation with others) and at least two restricted interests/repetitive behaviors, with an added behavior of hyper- or hypo-reactivity to sensory input or unique interests in sensory parts of the environment [56]. In the latest edition, the DSM-5-TR, some wordings were changed (e.g., “all of the following” criteria must be fulfilled) to tighten the diagnosis, thereby avoiding overdiagnosis.
Symptoms in Animal Models
To better understand the mechanism and identify new treatment options, animal models are needed. ASD models can be divided into two categories: genetical (e.g., mutation in OTR [57], NLGN, SRC homology 3 and multiple ankyrin repeat domains protein (SHANK), Contactin Associated Protein 2 (CNTNAP2), Melanoma Antigen Gene Family Member L2 (MAGEL2), a zinc-finger transcription factor TSHZ3, fragile-X syndrome [58,59]) and environmental [22]. The later can be drug-induced (e.g., VPA), immunological (polyriboinosinic: polyribocytidylic acid (poly I:C), a kind of maternal immune activation (MIA) [60]; at embryonic age E 11.5–13.5) or developmental, lesion-induced (presumably hippocampus lesion [61], the CA2 region [62], where V1b receptors might regulate aggression [63,64]).
A wide range of species is used. The most frequent are rodents (presumable transgenic mice [58] and rats), but avian [65], Drosophila melanogaster [11] and even zebrafish models [66,67] are also available [59].
Researchers can detect some of the behavioral components of ASD using a range of behavioral tasks in animal models (Table 1) [68]. Sociability, repetitive behavior, narrowness of the interest and associated symptoms (e.g., anxiety) are some of the parameters that are widely studied [69]. For example, the three-chamber sociability test [70] measures overall sociability and interest through direct social approach behaviors when a subject is given the option of spending time with a stimulus animal or an object. Measuring ultrasonic vocalizations [71] and grooming during an open-field test (OFT) [72] are two examples of behavioral tasks with face validity used to study communication deficits and repetitive behavior as well as comorbid anxiety in ASD animal models.

Table 1.
Comparison between human symptoms and animal models.
Table 1.
Comparison between human symptoms and animal models.
| Symptoms in Humans | Behavioral Test | Main Parameters in Rodents | References | |
|---|---|---|---|---|
| Impaired social interaction | Three-chamber sociability test | Time and fr. near stimulus animal | [70] | |
| Social interaction test | Time and fr. of social interactions | |||
| Deficient communication | MS-USV | USV | [71] | |
| Repetitive behavior | MBT | Number of buried marbles, time of digging | [73] | |
| Self-grooming | Grooming time and fr. in OFT | [74] | ||
| Comorbidity | Motor functions | Rotarod | Latency to fall | [75] |
| Erasmus Ladder | Number of missteps | [76] | ||
| DigiGait | Stance stride length and steps/sec | [77] | ||
| Delay eyeblink conditioning test | Successful conditioned response, blink amplitude and blinking speed | [77] | ||
| Anxiety | OFT | Total distance, time in center | [72] | |
| EPM | Time and fr. in arms | [72] | ||
| Pain sensitivity | Hot plate test | Withdraw latency | [78] | |
| Tail flick test | Withdraw latency | [78] | ||
Abbreviations: EPM: elevated plus maze; fr.: frequency; MBT: marble burying test; MS-USV: maternal separation-induced ultrasonic vocalization; OFT: open-field test; USV: ultrasonic vocalization.
3. Vasopressin
Our behavior is regulated by neuronal communication by means of more than 100 identified neurotransmitters [79]. While only 12 classical, small molecule neurotransmitters have been identified, there is an arsenal of oligopeptides that are also utilized by neurons to convey information. One of the best known pairs is the VP-OT, which are famous for their extensive role in physiology. These two neurotransmitters have highly similar amino acid sequences. Their characteristic is a disulfide bridge between position 1 and 6, thereby forming a ring. In mammals, VP and OT differ in two amino acids only: on position 3 (Phe for VP, Ile for OT) and 8 (Arg for VP, Leu for OT). Interestingly, this sequence seems to be exceptionally conserved throughout the species and even along different phylums. There is evidence of so-called VP- or OT-like peptides in invertebrates [80,81] going back on the phylogenetic tree as far as the Hydras of the Cnidarias [82]. The differences between them are also rather subtle, and all variants hold similar molecular properties (such as charge, hydrophobicity, polarity, etc.) [80]. According to our current knowledge, the common ancestral gene (along with their receptors [83]) went through duplication, and, over the course of the vertebrate evolution, the VP and OT genes gained their tail-to-tail orientation [84,85,86].
3.1. Vasopressin Receptors
When released from the neurohypophyseal nerve terminals of the magnocellular neurons of the paraventricular (PVN) and supraoptic nucleus (SON) of the hypothalamus to the blood, the main effect of the VP is osmoregulation in the kidney [87,88,89]. The activation of V2 receptors (Gs pathway) facilitates water reabsorption by increasing the expression and insertion of aquaporin 2 channel into the apical membranes of the collecting duct. Moreover, it also regulates the transcription of urea transporters and sodium channels, resulting in an overall increased water uptake from the urine (thus, the other name of VP is antidiuretic hormone, ADH).
There are two other known receptors of VP that activate the Gq (phosphatidylinositol) pathway. V1a receptors can be found in the vessels, liver and brain, with a widespread role in vasoconstriction, gluconeogenesis, blood clotting, social recognition and circadian rhythmicity, among others. On the other hand, V1b (or V3) receptors are mainly (but not exclusively) found on the anterior lobe of the pituitary, playing a role in stress adaptation.
Due to their shared structure, VP and OT can bind to each other’s receptors; however, this requires higher doses. The OT receptor (OTR) is also a G-protein coupled receptor [90]. Interestingly, its signalization may switch from Gq to Gi during pregnancy [91].
3.2. Vasopressin and Autism
Since 2006, a yearly average of 15.44 ± 1.34 articles are published on the possible contribution of VP in ASD (PubMed search with keywords “autism” and “vasopressin” and “publication date”), which shows a constant, stable interest. Vasopressin may influence ASD symptoms at several points, and is not restricted to the two major domains, the social skills and repetitive movements (see Section 2) (Figure 4).
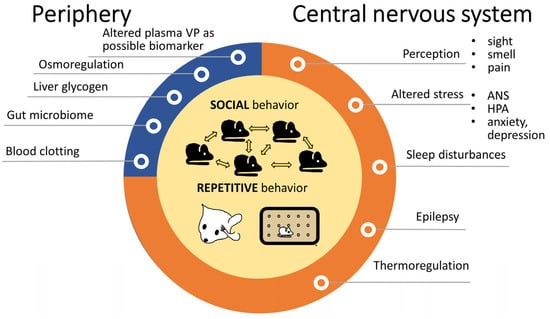
Figure 4.
Alterations in autism spectrum disorders with possible contribution of vasopressin. The observations were mainly in animals. Both social problems and repetitive behaviors—depicted in the middle—are core features of autism spectrum disorders and VP is obviously implicated in them. Peripheral VP functions (blue) might be only indirectly linked to autism, while other central VP effects (orange) might have a more important, although not yet fully clarified role. Abbreviations: ANS: autonomous nervous system; HPA: hypothalamic pituitary adrenocortical axis, VP: vasopressin.
3.2.1. Peripheral Vasopressin Function and Autism
In humans, the classical function of VP is the regulation of the salt-water homeostasis at the periphery (see earlier) and plasma levels are easier to monitor than brain levels. Therefore, human research initially focused on the correlation between peripheral VP concentration and ASD symptoms.
The VP-ASD connection was first raised in the abstract of a 1992 deWied article without further explanation [92]. In the same year, a brief report provided the first evidence for elevated VP plasma levels in autistic children (one girl and three boys, 11.75 ± 3.06 years old); however, it seemed to be a consequence of the disorder rather than a cause [93]. Moreover, in a later study, only 50% (out of 10) children had higher VP levels, while 70% showed a decrease in adrenocorticotropin (ACTH) levels, the hypophyseal component of the HPA axis [94], suggesting a blunted central VP effect. In some human cases, the blood VP levels correlated with the severity of certain aspects of ASD [95,96]. Maternal levels of VP were also associated with the symptoms of the offspring, with lower levels in the mother of ASD children [96,97]. Moreover, in human peripheral blood mononuclear cells, the expression of V1a receptor positively correlated with better social and behavioral function in ASD children (3–16-year-old) [98]. Despite this evidence, little is known about how altered peripheral VP homeostasis might influence the behavior in ASD patients.
A possible connection is through osmoregulation, although, for now, we can only talk about co-occurrence rather than causation. Nevertheless, NDDs are associated with higher rates of incontinence in children and adolescents, including nocturnal enuresis and daytime urinary incontinence [99]. In a small-scale study, urinary incontinence was observed in 85.1% of adults (out of 27) and 90% of children/teens (out of 20), and ASD patients had high prevalence of nocturnal enuresis [100]. Moreover, there is a possible association between kidney diseases and ASD as well [101,102]. Interestingly, in Caenorhabditis elegans, the ASD-related NLGN gene was found to also be important in osmoregulation [103] (Table 2). However, in NLGN3 KO mice, the OTergic system was found to be implicated in ASD-like symptoms [104]. In mice, heterozygous TSHZ3 +/− haploinsufficiency resulted in both ASD and renal abnormalities [105] (Table 2). The previously mentioned common genetic variants of the angiotensin receptor 2 may also contribute to urinary problems in ASD with a possible involvement of the VPergic system [15].
VP receptors are expressed in the liver as well [106], where the glycogen synthase produces glycogen to store energy [107]. Indeed, the glycogen synthase kinase 3β, one of the modulators, has been implicated in ASD [108], so much so that its inhibitors were even suggested for therapy [109]. VP resistance is observed in poorly controlled non-insulin-dependent diabetes mellitus subjects, which might contribute to their lower plasma volume [110]. Glucose transporter 3 (GLUT3) was coregulated in the neurohypophysis with VP [111] and its deficiency was also implicated in ASD, leading to social and communication problems and stereotyped behavior [112] (Table 2). In line, in V1a receptor KO mice, impaired glucose homeostasis was found [110].
VP might influence gut secretion [113]; however, gut microbiome can influence osmoregulation (at least in gerbils [114]). As the microbiome shapes—among others—the social behavior of the animals at several points from olfaction (with VP role [115,116]) to direct effect on social brain and social signaling molecules, e.g., VP-OT [117], we might assume a trilateral cooperation between VP, microbiome and ASD.
The coagulation pathways might also be altered in ASD [118,119]. Interestingly, desmopressin (1-desamino-8-D-arginine vasopressin, DDAVP), a V2 agonist, is widely used for promoting coagulation among others in von Willebrand disease (first line treatment) [120] and hemophilia [121].
However, all these peripheral VP-ASD connections are rather speculative and need further confirmations. Moreover, in the SHANK3 KO rat model, the plasma VP levels were normal [122]. Therefore, we concentrated on the central nervous system (CNS).
3.2.2. Social Behavior and Vasopressin with Implication in Autism
Impairments in social behavior are one of the main characteristics of ASD (Figure 3). Interestingly, both VP and OT have been connected to it and may regulate social behavior at the genetic, circulatory, neural functioning, and pharmacological levels [123]. Indeed, existing evidence suggests that VP can influence social functions already at the perceptional level and influence how sensory information is interpreted [124]. In this regard, the importance of VP in olfactory function was also confirmed before [115,116]. Moreover, VP was also found in the retina [125], VPergic fibers may project to the suprachiasmatic nucleus (SCN), the known center of circadian regulations (see later) [126,127].
Several animal models of ASD exist, where the contribution of VP was supposed mainly upon their effect on the social domain (Table 2). In the following section, we tried to discuss major findings along different phyla/species.

Table 2.
Animal models of autism with possible contribution of vasopressin.
Table 2.
Animal models of autism with possible contribution of vasopressin.
| Model | Major Problems | References | ||
|---|---|---|---|---|
| Type | Name/Implicated Molecule | |||
| Genetic models | KO | OTR | soc. | [57] |
| CNTNAP2 | soc., com. | [128] | ||
| MAGEL2 | soc. | [129] | ||
| OPRM1 | soc. | [130,131] | ||
| Klf7 | soc., rep. | [132] | ||
| Fragile X | FMR1 | soc., rep., motor problem, mood | [40] | |
| Rett syndrome | MECP2 | soc., com. | [133] | |
| Tuberous sclerosis | TSC1, TSC2 | soc., rep.; cerebellum; V2 antagonist | [134] | |
| Indirect evidence | NLGN mutations | soc., rest., com. | [103,135] | |
| TSHZ3 KO | soc., rep., narrowness of the field of interest | [68,105] | ||
| GLUT3 KO | soc., rep., com., memory problems | [111,112] | ||
| parvalbumin KO | soc., rep., com. | [136,137] | ||
| GAP43 | soc., resistance to change | [138,139] | ||
| SERT variants | soc., rep. | [140,141,142,143] | ||
| Environmental models | Drugs | VPA | soc., rep., com. | [144,145] |
| Maternal infection and inflammation | poly I:C | soc., rep. | [146,147] | |
| LPS | soc. | [148,149] | ||
| MIA | soc. | [147] | ||
Abbreviations: CNTNAP2: Contactin Associated Protein 2; Com: communication problems; Fragile Mental Retardation 1 locus (FMR1); GAP43: synaptic growth-associated protein-43; GLUT3: neuronal glucose transporter isoform 3; klf7: Krüppel-like factor 7; KO: knockout; LPS: lipopolysaccharide; MAGEL2: Melanoma Antigen Gene Family Member L2; MIA: maternal immune activation; methyl-CpG binding protein 2 (MECP2), NLGN: neuroligin; rep: repetitive behavior; poly I:C: polyriboinosinic: polyribocytidylic acid; OPRM1: μ opioid receptor; soc: social problems; TSC: tuberous sclerosis complex; TSHZ3: a zinc-finger transcription factor; VPA: valproate [59].
The VP-OT peptide affects social behavior already in ants [150]. Zebrafish (Danio rerio) is another good model for social behavior [66]. In relation to VP, after an aggressive encounter, the VP/vasotocin levels were higher in most brain areas of the winning zebrafish than in the losing ones. Moreover, fishes lacking OTRs showed antisocial-like behavior by the age of 8 weeks post fertilization [151]. A similar role can be identified in birds: pair bonding in zebra finches elevated their immunopositive V1a receptor numbers of selected brain areas [152]. VP/vasotocin was also critical for vocal learning in birds during development, a form of social communication [125]. In hamsters, it is theorized that VP serves as an ancestral molecule in scent marking and consequent territorial behaviors such as pair bonding [153,154,155].
Other studies showed decreased aggression after VP treatment in male mice reared in social isolation [156]. Mice are also widely used to study the effects of genetic alterations. In relation to ASD, direct manipulation (overexpression or knocking out (KO)) of the V1a gene may influence social behavioral outcomes [41]. Moreover, VP treatment has been shown to ameliorate social deficit via V1a receptors in OTR-deficient mice [57] (Table 2). CNTNAP2 is a key gene implicated in the manifestation of ASD symptoms, particularly in language disabilities (Table 2). In CNTNAP2-deficient mice, social deficits were improved after VP administration [128]. However, this effect was found to be mediated by OTRs, and not by V1a receptors. On the other hand, a deficiency of MAGEL2, a candidate gene for ASD and Prader Willi Syndrome, resulted in impaired social adaptation and discriminative social exploration caused by diminished VP, but not OT signaling in the lateral septum (LS) [129] (Table 2). μ opioid receptor (OPRM1)—possibly through a connection with the V1a receptors [130]—was also implicated in ASD with social motivation and skill problems in KO mice [131] (Table 2). Parvalbumin KO mice exhibited several ASD-like symptoms including social interactions and communication deficits [136] (Table 2). Linked to VP, developmentally, the V1a receptor modulates the number of parvalbumin positive interneurons in the cortex [137]. Among drug-induced ASD models, maternal viral infection (named maternal immune activation, MIA) can be mimicked via polyriboinosinic–polyribocytidylic acid (poly I:C) administration, which, in mice, induced changes in V1a mRNA expression in the hypothalamus of the offspring [147]. On the other hand, the bacterial infection model lipopolysaccharide (LPS) elevated maternal VP expression [157].
In rats, maternal aggression was also tied to VP, as high-anxiety-related behavior (HAB, based on elevated plus maze behavior) animals have an increased VP mRNA expression in PVN and limbic areas accompanied by increased maternal aggression putatively mediated by V1a receptors [158]. However, V1b receptors in the CA2 hippocampal region were also implicated in aggression [63,64]. One of the most famous rat KO models is the Brattleboro, which has a naturally occurring single-nucleotide deletion in the VP gene (exon 2), resulting in an inactive VP precursor. Thus, they have diabetes insipidus and urinate excessively due to missing the peripheral VP hormone. Moreover, they also exhibit behavioral alterations in social behavior, cognition [159,160] and stress response [159,161,162,163], making them an ideal model for SCZ [164,165,166]. However, during early development, they can serve as an ASD model as well. Indeed, we suggest that their social communication deficit during maternal-separation-induced ultrasound vocalization (MS-USV, Table 1) might provide a good test for new ASD treatment [167]. Based upon a KO mice model [168], as well as antagonist treatment in rat pups [169], V1b receptors might be responsible for this reduced communication. However, the reduced MS-USV was suggested to be a sign of anxiolysis. Nevertheless, maternal neglect of Brattleboro rats [170] might contribute to the disturbed development of the offspring, which might lead to the development of ASD-like symptoms in them [171]. Indeed, V1a receptor was associated with human maternal behavior [172]. In a VPA-induced ASD model, the subcutaneous (s.c.) administration of VP to adolescent rats alleviated social preference deficits and stereotyped behaviors, in parallel with an increase in cerebrospinal fluid VP concentration [173]. Moreover, in a rat MIA model with poly I:C administration, a reduced maternal VP level was found [146].
The role of VP in social behavior was also confirmed in an outdoor, ecologically relevant context, in free-living Richardson’s ground squirrels (Urocitellus richardsonii) [174]. In this species, chronic s.c. VP administration via an osmotic minipump increased male social vocalization and decreased their social aggression, thus supporting its pro-social role.
Despite general belief of the V1a receptor’s contribution to social behavior [175], in a study based on more than 3000 h of observation of 201 Rhesus macaques, the common genetic variation in the V1a receptor gene was not responsible for their social behavior [176]. Moreover, a recently developed V1a receptor KO hamster strain showed enhanced rather than reduced social communication, although this effect might be confounded by compensation in other systems [177]. Thus, we might assume some role of the V1b receptor as well in, e.g., maternal behavior [178]. Indeed, in humans, the V1b receptor participates in emotional empathy and social behavior [179]. Carriers of the G allele of V1b single-nucleotide polymorphism (SNP) rs28373064 have been reported to be more empathetic and pro-social [145]. Furthermore, the rs35369693 and rs28632197 SNPs of V1b were associated with ASD [180]. However, the variations in V1a receptors have been also significantly linked to ASD in humans [180]. Studies showed that rs11174815, rs7294536, rs3759292, and rs10877969 SNPs of the V1a receptor were correlated with ASD [181,182]. Moreover, an interaction between OPRM1, V1a receptor and social behavior was confirmed in students [130].
All in all, the aforementioned data clearly indicate VPergic contribution to social problems in ASD with V1a receptor implication. However, more human data are needed, and the sex/gender aspect should also be addressed.
3.2.3. Motor Signs: Repetitive Behavior and Convulsions
Beside social communication problems, alterations in motor function (especially stereotyped, repetitive behavior) are further core symptoms of ASD (see earlier), and vasopressin can also play a central role in the appearance of these.
Grooming
Already in 1981, the allo-grooming- and scratching-inducing effect of intracerebroventricular (i.c.v.), but not peripheral administration of a posterior pituitary extract was described in mice [183] (Figure 2). This short effect was not due to vasoconstriction [184], but was related to the vasoconstrictor properties of the analogues, suggesting the involvement of V1a receptors [185]. The grooming-inducing effect of low dose (below 100 pg) i.c.v. VP was also confirmed in rats, together with an inhibitory effect on exploration [186]. Later rat studies found even larger doses (30 ng) to be effective [187]. In hamster, both peripheral and central (intra-SCN) injection promoted grooming [188]. In line with this, the male [189], but not female [190], VP-deficient Brattleboro rat strain also showed reduced grooming, and in male squirrel monkeys, i.c.v. VP administration increased both grooming and scent-marking stereotyped behaviors [191]. However, this effect was not specific to VP, as a similar dose of OT could also elicit grooming both in male and female rats [192].
In contrast, in male rats, peripheral s.c. VP administration reduced grooming in open-field conditions 15 min, but not 60 min, after its administration [193]. A subsequent study found arginine VP (major form in humans and rodents) to be ineffective, while lysine VP had a similar reducing effect on grooming [194]. These discrepancies might be easily explained by the different peripheral and central role and receptor repertoire of VP.
Marble Burying
Marble burying might also reflect a repetitive behavior (Figure 3), often modeling OCD [195]. Although we could not detect any genotype difference in female VP-deficient Brattleboro rats [190], male Brattleboro and V1a KO mice buried fewer marbles than the controls [196,197], an effect not influenced by manipulation of the peripheral V2 receptors [197]. Moreover, during the adolescent period, both male and female Brattleboro rats displayed reduced marble burying [198]. However, these alterations were interpreted as anxiolysis and not reduced repetitive behavior.
Epilepsy—A Comorbidity
Nearly one-half of the individuals diagnosed with ASD have also been diagnosed with comorbid epilepsy [199]. Especially important, temporal lobe epilepsy (TLE) is highly prevalent, observable in one-third of ASD patients [200]. This might explain unpredictable emotional outbursts, hypersensitivity and hyperreactivity to trifling noises; thus, not only motor symptoms will occur.
I.c.v. administration of VP into rats induced dose-dependent (1–10 ng/rat) barrel rotations, a violent and apparently uncontrolled motor activity, suggesting a connection between VP and epilepsy [186]. Furthermore, VP administered s.c. (1 and 3 μg/rat) potentiated pilocarpine-induced seizures [201]. Even in febrile seizures (see later via thermoregulation), high VP doses had a pro-convulsant effect [202]. Thus, it was generally considered that VP is a pro-convulsant [175,202]. In accordance, antiepileptic drugs, including VPA, reduced VP-induced barrel rotation [203], without influencing serum VP levels [204]. Interestingly, the peripheral (intraperitoneal, i.p.) administration of low dose VP (0.1 μg/kg) lowered the pentylenetetrazol-induced seizures threshold, thus being pro-convulsant, while higher doses (10 and 20 μg/kg) increased it, thus being anti-convulsant [205]. The pro-convulsant effect was antagonized by both the V1a and the V1b antagonists, as well as the V2 antagonist, while only the V1b (and OTR) antagonist prevented the anti-convulsant action [175,201,205]. This suggests an atypical receptor activation of the higher doses, and a putative endogenous pro-convulsant effect of VP.
Stress in Autism—The Third “Hit”
Despite atypical ANS and HPA functioning of ASD patients both at resting state and during the presence of social and/or non-social stressors being generally accepted [30], there are still some controversies.
- The Autonomic Nervous System
For the correct interpretation of emotions, feedback from the ANS is essential as formulated by the often debated James–Lange theory [206]. Indeed, recent studies examining the heart rate variability confirmed dysregulation of the ANS in adult [207] and adolescent [208] ASD patients with reduced parasympathetic and increased sympathetic activity. The dysregulation of the ANS might lead to both hypo- and hyperarousal [209]. As social abilities are optimal when arousal is normal, when arousal increases due to misinterpretation of danger signals from the environment, social behaviors are compromised. Thus, ANS problems may contribute to socio-communication deficits in ASD.
Although VP and ANS were mostly examined separately in ASD, based on the cardiovascular peptide nature of VP and its strong interaction with ANS [210], we might assume a trilateral collaboration between VP, ANS and ASD. Indeed, VP is considered to be part of the “extended” ANS [211].
However, some authors argue that the reported ANS dysfunction in ASD patients is confounded by high anxiety related to the study situation or might be due to other comorbidities [212]. Thus, the question remains open.
- Vasopressin in Thermoregulation with Implication in Autism
Thermoregulation is accomplished via autonomic and behavioral responses controlled by the ANS [213].
There is no evidence to suggest that individuals with ASD are more prone to fevers than others. However, fever management may be more challenging in them due to their sensory hyper-sensitivity [214].
According to some observations, children with ASD show improved communication and social behavior during their febrile episodes [215,216]. The mechanism behind this has not yet been fully elucidated. Nevertheless, VP is involved in thermoregulation during fever: an early study found that central VP release increases during fever in sheep [217]. This phenomenon was later supported by a human study: plasma and cerebrospinal fluid VP concentrations were found to be elevated in febrile individuals compared to those in controls [218]. In the literature, VP is also referred to as an endogenous antipyretic: it influences thermoregulatory neurons in the anterior hypothalamus, preoptic, and septal areas [219,220] and can participate in tolerance to pyrogens in these areas [221]. However, in rabbits, the contribution of the peripheral VP effect through the V1 receptors was also suggested [222]. Thus, VP signaling might contribute to the transient beneficial effect of febrile episodes in ASD.
However, despite a beneficial antipyretic effect, high levels of VP might even induce febrile convulsions [202]. Yet, the receptor specificity is still questionable.
- The Hypothalamic–Pituitary–Adrenocortical Axis
It is textbook knowledge, that—together with corticotropin-releasing hormone (CRH)—VP from the PVN is a major regulator of the HPA axis, stimulating the ACTH secretion in the anterior lobe of the pituitary (AL) through V1b receptors [163,220]. There are reports on blunted [223] or exaggerated [224,225] cortisol (the end-hormone of the HPA axis) release in ASD patients in response to ACTH or perceived stressors with high individual variability [226]. Besides methodological differences (note that the measured stress-hormone levels are very sensitive to the sampling methods), the selective contribution of central–not only AL-V1b–receptors might shade the picture resulting in stressor-specific effects.
The synaptic growth-associated protein-43 (GAP43), an ASD candidate gene of interest, is deeply involved in the regeneration of VP production after injury [138], and, at the same time, its deficiency in GAP43 KO mice leads to resistance to changes as well as to stress vulnerability [139], further supporting VPerg’s contribution to an atypical ASD stress response (Table 2). We might even assume that altered stress reactivity designates a subpopulation of ASD patients as sensitive to VPergic manipulations.
- Mood Disorders and Autism—A Comorbidity
A meta-analysis conducted in 2019 showed that in adult ASD patients, the current prevalence of anxiety disorders was 27% (in comparison to 19.1% in a normal population), while that of depressive disorders was 23% (compared to 5% among neurotypical adults) [33,34]. VP was considered an ‘endogenous anxiogenic/depressogenic substance’ [220] based upon its prevalent role in HPA regulation and the stress-related nature of anxiety and depression [161,227].
- Anxiety
In a rodent model of anxiety (HAB and low anxiety behavior (LAB) mice and rats, see earlier), the VP gene was found to be the candidate gene for inborn anxiety [228]. In line with this, the VP-deficient male Brattleboro rats showed reduced anxiety- and depression-like symptoms [189]. Furthermore, the V1a receptor KO mice exhibited reduced anxiety [196,229] and the V1a receptor antagonist was found to be anxiolytic [230]. These results support the theory that the V1a receptor subtype plays an important role in the regulation of anxiety-like behavior. In support, a newly developed V1a receptor antagonist reduced anxiety-potentiated startle independently of fear-potentiated startle in healthy volunteers [231].
At present, we might only assume that VP also contributes to anxiety in ASD, which raises the possibility of a V1a antagonist treatment. However, its systemic administration might be questionable as V1a receptors are present in blood vessels; thus, these antagonists might lead to vasodilatation and hypotension.
- Depression and Serotonin
In relation to depression, many preclinical studies supported the involvement of the V1b receptor, the major regulator of the HPA axis in the development of the symptoms [220,232]. However, clinical studies did not support this notion [233]; therefore, most investigations following this direction were suspended [234]. For now, we can assume that V1b antagonists might be effective only in a selected subpopulation or gender.
On the other hand, serotonin is highly implicated in depression, as presently prescribed drugs are mainly selective serotonin reuptake inhibitors (SSRI). On the other hand, an average of a 50% increase was found in plasma-based serotonin levels in one-third of ASD individuals, supporting the connection between serotonin and ASD [235]. This increased peripheral level is thought to reduce central serotonin function due to negative feedback [200]. Moreover, genetic variation in the serotonin transporter (SERT) in mice led to—among others—ASD-like behavioral changes [140,141] (Table 2). However, this is a rather complex topic and different players of the serotoninergic systems might be differentially implicated [236]. In terms of the role of VP, abnormal HPA axis function might lead to mood disorders or even to suicidal behavior via the dysregulation of the serotonergic system [142]. There is a strong interaction between VP and serotonin in social contexts, modulating the appearance of aggressive behavior [143]. Indeed, serotonin might antagonize VP activity in the CNS [237,238]. In hamsters, VP induced repetitive aggressive behaviors, which were inhibited by the simultaneous use of a serotonin (5HT1a) agonist [239]. Thus, VP might contribute to the effectiveness of SSRIs in ASD (see later).
Sleep Disturbances in Autism—Another Third “Hit”?
One of the most frequent comorbidities in patients with ASD are sleep disorders (prevalence between 50% and 83%) [240,241], which contribute to a decreased quality of life [242]. Indeed, problems in the development of the sleep–wake rhythm might deeply contribute to the appearance of the NDDs [243].
In the literature, VP has been associated with sleep mostly in relation to mood disorders [244], but not to ASD. Nevertheless, VP is deeply implicated in circadian regulation (see Section 3.3.3), which might also be disturbed in ASD [245]. Even a causal gene for ASD, the Krüppel-like factor 7 (klf7), a transcription factor in the CNS (Table 2), might induce ASD-like behavior by regulating the circadian rhythm [132], indicating that sleep disturbances in ASD are causes more than they are consequences. Interestingly, besides direct regulatory role in the SCN, the potentiating effect of VP on melatonin secretion in pineal gland was also described in rats [246]. However, it has been disproved in a human study [247].
Vasopressinergic Pain Regulation with Implication in Autism
In patients with ASD, perceptional disturbances are common and hypersensitivity to pain might also occur [248]. Interestingly, more recent studies suggest that peripheral VP plays a direct role in the regulation of pain [249] and might be used even for postoperative analgesia in humans [250]. Furthermore, the V1a receptor subtype has been described in mice on the dorsal root ganglia, the major sensory ganglion, which might contribute to the analgesic effect of VP (as well as OT) [251].
However, another study suggested that VP modulates pain perception through brain areas outside the pain matrix [252], at least in rats [253]. In support, pain might increase the expression of VP in the rat PVN, suggesting an increased stress state [254]. We might assume that alterations in the cerebrospinal fluid VP content described in ASD patients might also contribute to their pain hypersensitivity, possibly through the PVN [255].
Although there are mainly rodent studies in connection with pain, we can hypothesize that evolutionarily well-conserved signaling pathways also regulate pain sensitivity in humans with VP contribution.
3.3. Brain Areas as Possible Links between Vasopressin and Autism
Considering the aforementioned wide range of processes, VP might influence the development of ASD (or ASD-like) symptoms at several points. We tried to summarize the available literature on brain areas with VPergic contribution along the two major domains (social skills and repetitive behavior) and added stress and circadian regulation as possible third “hits”.
3.3.1. Social Behavioral Network
The signaling mechanisms behind the social role of VP have been studied well. Indeed, VP receptors have been found in brain areas of the social behavior neural network (SBNN) [124,256] as well as in the mesocorticolimbic dopamine system [257]. These networks control social behavior in mammals according to the hypothesis of Newman [256] (Figure 5).
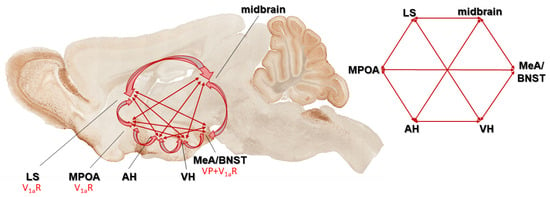
Figure 5.
According to the hypothesis of Newman, these brain areas form an integrated social behavior circuit, a subcortical limbic network (social behavior neural network (SBNN)) that subserves the entire spectrum of social behaviors. All these areas have been identified as an important place of activation or regulation of more than one social behavior and each is reciprocally interconnected with all others. Vasopressin (VP) and its V1a receptor can be found on many parts of this network. Abbreviations: AH: anterior hypothalamus; BNST: bed nucleus of stria terminalis; LS: lateral septum; MeA: medial part of the amygdala; MPOA: medial preoptic area; VH: ventromedial hypothalamus.
The medial preoptic area (MPOA)—part of the SBNN—might have a role in regulating social communication and social touch. In this context, MPOA-V1a receptors were found to be important in the olfactory communication in hamsters, called flank marking [153,258]. Recently, a thalamo-preoptic pathway was found to regulate social touch in female rats, with an existing human analogue [259]. As VP is released from MPOA [260] and regulates maternal care, we might assume its involvement in this process as well.
Another SBNN area, the lateral septum (LS), seems to have the most dominant role in regulating social recognition and social memory through VP [261,262,263]. Re-expressing V1a in the LS of KO mice normalized, while the overexpression in WT increased social memory [261]. Furthermore, in prairie vole (Microtus ochrogaster), the most studied social animal model, the septal VP fiber density showed alterations according to the paternal behavior, and V1a antagonist reduced the appearance of paternal responsiveness [155]. As a part of the paternal repertoire, the grooming of offspring was also elicited by LS injection of VP [155]. Septal VP may underlie the species differences and different life strategies of monogamous prairie vole and the polygamous montane vole (Microtus montanus) as well [264]. In a monogamous mice species (Peromyscus californicus), more VP receptors were found in the LS compared to a polygamous one (Peromyscus maniculatus) [265]. A recent study in an animal model of ASD has described a direct relationship between LS, VP, and social behavior [266]. A similar suggestion can be made based on a placebo-controlled study in healthy adult volunteers, as intranasal (i.n.) VP administration increased LS activity while viewing facial photographs [267].
In mice, the medial part of the amygdala (MeA) receives VP innervation [268], but it contains VP producing neurons as well and is implicated in the regulation of social behavior [269]. Moreover, VP was able to alter VPA-induced genetic alterations in the amygdala [144]. A human functional magnetic resonance imaging (fMRI) study suggested that VP modulates prefrontal cortex (PFC)-amygdala circuitry during emotion processing using facial emotion recognition [270]. Furthermore, in 3–5-year-old ASD children, negative functional connectivity of left amygdala and left supramarginal gyrus was accompanied by lower plasma VP levels; however, this was only detectable in boys [271]. In addition, an increased volume of the left amygdala has been found in children with ASD, and this was positively correlated with plasma VP levels. As a possible background, the amygdala of ASD patients showed an initial increase in the number of mature neurons followed by a decline into adulthood compared to healthy controls [272].
The extended amygdala region, the bed nucleus of stria terminalis (BNST) [273], also contains VP-producing cells [274]. Among others, BNST is associated with anxiety, addiction [275], and an innate fear response [276]. Moreover, a 2019 human study described it as one of the central brain areas of social anxiety [277]. Investigations on juvenile rats showed that VP is important in social play, and the PVN and BNST VP systems, in particular, regulate this behavior [274]. In the VPA model of ASD, VPergic BNST projection was significantly altered [278]. Moreover, maternal LPS injection-induced reduction in juvenile play was accompanied by reduced VP mRNA content here as well as in the MeA [149].
The PFC plays a pivotal role in social interactions, serving as a hub for motivation, affiliation, empathy, and social hierarchy [279]. In maternal VPA-treated monogamous prairie voles, PFC expressed less V1a receptor mRNA together with reduced sociability [280]. Additionally, VP altered VPA-induced genetic alterations in the PFC [281].
An often-neglected brain area is the olfactory bulb, containing VP as well [115,116]. Indeed, olfactory bulb dysgenesis was described in ASD and—according to the theory of Brang and Ramachandran [200]—might even be causally involved in mirror neuron system malfunction.
There are interactions between the above-mentioned areas, as LS receives VPergic innervations from BNST and MeA, forming a regulatory network for shaping social behavior [282,283,284]. The role of VP in aggression, a specific social behavior often observable in ASD patients, is brain-area-specific as its release in the LS facilitates, while BNST decreases, intermale aggression in rats [285]. Moreover, a fine balance exists between different areas, as SON replacement of VP in Brattleboro rats led to an increase in friendly interactions, which were originally normal in KO animals [159]. The VP immunoreactivity in both the LS and BNST is sexually dimorphic, and, in males, it is dependent on neonatal testosterone levels, later shaping aggressive behavior in mice [286]. In hamsters, VP injections to the ventrolateral hypothalamus facilitated aggression, an effect which was antagonized by i.p. SSRI administration [237].
Maternal behavior is another interesting social behavioral type, strongly influencing infant development, where VP is also deeply implicated [172]. Interestingly, MPOA administration of V1b antagonist increased, while its BNST injection decreased offspring care [178]. However, it is mostly the LS V1a receptors that are assumed to be involved in this process [287].
In summary, the dysfunction of the social behavioral network occurring during ASD might be related to VP at many points, among which the role of LS is best clarified.
3.3.2. Motor Behavior and Vasopressin
The previously mentioned flank marking in hamsters is, in fact, a stereotyped behavior, which was elicited by VP injection into the MPOA [288] and was antagonized by the V1 antagonist in the LS and BNST [154]. Furthermore, periaqueductal grey (PAG) injections of VP were also able to elicit this behavior both in male and female hamsters [289]. Moreover, SCN injection of VP in hamsters reduced spontaneous nocturnal running [188].
Interestingly, a recent study using optogenetic technique in mice elicited immediate grooming via the stimulation of the PVN VPergic cells [290]. On the other hand, grooming was elicited via amygdalar VP injection in male rats as well [291,292]. However, a repeated injection induced not only grooming, but also barrel rotations and myoclonic/myotonic-like convulsive behavior [293].
Barrel rotation was also induced by nodular cerebellum VP injections [294]. Indeed, cerebellum has attracted renewed interest as a brain area at the crossroads of cognitive and motor symptoms characteristic of ASD [295]. The cerebellum is not only critical for the coordination and adjustment of movement but is also involved in higher functions such as cognition, speech and emotion, all of which are altered in ASD [296]. Of interest is the fact that perinatal cerebellar injury is the highest risk factor for ASD, apart from an affected monozygotic twin [22,297]. In the tuberous sclerosis model of ASD, Purkinje cell damage was associated with ASD-like symptoms [298]. VP administration increased cerebellar activation to infant cry, supporting its contribution to emotional regulation [299].
The hippocampus is a prominent source of epileptic seizures. It contains both V1a and V1b (as well as OTR) receptors and both were shown to contribute to hippocampal excitability [300]. Moreover, the magnocellular PVN and SON, as well as parvocellular BNST and amygdala, project to the hippocampus.
3.3.3. Vasopressinergic Link to Stress, and Related Disorder
The anxiolytic effect of VP is presumably connected to V1a receptors localized in BNST [301]. Moreover, its effectiveness, similar to that of SSRIs, may be due to a BNST-derived VP-ergic innervation into the dorsal raphe nucleus (one of the main serotoninergic nuclei), but this pathway has only been described in mice to date.
On the other hand, the depressive-like behavior of HAB rats was accompanied by the overexpression of the VP gene in the PVN [302]. Differences in PVN have also been reported in human depressed patients compared to healthy controls, where both the VP genes and the V1a receptor were overexpressed [303,304].
PVN is also a major regulator of the ANS, being a concertmaster and providing a place for VP-ANS-ASD interaction [210,305].
3.3.4. Circadian Rhythm and Vasopressin
Electroencephalography (EEG) studies have suggested that ASD patients show alterations in rapid eye movement (REM) sleep [306], possibly due to a disturbance of the SCN, the center of endogenous clock. Indeed, in the VPA-induced animal model of autism, circadian dysregulation was found due to alterations within the core clockwork of SCN [307].
One of the first discovered neurotransmitters of the SCN was VP, confirmed later in many species, including humans [308]. The neuronal activity of VPergic cells of the SCN shows daily variation and may control neuroendocrine (stress and gonadal axis) and other (e.g., sleep–wake) rhythmic changes [309]. Research on hamsters showed that SCN VP cells might possibly respond to melatonin signals [310]. Furthermore, in rats, VP administration into the PVN elevated the plasma melatonin levels [311]. This might strengthen the hypothesis that VP can mediate circadian responses to melatonin in the SCN. Investigations into melatoninergic VP regulation in the human SCN may pave the way for new perspectives to understand the mechanism of sleep disturbances in ASD [312].
All in all, VP may play a key role in the onset of ASD symptoms and comorbidities in several brain areas, which can be different depending on the symptoms. In fact, ASD is a network disorder; thus, highlighting an area in particular would be difficult. For a summary of the possible brain areas with VPergic contributions to ASD symptoms, see Figure 6.
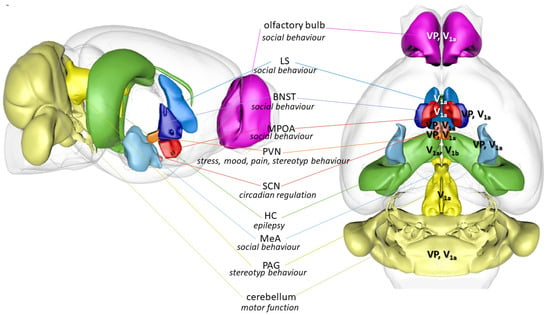
Figure 6.
Brain areas containing vasopressin producing neurons or vasopressin receptors with possible involvement in autistic behavior. Major role of the nucleus is given. Abbreviations: BNST: bed nucleus of stria terminalis; HC: hippocampus; LS: lateral septum; MeA: medial amygdala; MPOA: medial preoptic area; PAG: periaqueductal grey; PVN: paraventricular nucleus of the hypothalamus; SCN: suprachiasmatic nucleus; V1a: vasopressin receptor; V1b: vasopressin receptor; VP: vasopressin producing cells (based upon a mouse brain in https://scalablebrainatlas.incf.org/composer/index.php, accessed on 1 August 2023).
4. Sex Differences in Autism with Focus on Vasopressin
The higher prevalence of ASD in males [313] suggests sex-dependent regulatory processes. However, ASD can be under-diagnosed in females since they can better compensate for the lacking social skills or might show atypical signs [314,315]. Nevertheless, prominent theories have been proposed to explain sex biases, like genetic factors, sex hormones, sociological factors, cognitive differences between the sexes, and environmental insult [316].
In relation to our present topic, differences in the VP and OT system could be one of the underlying causes of the male bias. Since ASD is known as a neurodevelopmental disorder, the disruption of the VP system during early development may play an important role in the pathomechanism disrupting sex-specific neural circuits that are responsible for the sexually dimorphic nature of the social behavior [317]. Excess VP or dysregulation in the VP system could contribute to male vulnerability, while processes mediated via the OT system may explain the resistance in females [318].
In adult rodents, there are gender differences in the amount of VP produced and the density of V1a receptors in specific brain regions [317]. In these regions, the level of VP tends to be higher in male rats and mice than in females [268,283,319]. Of note, the LS of adult male rats have denser VP fibers but fewer V1a bindings than females [320,321]. This is in good agreement with the idea of brain-based sex differences in ASD; however, the authors suggested different neurodevelopmental trajectories leading to the masculinization or feminization of the brain [322]. Indeed, cortico-cerebellar hyperconnectivity was found in ASD females, while hypoconnectivity was found in males [323]. Nevertheless, the effect of VP treatment can also be sex-dependent, as, in free-living, female Richardson’s ground squirrels, it resulted in increased “anxiety-like” behaviors during social challenge, while, in males, it increased social communication and reduced aggression [174].
Alterations in sex hormones may also contribute to sex differences in ASD, as androgen receptors can be found in ASD-related brain regions [316]. On the other hand, sex hormones can affect the levels of VP, and could thus further contribute to male vulnerability in ASD. In the BNST and MeA, more than 90% of the VPergic neurons contain androgen receptors in rats, and VP synthesis was also androgen-dependent in those areas [324]. PVN also has a higher number of androgen receptors in human males compared to females [325]. Testosterone injected in female or neonatally castrated male rats on the first, second or third week of life resulted in an increase in VP fiber density [326]. In contrast, progesterone could inhibit the synthesis of VP in the BNST, the central nucleus of the amygdala and the LS in male rats [327].
In line with this predominant role of VP in males, the VP-deficient male, but not female Brattleboro rats, showed reduced social abilities [166,190]. To date, social deficit has been described in male V1a KO mice only [196,229,261]. In adolescent autistic patients, plasma VP showed negative correlation with repetitive behaviors in boys, while, in girls, the association was positive [328]. In relation to VP-serotonin interaction, gender differences in the response to SSRIs have been also reported [329].
The effects of exogenous VP treatment are also sexually dimorphic and dose-dependent. In prairie voles, i.c.v. low dose VP (0.5 ng) heightened the partner preference in males [330], but had no effect in females [331], further supporting the dominant role of VP in males. However, when 100 ng VP was administered, partner preference was enhanced in both genders [332].
5. Vasopressin-Related Possible Therapies in Autism
There is no cure for ASD, and there is currently no medication to treat it. The medications are prescribed mainly to treat self-injury, inability to focus, anxiety and depression (SSRIs), aggression (alpha-2 adrenergic agonist, Clonidine) and hyperactivity (dopamine and noradrenaline stimulant methylphenidate, Ritalin) [333]. Currently, strategies to treat the core symptoms of ASD are directed to correct synaptic dysfunctions, abnormalities in central VP, OT and serotonin neurotransmission, and neuroinflammation [42]. Although, for now, only two antipsychotics (risperidone and aripriprazole) are approved by the American Food and Drug Administration (FDA) for the treatment of some ASD symptoms, there are multiple drugs undergoing active investigation and trials to assess their safety and efficacy, including both VP agonists and—surprisingly—antagonists. However, OT is more intensively studied, even called a pro-social pill [334].
5.1. Available Therapies with Possible Vasopressinergic Contribution
Among the most prescribed medications for autism [333], the following VP interactions can be supposed:
From the second-generation antipsychotics used for the treatment of irritability, cariprazine is promising and their serotoninergic effect suggest a possible VPergic contribution [335].
For the improvement of mood, as well as to reduce the frequency and intensity of repetitive behaviors and improve eye contact, SSRIs are often used. In this regard, VP–serotonin interaction might contribute to the possible effectiveness of aggression treatment using SSRIs [238].
As regards methylphenidate (Ritalin), a dopamine (DA) reuptake inhibitor, it is used as a stimulant for the treatment of hyperactivity (paradoxically) and lack of attention in ASD. It was shown that it may influence the VP system [336] and it acts—at least partly—via the V1a receptor [337].
Alpha2-agonist (e.g., Clonidine) may be used for ASD-related hyperactivity, attention deficit, and aggression, and may interact with VP on the cardiovascular function. Indeed, i.c.v. Clonidine administration-induced pressor response was prevented by i.c.v. V1 antagonist administration in rats [338]. Interestingly, in humans, Clonidine administration decreased plasma VP levels [339]. In horses, no interaction was found between Clonidine and VP on HPA axis [340]; however, in rats, Clonidine reduced the firing of SON VPergic cells, further supporting an interaction at the level of water balance [341].
As for applied behavior analysis (ABA), in a backtranslation study using ASD model mice, this intervention normalized VP and V1a expression in several brain areas, including MeA [342].
Even transcutaneous electrical acupoint stimulation elevated VP levels in connection with an improvement of ASD symptoms [343].
Although experts do not recommend any specific diets for children (not even gluten- or casein-free), some probiotics might improve gastrointestinal symptoms [344]. As a possible link to VP, in prairie voles, Limosilactobacillus reuteri administration resulted in lower anxiety, but also lower social affiliation in female but not male individuals, with a decrease in PVN V1a expression [345].
For a summary, see Figure 7.
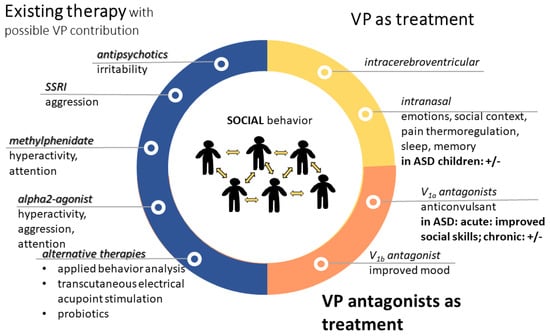
Figure 7.
Treatment options in autism with contribution of vasopressin. VP might contribute to the effectiveness of presently available therapies (blue). However, VP alone (yellow) or its antagonists (orange) can be used for therapy. Most of the treatments aim to improve social skills; however, sometimes the results are questionable (+/−). Abbreviations: ASD: autism spectrum disorder; SSRI: selective serotonin reuptake inhibitor; V1a: vasopressin 1a receptor; VP: vasopressin.
5.2. Influencing the Vasopressinergic System in Autism-Related Problems
Besides the aforementioned indirect effects, the direct influence on the VP pathway might have therapeutic potential on its own.
As VP does not cross the blood–brain barrier [175], for influencing the central VPergic system, i.c.v. or i.n. application is preferable.
In a rat VPA model, acute i.c.v. VP administration prevented social-interaction-induced brain activation based on blood oxygenation level (BOLD) signal in fMRI [346].
5.2.1. Intranasal Vasopressin Application
In rats, i.n. VP treatment (from PND 21 for 3 weeks) improved maternal VPA injection-induced (E12.5) social deficit, elevated the serum VP level and corrected expression changes related to synaptic and axon dysplasia and oligodendrocyte development in the PFC [281] and amygdala [144].
In male, but not female, marmosets, i.n. VP administration reduced food sharing with increased aggressive vocalization [347]. Accordingly, in monogamous male prairie voles [348], as well as in the coppery titi monkey (Callicebus cupreus) [349], a similar treatment reduced partner preference. These preclinical results did not suggest a possible positive effect on ASD symptoms.
However, when VP was administered i.n. for 4 weeks in ASD children aged 6–13 years in a phase 2 randomized clinical trial, improved social responsiveness and social abilities with decreased anxiety and limited repetitive behavior were reported [350]. The response was the strongest in high-plasma VP patients, and depended on the expression pattern of the V1a and OTR receptors. The latter might explain the controversially decreased anxiety, as V1b receptors were more involved in this stress-related disorder. In contrast, a randomized, double-blind, placebo controlled, between-subjects design on 125 undergraduate students (with 41 placebo, 30 females in each), using i.n. VP administration, did not find any effect on social outcomes [351]. In support, i.n. VP administration in rats failed to influence social recognition [352], despite previous effectiveness of the direct olfactory bulb manipulation [116]. Moreover, in healthy male volunteers, i.n. VP administration decreased goal-directed top-down attention control to social salient stimuli with an increase in bottom-up social attentional processing [353]. This effect was similar to OT administration and accompanied by an anxiolytic effect as well. In another study on face processing, a single low-dose i.n. VP (20 IU) administration to men decreased social assessments with a most pronounced effect in V1a risk allele carrier subjects [354]. This suggest that via i.n. application, significant amounts of VP might not reach behaviorally relevant areas in the brain described previously as targets for the central administration of the peptide [352].
For other ASD-related alterations, where we suggested possible VP contribution (Figure 4), the following treatment effects were found:
The activity of brain regions implicated in emotion processing was altered by i.n. VP treatment [41]. In this regard, in humans, i.n. VP regulated the processing of infant cry sounds with emotional contextual information in fathers [299]. In male volunteers, i.n. VP administration increased approaching ratings to some faces, together with increased processing suggested by higher N1 amplitude on the electroencephalograph; however, this effect was highly context-dependent [355]. Another study using fMRI in healthy male subjects reported reduced amygdalar activation to emotional faces after i.n. VP administration [356]. In contrast, another study reported enhanced neural pattern in the right amygdala to social–emotional stimuli observed via MRI [357].
As mentioned before, i.n. VP administration was also able to reduce pain in relation to postoperative orthopedic surgery [250].
Regarding its thermoregulatory role, i.n. VP (more specifically desmopressin, a V2 receptor-selective agonist) reduced persisting coldness after brain injury in six patients [358].
In contrast, i.n. VP administration exacerbated physiological ANS parameters in combat veterans [359].
In healthy, elderly subjects, i.n. VP promoted sleep time and improved sleep architecture [360], reinforcing the potential beneficial effect of VP in ASD treatment. However, it was ineffective as regards verbal memory function [361].
5.2.2. Vasopressin Antagonist Treatment
In recent years, vasopressin receptor antagonists have been in the spotlight of drug discovery, especially V1a selective molecules [362]. Publishing Balovaptan as a possible treatment for ASD greatly increased the interest in CNS-acting vasopressin antagonists. Although clinical trials were unsuccessful in many cases, there is still potential in the VP antagonists as shown by several currently ongoing clinical studies.
The main focus is on V1a receptor antagonists. In this context, SRX246, a V1a receptor antagonist, blocked the effect of i.n. VP administration-induced reduced amygdalar activation to angry faces [356]. Moreover, in 2017, a multicenter double-blinded crossover study found that single-dose intravenous (i.v.) infusion of RG7713, a highly selective V1a antagonist in adult males with high-functioning ASD, resulted in a subtle but statistically significant improvement in social communications and social sensitivity [363]. As a follow up, the VP Antagonist to Improve Social Communication in Autism (VANILLA), a double-blinded placebo controlled clinical trial, examined 223 adult men with high-functioning ASD using another selective V1a receptor antagonist, RG7714 (commercially known as Balovaptan) for 3 months [364]. The treatment was well tolerated and resulted in improvement in communication and socialization scores, though not in all aspects of the ASD spectrum (e.g., social responsiveness was not improved). Despite effectiveness during the phase 2 trial [365], in subsequent phase 3 trials in high-functioning children (5–17-year) [366] and adults (above 18-year) [367], the 6-month Balovaptan treatment was ineffective as regards social communication.
Other selective V1a receptor antagonists (like the orally active Relcovaptan) might be effective as regards comorbid epilepsy [175]. On the other hand, for many years, V1b receptor antagonists were developed to treat mood disorders. Despite previous ineffectiveness in major depression [233], V1b receptor antagonists might be effective in subpopulations [368,369] and are therefore still under development (e.g., THY1773 [370], TS-121 [371], ABT-436 [372]). We cannot ignore V2 receptors either, as Tolvaptan, a V2 antagonist was implicated in the treatment of tuberous sclerosis, a genetic ASD, in a case report [134] (Table 2).
5.2.3. Oxytocin Treatment
As VP might bind to OTRs (see earlier), it is important to note that several animal trials of OT treatment suggested beneficial effects. In children, even a single intranasal OT administration increased the nonverbal information-based judgments [373]. Despite mixed results, a recent meta-analysis found moderate evidence that a 6-week OT treatment might improve the reduced interest and repetitive behavior of ASD children and the effect lasted for at least 6 months [374].
5.2.4. Contradiction
There is an apparent contradiction between the effectiveness of VP as well as its antagonist. A possible explanation can be the age of the participants as well as the method used for drug administration (i.n. for children, other peripheral routes for adults), thereby targeting central or peripheral receptors. Moreover, although VP may stimulate all receptors including OTRs, its effectiveness can be different on them, while antagonists are highly selective, which might shift balance between the VP receptor actions.
6. Conclusions
VP as a social hormone with a ubiquitous role might influence the development of ASD symptoms at several points (Figure 4). Although we might even suppose a causative role of VP, at present, only a symptomatic treatment can be assumed. The available treatment options might also influence the VPergic system; however, VP or antagonist administration can also be considered. Controversially, both VP itself and V1a antagonist have already been proven to ameliorate several symptoms (Figure 7). This discrepancy drew our attention to the possibility of subpopulation (i.e., stress-sensitive, male individuals) and/or brain-area-specific manipulation, which was not emphasized before. One may suppose that ASD patients with low blood VP level might benefit more from VP therapy [350,375], and the doses should thus be adjusted accordingly. Although, in humans, systematic treatments are preferable, viral vectors are widely used in therapy nowadays to provide a possible life-long treatment with a single injection [376]. However, it would not be easy to dissect a certain brain area, as the role of VP in socioemotional functioning recruits multiple brain networks distributed across the whole brain [377] (Figure 5 and Figure 6). As the aforementioned clinical studies have limitations (e.g., low sample size, male-biased sample, short treatment duration, not medication-free patients [350]), further preclinical and clinical trials are needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biomedicines11102603/s1, Figure S1: Statistical data about the importance of autism spectrum disorder (ASD) [1,2,3,378,379,380].
Author Contributions
Conceptualization, K.L. and D.Z.; methodology, K.L., D.V., P.C., C.L.F., B.T., I.P. and D.Z.; investigation, K.L., D.V., P.C., C.L.F., B.T., I.P. and D.Z.; writing—original draft preparation, K.L., D.V., P.C., C.L.F., B.T., I.P. and D.Z.; writing—review and editing, K.L., D.V., P.C., C.L.F., B.T., I.P. and D.Z.; visualization, D.V., P.C., C.L.F., B.T., I.P. and D.Z.; supervision, K.L. and D.Z.; funding acquisition, D.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Brain Research Program (NAP 3.0) of the Hungarian Academy of Sciences; Mobility program between the Hungarian and Slovak Academy of Sciences (NKM2023-6/2023); the National Research Development and Innovation Office of Hungary (grant numbers K141934 and K138763), as well as the Thematic Excellence Program 2021 Health Sub-program of the Ministry for Innovation and Technology in Hungary (within the framework of the TKP2021-EGA-16 project of the Pécs University).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cakir, J.; Frye, R.E.; Walker, S.J. The lifetime social cost of autism: 1990–2029. Res. Autism Spectr. Disord. 2020, 72, 101505. [Google Scholar] [CrossRef]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S.; Andrews, J.G.; et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Rasoulpoor, S.; Rasoulpoor, S.; Shohaimi, S.; Jafarpour, S.; Abdoli, N.; Khaledi-Paveh, B.; Mohammadi, M. The global prevalence of autism spectrum disorder: A comprehensive systematic review and meta-analysis. Ital. J. Pediatr. 2022, 48, 112. [Google Scholar] [CrossRef]
- Wing, L.; Potter, D. The epidemiology of autistic spectrum disorders: Is the prevalence rising? Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 151–161. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Bearman, P. Diagnostic change and the increased prevalence of autism. Int. J. Epidemiol. 2009, 38, 1224–1234. [Google Scholar] [CrossRef]
- Fombonne, E. Epidemiology of pervasive developmental disorders. Pediatr. Res. 2009, 65, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.S.; Maenner, M.J.; Newschaffer, C.J.; Lee, L.C.; Cunniff, C.M.; Daniels, J.L.; Kirby, R.S.; Leavitt, L.; Miller, L.; Zahorodny, W.; et al. Advanced parental age and the risk of autism spectrum disorder. Am. J. Epidemiol. 2008, 168, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Durkin, M.S.; Maenner, M.J.; Meaney, F.J.; Levy, S.E.; DiGuiseppi, C.; Nicholas, J.S.; Kirby, R.S.; Pinto-Martin, J.A.; Schieve, L.A. Socioeconomic inequality in the prevalence of autism spectrum disorder: Evidence from a U.S. cross-sectional study. PLoS ONE 2010, 5, e11551. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; Bagot, R.C.; Parker, K.J.; Vinkers, C.H.; de Kloet, E.R. The three-hit concept of vulnerability and resilience: Toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 2013, 38, 1858–1873. [Google Scholar] [CrossRef]
- Leblond, C.S.; Le, T.L.; Malesys, S.; Cliquet, F.; Tabet, A.C.; Delorme, R.; Rolland, T.; Bourgeron, T. Operative list of genes associated with autism and neurodevelopmental disorders based on database review. Mol. Cell. Neurosci. 2021, 113, 103623. [Google Scholar] [CrossRef]
- Ueoka, I.; Pham, H.T.N.; Matsumoto, K.; Yamaguchi, M. Autism Spectrum Disorder-Related Syndromes: Modeling with Drosophila and Rodents. Int. J. Mol. Sci. 2019, 20, 4071. [Google Scholar] [CrossRef] [PubMed]
- Jamain, S.; Quach, H.; Betancur, C.; Rastam, M.; Colineaux, C.; Gillberg, I.C.; Soderstrom, H.; Giros, B.; Leboyer, M.; Gillberg, C.; et al. Mutations of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with autism. Nat. Genet. 2003, 34, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Cox, K.H.; Quinnies, K.M.; Eschendroeder, A.; Didrick, P.M.; Eugster, E.A.; Rissman, E.F. Number of X-chromosome genes influences social behavior and vasopressin gene expression in mice. Psychoneuroendocrinology 2015, 51, 271–281. [Google Scholar] [CrossRef]
- Tick, B.; Bolton, P.; Happe, F.; Rutter, M.; Rijsdijk, F. Heritability of autism spectrum disorders: A meta-analysis of twin studies. J. Child. Psychol. Psychiatry 2016, 57, 585–595. [Google Scholar] [CrossRef]
- Sajdel-Sulkowska, E.M.; Makowska-Zubrycka, M.; Czarzasta, K.; Kasarello, K.; Aggarwal, V.; Bialy, M.; Szczepanska-Sadowska, E.; Cudnoch-Jedrzejewska, A. Common Genetic Variants Link the Abnormalities in the Gut-Brain Axis in Prematurity and Autism. Cerebellum 2019, 18, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Ferreira, H.; Martins, J.; Goncalves, J.; Castelo-Branco, M. Male sex bias in early and late onset neurodevelopmental disorders: Shared aspects and differences in Autism Spectrum Disorder, Attention Deficit/hyperactivity Disorder, and Schizophrenia. Neurosci. Biobehav. Rev. 2022, 135, 104577. [Google Scholar] [CrossRef]
- Gardener, H.; Spiegelman, D.; Buka, S.L. Perinatal and neonatal risk factors for autism: A comprehensive meta-analysis. Pediatrics 2011, 128, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, C.N.; Tsimis, M.; Burd, I. Infections and Brain Development. Obstet. Gynecol. Surv. 2015, 70, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Beversdorf, D.Q.; Stevens, H.E.; Margolis, K.G.; Van de Water, J. Prenatal Stress and Maternal Immune Dysregulation in Autism Spectrum Disorders: Potential Points for Intervention. Curr. Pharm. Des. 2019, 25, 4331–4343. [Google Scholar] [CrossRef]
- Vohr, B.R.; Poggi Davis, E.; Wanke, C.A.; Krebs, N.F. Neurodevelopment: The Impact of Nutrition and Inflammation During Preconception and Pregnancy in Low-Resource Settings. Pediatrics 2017, 139 (Suppl. S1), S38–S49. [Google Scholar] [CrossRef]
- Ornoy, A. Valproic acid in pregnancy: How much are we endangering the embryo and fetus? Reprod. Toxicol. 2009, 28, 1–10. [Google Scholar] [CrossRef]
- Jaber, M. Genetic and environmental mouse models of autism reproduce the spectrum of the disease. J. Neural Transm. 2023, 130, 425–432. [Google Scholar] [CrossRef]
- Tartaglione, A.M.; Schiavi, S.; Calamandrei, G.; Trezza, V. Prenatal valproate in rodents as a tool to understand the neural underpinnings of social dysfunctions in autism spectrum disorder. Neuropharmacology 2019, 159, 107477. [Google Scholar] [CrossRef]
- Coiro, V.; Chiodera, P. Inhibition by sodium valproate of the arginine vasopressin and adrenocorticotropin responses to angiotensin II in normal men. Brain Res. 1989, 491, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Chiodera, P.; Gnudi, A.; Volpi, R.; Marchesi, C.; Marchesi, M.; Davoli, D.; Capretti, L.; Coiro, V. Effects of the GABAergic agent sodium valproate on the arginine vasopressin responses to hypertonic stimulation and upright posture in man. Clin. Endocrinol. 1989, 30, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, A.J.; Murch, S.H.; Anthony, A.; Linnell, J.; Casson, D.M.; Malik, M.; Berelowitz, M.; Dhillon, A.P.; Thomson, M.A.; Harvey, P.; et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998, 351, 637–641. [Google Scholar] [CrossRef]
- Taylor, L.E.; Swerdfeger, A.L.; Eslick, G.D. Vaccines are not associated with autism: An evidence-based meta-analysis of case-control and cohort studies. Vaccine 2014, 32, 3623–3629. [Google Scholar] [CrossRef]
- Maglione, M.A.; Das, L.; Raaen, L.; Smith, A.; Chari, R.; Newberry, S.; Shanman, R.; Perry, T.; Goetz, M.B.; Gidengil, C. Safety of vaccines used for routine immunization of U.S. children: A systematic review. Pediatrics 2014, 134, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Gonzalez, O.; Fresno-Rodriguez, A.; Spencer-Contreras, R.E.; Tarraga-Minguez, R.; Gonzalez-Fernandez, D.; Sepulveda-Opazo, F. Research Mapping of Trauma Experiences in Autism Spectrum Disorders: A Bibliometric Analysis. Healthcare 2023, 11, 1267. [Google Scholar] [CrossRef]
- Makris, G.; Agorastos, A.; Chrousos, G.P.; Pervanidou, P. Stress System Activation in Children and Adolescents with Autism Spectrum Disorder. Front. Neurosci. 2021, 15, 756628. [Google Scholar] [CrossRef]
- Thoen, A.; Steyaert, J.; Alaerts, K.; Evers, K.; Van Damme, T. A Systematic Review of Self-Reported Stress Questionnaires in People on the Autism Spectrum. Rev. J. Autism Dev. Disord. 2023, 10, 295–318. [Google Scholar] [CrossRef]
- de Bruin, E.I.; Ferdinand, R.F.; Meester, S.; de Nijs, P.F.; Verheij, F. High rates of psychiatric co-morbidity in PDD-NOS. J. Autism Dev. Disord. 2007, 37, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Hollocks, M.J.; Lerh, J.W.; Magiati, I.; Meiser-Stedman, R.; Brugha, T.S. Anxiety and depression in adults with autism spectrum disorder: A systematic review and meta-analysis. Psychol. Med. 2019, 49, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Simonoff, E.; Pickles, A.; Charman, T.; Chandler, S.; Loucas, T.; Baird, G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. J. Am. Acad. Child. Adolesc. Psychiatry 2008, 47, 921–929. [Google Scholar] [CrossRef]
- Lukmanji, S.; Manji, S.A.; Kadhim, S.; Sauro, K.M.; Wirrell, E.C.; Kwon, C.S.; Jette, N. The co-occurrence of epilepsy and autism: A systematic review. Epilepsy Behav. 2019, 98 Pt A, 238–248. [Google Scholar] [CrossRef]
- Leyfer, O.T.; Folstein, S.E.; Bacalman, S.; Davis, N.O.; Dinh, E.; Morgan, J.; Tager-Flusberg, H.; Lainhart, J.E. Comorbid psychiatric disorders in children with autism: Interview development and rates of disorders. J. Autism Dev. Disord. 2006, 36, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Pezzimenti, F.; Han, G.T.; Vasa, R.A.; Gotham, K. Depression in Youth with Autism Spectrum Disorder. Child. Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Postorino, V.; Kerns, C.M.; Vivanti, G.; Bradshaw, J.; Siracusano, M.; Mazzone, L. Anxiety Disorders and Obsessive-Compulsive Disorder in Individuals with Autism Spectrum Disorder. Curr. Psychiatry Rep. 2017, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Berenguer-Forner, C.; Miranda-Casas, A.; Pastor-Cerezuela, G.; Rosello-Miranda, R. Comorbidity of autism spectrum disorder and attention deficit with hyperactivity. A review study. Rev. Neurol. 2015, 60 (Suppl. S1), S37–S43. [Google Scholar]
- Francis, S.M.; Sagar, A.; Levin-Decanini, T.; Liu, W.; Carter, C.S.; Jacob, S. Oxytocin and vasopressin systems in genetic syndromes and neurodevelopmental disorders. Brain Res. 2014, 1580, 199–218. [Google Scholar] [CrossRef]
- Frye, R.E. Social Skills Deficits in Autism Spectrum Disorder: Potential Biological Origins and Progress in Developing Therapeutic Agents. CNS Drugs 2018, 32, 713–734. [Google Scholar] [CrossRef] [PubMed]
- Lacivita, E.; Perrone, R.; Margari, L.; Leopoldo, M. Targets for Drug Therapy for Autism Spectrum Disorder: Challenges and Future Directions. J. Med. Chem. 2017, 60, 9114–9141. [Google Scholar] [CrossRef] [PubMed]
- Werner, E.; Dawson, G.; Osterling, J.; Dinno, N. Brief report: Recognition of autism spectrum disorder before one year of age: A retrospective study based on home videotapes. J. Autism Dev. Disord. 2000, 30, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Osterling, J.; Dawson, G. Early recognition of children with autism: A study of first birthday home videotapes. J. Autism Dev. Disord. 1994, 24, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Cook, E.H.; Leventhal, B.L.; Amaral, D.G. Autism spectrum disorders. Neuron 2000, 28, 355–363. [Google Scholar] [CrossRef]
- Kelley, M.E.; Nadler, C.B.; Rey, C.; Cowie, S.; Podlesnik, C.A. Noncontingent reinforcement competes with response performance. J. Exp. Anal. Behav. 2017, 107, 343–353. [Google Scholar] [CrossRef] [PubMed]
- De Giacomo, A.; Fombonne, E. Parental recognition of developmental abnormalities in autism. Eur. Child. Adolesc. Psychiatry 1998, 7, 131–136. [Google Scholar] [CrossRef]
- Rutter, M. Concepts of autism: A review of research. J. Child. Psychol. Psychiatry 1968, 9, 1–25. [Google Scholar] [CrossRef]
- Rosen, N.E.; Lord, C.; Volkmar, F.R. The Diagnosis of Autism: From Kanner to DSM-III to DSM-5 and Beyond. J. Autism Dev. Disord. 2021, 51, 4253–4270. [Google Scholar] [CrossRef]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders, 3rd ed.; American Psychiatric Association: Washington, DC, USA, 1980. [Google Scholar]
- Rapoport, J.; Chavez, A.; Greenstein, D.; Addington, A.; Gogtay, N. Autism spectrum disorders and childhood-onset schizophrenia: Clinical and biological contributions to a relation revisited. J. Am. Acad. Child. Adolesc. Psychiatry 2009, 48, 10–18. [Google Scholar] [CrossRef]
- Trevisan, D.A.; Foss-Feig, J.H.; Naples, A.J.; Srihari, V.; Anticevic, A.; McPartland, J.C. Autism Spectrum Disorder and Schizophrenia Are Better Differentiated by Positive Symptoms Than Negative Symptoms. Front. Psychiatry 2020, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- Sharma, S.R.; Gonda, X.; Tarazi, F.I. Autism Spectrum Disorder: Classification, diagnosis and therapy. Pharmacol. Ther. 2018, 190, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Sala, M.; Braida, D.; Lentini, D.; Busnelli, M.; Bulgheroni, E.; Capurro, V.; Finardi, A.; Donzelli, A.; Pattini, L.; Rubino, T.; et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: A neurobehavioral model of autism. Biol. Psychiatry 2011, 69, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Willemsen, R.; Kooy, R.F. Mouse models of fragile X-related disorders. Dis. Model. Mech. 2023, 16, dmm049485. [Google Scholar] [CrossRef]
- Ergaz, Z.; Weinstein-Fudim, L.; Ornoy, A. Genetic and non-genetic animal models for autism spectrum disorders (ASD). Reprod. Toxicol. 2016, 64, 116–140. [Google Scholar] [CrossRef]
- Bucknor, M.C.; Gururajan, A.; Dale, R.C.; Hofer, M.J. A comprehensive approach to modeling maternal immune activation in rodents. Front. Neurosci. 2022, 16, 1071976. [Google Scholar] [CrossRef]
- Heuer, E.; Kazama, A.; Bachevalier, J. Acoustic startle and prepulse inhibition deficits in adult monkeys with neonatal lesions of the hippocampus, amygdala and orbital frontal cortex. Behav. Brain Res. 2023, 438, 114170. [Google Scholar] [CrossRef]
- Hitti, F.L.; Siegelbaum, S.A. The hippocampal CA2 region is essential for social memory. Nature 2014, 508, 88–92. [Google Scholar] [CrossRef]
- Pagani, J.H.; Zhao, M.; Cui, Z.; Avram, S.K.; Caruana, D.A.; Dudek, S.M.; Young, W.S. Role of the vasopressin 1b receptor in rodent aggressive behavior and synaptic plasticity in hippocampal area CA2. Mol. Psychiatry 2015, 20, 490–499. [Google Scholar] [CrossRef]
- Stevenson, E.L.; Caldwell, H.K. The vasopressin 1b receptor and the neural regulation of social behavior. Horm. Behav. 2012, 61, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Csillag, A.; Adam, A.; Zachar, G. Avian models for brain mechanisms underlying altered social behavior in autism. Front. Physiol. 2022, 13, 1032046. [Google Scholar] [CrossRef] [PubMed]
- Kareklas, K.; Teles, M.C.; Nunes, A.R.; Oliveira, R.F. Social zebrafish: Danio rerio as an emerging model in social neuroendocrinology. J. Neuroendocrinol. 2023, in press. [CrossRef]
- Tayanloo-Beik, A.; Hamidpour, S.K.; Abedi, M.; Shojaei, H.; Tavirani, M.R.; Namazi, N.; Larijani, B.; Arjmand, B. Zebrafish Modeling of Autism Spectrum Disorders, Current Status and Future Prospective. Front. Psychiatry 2022, 13, 911770. [Google Scholar] [CrossRef] [PubMed]
- Roubertoux, P.L.; Tordjman, S.; Caubit, X.; di Cristopharo, J.; Ghata, A.; Fasano, L.; Kerkerian-Le Goff, L.; Gubellini, P.; Carlier, M. Construct Validity and Cross Validity of a Test Battery Modeling Autism Spectrum Disorder (ASD) in Mice. Behav. Genet. 2020, 50, 26–40. [Google Scholar] [CrossRef]
- Crawley, J.N. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007, 17, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Silverman, J.L.; Crawley, J.N. Automated three-chambered social approach task for mice. Curr. Protoc. Neurosci. 2011, 8, 8–26. [Google Scholar] [CrossRef]
- Penagarikano, O.; Abrahams, B.S.; Herman, E.I.; Winden, K.D.; Gdalyahu, A.; Dong, H.; Sonnenblick, L.I.; Gruver, R.; Almajano, J.; Bragin, A.; et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 2011, 147, 235–246. [Google Scholar] [CrossRef]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Angoa-Perez, M.; Kane, M.J.; Briggs, D.I.; Francescutti, D.M.; Kuhn, D.M. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 2013, 82, 50978. [Google Scholar]
- Silverman, J.L.; Tolu, S.S.; Barkan, C.L.; Crawley, J.N. Repetitive self-grooming behavior in the BTBR mouse model of autism is blocked by the mGluR5 antagonist MPEP. Neuropsychopharmacology 2010, 35, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, P.E.; Fuccillo, M.V.; Maxeiner, S.; Hayton, S.J.; Gokce, O.; Lim, B.K.; Fowler, S.C.; Malenka, R.C.; Sudhof, T.C. Autism-associated neuroligin-3 mutations commonly impair striatal circuits to boost repetitive behaviors. Cell 2014, 158, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Ten Brinke, M.M.; Stedehouder, J.; Reinelt, C.M.; Wu, B.; Zhou, H.; Zhou, K.; Boele, H.J.; Kushner, S.A.; Lee, M.G.; et al. Dysfunctional cerebellar Purkinje cells contribute to autism-like behaviour in Shank2-deficient mice. Nat. Commun. 2016, 7, 12627. [Google Scholar] [CrossRef]
- Piochon, C.; Kloth, A.D.; Grasselli, G.; Titley, H.K.; Nakayama, H.; Hashimoto, K.; Wan, V.; Simmons, D.H.; Eissa, T.; Nakatani, J.; et al. Cerebellar plasticity and motor learning deficits in a copy-number variation mouse model of autism. Nat. Commun. 2014, 5, 5586. [Google Scholar] [CrossRef]
- Silverman, J.L.; Turner, S.M.; Barkan, C.L.; Tolu, S.S.; Saxena, R.; Hung, A.Y.; Sheng, M.; Crawley, J.N. Sociability and motor functions in Shank1 mutant mice. Brain Res. 2011, 1380, 120–137. [Google Scholar] [CrossRef]
- Cuevas, J. Neurotransmitters and Their Life Cycle. Ref. Modul. Biomed. Sci. 2019, 1–7. [Google Scholar] [CrossRef]
- Acher, R. Neurohypophysial peptide systems: Processing machinery, hydroosmotic regulation, adaptation and evolution. Regul. Pept. 1993, 45, 1–13. [Google Scholar] [CrossRef]
- Acher, R.; Chauvet, J.; Chauvet, M.T. Man and the chimaera. Selective versus neutral oxytocin evolution. Adv. Exp. Med. Biol. 1995, 395, 615–627. [Google Scholar] [PubMed]
- Grimmelikhuijzen, C.J.; Graff, D. Arg-Phe-amide-like peptides in the primitive nervous systems of coelenterates. Peptides 1985, 6 (Suppl. S3), 477–483. [Google Scholar] [CrossRef]
- Ocampo Daza, D.; Bergqvist, C.A.; Larhammar, D. The Evolution of Oxytocin and Vasotocin Receptor Genes in Jawed Vertebrates: A Clear Case for Gene Duplications Through Ancestral Whole-Genome Duplications. Front. Endocrinol. 2021, 12, 792644. [Google Scholar] [CrossRef] [PubMed]
- Elphick, M.R.; Mirabeau, O.; Larhammar, D. Correction: Evolution of neuropeptide signalling systems. J. Exp. Biol. 2018, 221 Pt 19, jeb151092. [Google Scholar] [CrossRef]
- Gwee, P.C.; Tay, B.H.; Brenner, S.; Venkatesh, B. Characterization of the neurohypophysial hormone gene loci in elephant shark and the Japanese lamprey: Origin of the vertebrate neurohypophysial hormone genes. BMC Evol. Biol. 2009, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Kochman, K. Neurohormones: Oxytocin, vasopressin and related peptides—Structure, genes, receptors, and evolution. J. Anim. Feed. Sci. 2013, 22, 283–294. [Google Scholar] [CrossRef][Green Version]
- Bankir, L.; Bichet, D.G.; Morgenthaler, N.G. Vasopressin: Physiology, assessment and osmosensation. J. Intern. Med. 2017, 282, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; Hurtado-Alvarado, G.; Soto-Tinoco, E. Vasopressin: An output signal from the suprachiasmatic nucleus to prepare physiology and behaviour for the resting phase. J. Neuroendocrinol. 2021, 33, e12998. [Google Scholar] [CrossRef]
- Danziger, J.; Zeidel, M.L. Osmotic homeostasis. Clin. J. Am. Soc. Nephrol. 2015, 10, 852–862. [Google Scholar] [CrossRef]
- McKay, E.C.; Counts, S.E. Oxytocin Receptor Signaling in Vascular Function and Stroke. Front. Neurosci. 2020, 14, 574499. [Google Scholar] [CrossRef]
- Zhou, X.B.; Lutz, S.; Steffens, F.; Korth, M.; Wieland, T. Oxytocin receptors differentially signal via Gq and Gi proteins in pregnant and nonpregnant rat uterine myocytes: Implications for myometrial contractility. Mol. Endocrinol. 2007, 21, 740–752. [Google Scholar] [CrossRef] [PubMed]
- de Wied, D. Neuropeptides as psychotropic drugs. Acta Neuropsychiatr. 1992, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Leboyer, M.; Bouvard, M.P.; Launay, J.M.; Tabuteau, F.; Waller, D.; Dugas, M.; Kerdelhue, B.; Lensing, P.; Panksepp, J. Brief report: A double-blind study of naltrexone in infantile autism. J. Autism Dev. Disord. 1992, 22, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, M.P.; Leboyer, M.; Launay, J.M.; Recasens, C.; Plumet, M.H.; Waller-Perotte, D.; Tabuteau, F.; Bondoux, D.; Dugas, M.; Lensing, P.; et al. Low-dose naltrexone effects on plasma chemistries and clinical symptoms in autism: A double-blind, placebo-controlled study. Psychiatry Res. 1995, 58, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Carson, D.S.; Garner, J.P.; Hyde, S.A.; Libove, R.A.; Berquist, S.W.; Hornbeak, K.B.; Jackson, L.P.; Sumiyoshi, R.D.; Howerton, C.L.; Hannah, S.L.; et al. Arginine Vasopressin Is a Blood-Based Biomarker of Social Functioning in Children with Autism. PLoS ONE 2015, 10, e0132224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.F.; Dai, Y.C.; Wu, J.; Jia, M.X.; Zhang, J.S.; Shou, X.J.; Han, S.P.; Zhang, R.; Han, J.S. Plasma Oxytocin and Arginine-Vasopressin Levels in Children with Autism Spectrum Disorder in China: Associations with Symptoms. Neurosci. Bull. 2016, 32, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.J.; Shou, X.J.; Li, J.; Jia, M.X.; Zhang, J.S.; Guo, Y.; Wei, Q.Y.; Zhang, X.T.; Han, S.P.; Zhang, R.; et al. Mothers of autistic children: Lower plasma levels of oxytocin and Arg-vasopressin and a higher level of testosterone. PLoS ONE 2013, 8, e74849. [Google Scholar] [CrossRef]
- Voinsky, I.; Bennuri, S.C.; Svigals, J.; Frye, R.E.; Rose, S.; Gurwitz, D. Peripheral Blood Mononuclear Cell Oxytocin and Vasopressin Receptor Expression Positively Correlates with Social and Behavioral Function in Children with Autism. Sci. Rep. 2019, 9, 13443. [Google Scholar] [CrossRef]
- von Gontard, A.; Hussong, J.; Yang, S.S.; Chase, J.; Franco, I.; Wright, A. Neurodevelopmental disorders and incontinence in children and adolescents: Attention-deficit/hyperactivity disorder, autism spectrum disorder, and intellectual disability-A consensus document of the International Children’s Continence Society. Neurourol. Urodyn. 2022, 41, 102–114. [Google Scholar] [CrossRef]
- Gubbiotti, M.; Elisei, S.; Bedetti, C.; Marchiafava, M.; Giannantoni, A. Urinary and bowel disfunction in autism spectrum disorder: A prospective, observational study. Psychiatr. Danub. 2019, 31 (Suppl. S3), 475–478. [Google Scholar]
- Clothier, J.; Absoud, M. Autism spectrum disorder and kidney disease. Pediatr. Nephrol. 2021, 36, 2987–2995. [Google Scholar] [CrossRef]
- Miot, S.; Akbaraly, T.; Michelon, C.; Couderc, S.; Crepiat, S.; Loubersac, J.; Picot, M.C.; Pernon, E.; Gonnier, V.; Jeandel, C.; et al. Comorbidity Burden in Adults with Autism Spectrum Disorders and Intellectual Disabilities-A Report From the EFAAR (Frailty Assessment in Ageing Adults with Autism Spectrum and Intellectual Disabilities) Study. Front. Psychiatry 2019, 10, 617. [Google Scholar] [CrossRef]
- Calahorro, F.; Alejandre, E.; Ruiz-Rubio, M. Osmotic avoidance in Caenorhabditis elegans: Synaptic function of two genes, orthologues of human NRXN1 and NLGN1, as candidates for autism. J. Vis. Exp. 2009, 34, e1616. [Google Scholar]
- Hornberg, H.; Perez-Garci, E.; Schreiner, D.; Hatstatt-Burkle, L.; Magara, F.; Baudouin, S.; Matter, A.; Nacro, K.; Pecho-Vrieseling, E.; Scheiffele, P. Rescue of oxytocin response and social behaviour in a mouse model of autism. Nature 2020, 584, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martin, I.; Magalhaes, P.; Ranjzad, P.; Fatmi, A.; Richard, F.; Manh, T.P.V.; Saurin, A.J.; Feuillet, G.; Denis, C.; Woolf, A.S.; et al. Haploinsufficiency of the mouse Tshz3 gene leads to kidney defects. Hum. Mol. Genet. 2021, 31, 1921–1945. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, N.L.; Lolait, S.J.; Bradley, D.J.; O’Carroll, A.M.; Brownstein, M.J.; Young, W.S., 3rd. Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinol. 1992, 131, 533–535. [Google Scholar] [CrossRef]
- Frizzo, M.E. Putative role of glycogen as a peripheral biomarker of GSK3beta activity. Med. Hypotheses 2013, 81, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Kalkman, H.O. Potential opposite roles of the extracellular signal-regulated kinase (ERK) pathway in autism spectrum and bipolar disorders. Neurosci. Biobehav. Rev. 2012, 36, 2206–2213. [Google Scholar] [CrossRef]
- Arciniegas Ruiz, S.M.; Eldar-Finkelman, H. Glycogen Synthase Kinase-3 Inhibitors: Preclinical and Clinical Focus on CNS-A Decade Onward. Front. Mol. Neurosci. 2021, 14, 792364. [Google Scholar] [CrossRef]
- Aoyagi, T.; Birumachi, J.; Hiroyama, M.; Fujiwara, Y.; Sanbe, A.; Yamauchi, J.; Tanoue, A. Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice. Endocrinology 2007, 148, 2075–2084. [Google Scholar] [CrossRef]
- Vannucci, S.J.; Maher, F.; Koehler, E.; Simpson, I.A. Altered expression of GLUT-1 and GLUT-3 glucose transporters in neurohypophysis of water-deprived or diabetic rats. Am. J. Physiol. 1994, 267, E605–E611. [Google Scholar] [CrossRef]
- Zhao, Y.; Fung, C.; Shin, D.; Shin, B.C.; Thamotharan, S.; Sankar, R.; Ehninger, D.; Silva, A.; Devaskar, S.U. Neuronal glucose transporter isoform 3 deficient mice demonstrate features of autism spectrum disorders. Mol. Psychiatry 2010, 15, 286–299. [Google Scholar] [CrossRef]
- Pais, R.; Rievaj, J.; Meek, C.; De Costa, G.; Jayamaha, S.; Alexander, R.T.; Reimann, F.; Gribble, F. Role of enteroendocrine L-cells in arginine vasopressin-mediated inhibition of colonic anion secretion. J. Physiol. 2016, 594, 4865–4878. [Google Scholar] [CrossRef]
- Nouri, Z.; Zhang, X.Y.; Khakisahneh, S.; Degen, A.A.; Wang, D.H. The microbiota-gut-kidney axis mediates host osmoregulation in a small desert mammal. NPJ Biofilms Microbiomes 2022, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Wacker, D.W.; Engelmann, M.; Tobin, V.A.; Meddle, S.L.; Ludwig, M. Vasopressin and social odor processing in the olfactory bulb and anterior olfactory nucleus. Ann. N. Y. Acad. Sci. 2011, 1220, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Tobin, V.A.; Hashimoto, H.; Wacker, D.W.; Takayanagi, Y.; Langnaese, K.; Caquineau, C.; Noack, J.; Landgraf, R.; Onaka, T.; Leng, G.; et al. An intrinsic vasopressin system in the olfactory bulb is involved in social recognition. Nature 2010, 464, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Harty, S.; Johnson, K.V.; Moeller, A.H.; Carmody, R.N.; Lehto, S.M.; Erdman, S.E.; Dunbar, R.I.M.; Burnet, P.W.J. The role of the microbiome in the neurobiology of social behaviour. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1131–1166. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tang, X.; Feng, C.; Lin, J.; Zhang, H.; Liu, Q.; Zheng, Q.; Zhuang, H.; Liu, X.; Li, H.; et al. A Systematic Investigation of Complement and Coagulation-Related Protein in Autism Spectrum Disorder Using Multiple Reaction Monitoring Technology. Neurosci. Bull. 2023, in press. [CrossRef]
- Coban, N.; Gokcen, C.; Akbayram, S.; Calisgan, B. Evaluation of Platelet Parameters in Children with Autism Spectrum Disorder: Elongated Collagen-Adenosine Diphosphate and Collagen-Epinephrine Closure Times. Autism Res. 2019, 12, 1069–1076. [Google Scholar] [CrossRef]
- Chandrakumaran, P.; Hews-Girard, J.; Poon, M.C. Desmopressin (DDAVP) use in patients with von Willebrand disease: A single-centre retrospective review of test response and clinical outcomes. Haemophilia 2023, 29, 1095–1103. [Google Scholar] [CrossRef]
- Preijers, T.; Schutte, L.M.; Kruip, M.; Cnossen, M.H.; Leebeek, F.W.G.; van Hest, R.M.; Mathot, R.A.A. Strategies for Individualized Dosing of Clotting Factor Concentrates and Desmopressin in Hemophilia A and B. Ther. Drug Monit. 2019, 41, 192–212. [Google Scholar] [CrossRef]
- Song, T.J.; Lan, X.Y.; Wei, M.P.; Zhai, F.J.; Boeckers, T.M.; Wang, J.N.; Yuan, S.; Jin, M.Y.; Xie, Y.F.; Dang, W.W.; et al. Altered Behaviors and Impaired Synaptic Function in a Novel Rat Model with a Complete Shank3 Deletion. Front. Cell Neurosci. 2019, 13, 111. [Google Scholar] [CrossRef]
- Young, L.J.; Flanagan-Cato, L.M. Editorial comment: Oxytocin, vasopressin and social behavior. Horm. Behav. 2012, 61, 227–229. [Google Scholar] [CrossRef]
- Albers, H.E. The regulation of social recognition, social communication and aggression: Vasopressin in the social behavior neural network. Horm. Behav. 2012, 61, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Baran, N.M.; Peck, S.C.; Kim, T.H.; Goldstein, M.H.; Adkins-Regan, E. Early life manipulations of vasopressin-family peptides alter vocal learning. Proc. Biol. Sci. 2017, 284, 20171114. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Allchorne, A.J.; Zhang, M.; Tsuji, C.; Tobin, V.A.; Pineda, R.; Raftogianni, A.; Stern, J.E.; Grinevich, V.; Leng, G.; et al. Vasopressin casts light on the suprachiasmatic nucleus. J. Physiol. 2017, 595, 3497–3514. [Google Scholar] [CrossRef] [PubMed]
- Hume, C.; Allchorne, A.; Grinevich, V.; Leng, G.; Ludwig, M. Effects of optogenetic stimulation of vasopressinergic retinal afferents on suprachiasmatic neurones. J. Neuroendocrinol. 2019, 31, e12806. [Google Scholar] [CrossRef]
- Penagarikano, O.; Lazaro, M.T.; Lu, X.H.; Gordon, A.; Dong, H.; Lam, H.A.; Peles, E.; Maidment, N.T.; Murphy, N.P.; Yang, X.W.; et al. Exogenous and evoked oxytocin restores social behavior in the Cntnap2 mouse model of autism. Sci. Transl. Med. 2015, 7, 271ra8. [Google Scholar] [CrossRef]
- Borie, A.M.; Theofanopoulou, C.; Andari, E. The promiscuity of the oxytocin-vasopressin systems and their involvement in autism spectrum disorder. Handb. Clin. Neurol. 2021, 182, 121–140. [Google Scholar]
- Tanaka, H.; Nishina, K.; Shou, Q.; Takahashi, H.; Sakagami, M.; Matsuda, T.; Inoue-Murayama, M.; Takagishi, H. Association between arginine vasopressin receptor 1A (AVPR1A) polymorphism and inequity aversion. Proc. Biol. Sci. 2023, 290, 20230378. [Google Scholar] [CrossRef]
- Garbugino, L.; Centofante, E.; D’Amato, F.R. Early Social Enrichment Improves Social Motivation and Skills in a Monogenic Mouse Model of Autism, the Oprm1 (-/-) Mouse. Neural Plast. 2016, 2016, 5346161. [Google Scholar] [CrossRef]
- Tian, H.; Jiao, Y.; Guo, M.; Wang, Y.; Wang, R.; Wang, C.; Chen, X.; Tian, W. Kruppel-like factor 7 deficiency causes autistic-like behavior in mice via regulating Clock gene. Cell Biosci. 2022, 12, 166. [Google Scholar] [CrossRef]
- Martinez-Rodriguez, E.; Martin-Sanchez, A.; Kul, E.; Bose, A.; Martinez-Martinez, F.J.; Stork, O.; Martinez-Garcia, F.; Lanuza, E.; Santos, M.; Agustin-Pavon, C. Male-specific features are reduced in Mecp2-null mice: Analyses of vasopressinergic innervation, pheromone production and social behaviour. Brain Struct. Funct. 2020, 225, 2219–2238. [Google Scholar] [CrossRef]
- Guerra-Torres, X.E. A Case Report of Tuberous Sclerosis and Autosomal Dominant Polycystic Kidney Disease in the Era of Tolvaptan. Curr. Rev. Clin. Exp. Pharmacol. 2023, 18, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Jamain, S.; Radyushkin, K.; Hammerschmidt, K.; Granon, S.; Boretius, S.; Varoqueaux, F.; Ramanantsoa, N.; Gallego, J.; Ronnenberg, A.; Winter, D.; et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. USA 2008, 105, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Wohr, M.; Orduz, D.; Gregory, P.; Moreno, H.; Khan, U.; Vorckel, K.J.; Wolfer, D.P.; Welzl, H.; Gall, D.; Schiffmann, S.N.; et al. Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Transl. Psychiatry 2015, 5, e525. [Google Scholar] [CrossRef] [PubMed]
- Hammock, E.A.; Levitt, P. Modulation of parvalbumin interneuron number by developmentally transient neocortical vasopressin receptor 1a (V1aR). Neuroscience 2012, 222, 20–28. [Google Scholar] [CrossRef]
- Feng, Z.; Ou, Y.; Zhou, M.; Wu, G.; Ma, L.; Zhang, Y.; Liu, Y.; Qi, S. Functional ectopic neural lobe increases GAP-43 expression via PI3K/AKT pathways to alleviate central diabetes insipidus after pituitary stalk lesion in rats. Neurosci. Lett. 2018, 673, 1–6. [Google Scholar] [CrossRef]
- Zaccaria, K.J.; Lagace, D.C.; Eisch, A.J.; McCasland, J.S. Resistance to change and vulnerability to stress: Autistic-like features of GAP43-deficient mice. Genes. Brain Behav. 2010, 9, 985–996. [Google Scholar] [CrossRef]
- Kyzar, E.J.; Pham, M.; Roth, A.; Cachat, J.; Green, J.; Gaikwad, S.; Kalueff, A.V. Alterations in grooming activity and syntax in heterozygous SERT and BDNF knockout mice: The utility of behavior-recognition tools to characterize mutant mouse phenotypes. Brain Res. Bull. 2012, 89, 168–176. [Google Scholar] [CrossRef]
- Veenstra-VanderWeele, J.; Muller, C.L.; Iwamoto, H.; Sauer, J.E.; Owens, W.A.; Shah, C.R.; Cohen, J.; Mannangatti, P.; Jessen, T.; Thompson, B.J.; et al. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc. Natl. Acad. Sci. USA 2012, 109, 5469–5474. [Google Scholar] [CrossRef]
- Pompili, M.; Serafini, G.; Innamorati, M.; Moller-Leimkuhler, A.M.; Giupponi, G.; Girardi, P.; Tatarelli, R.; Lester, D. The hypothalamic-pituitary-adrenal axis and serotonin abnormalities: A selective overview for the implications of suicide prevention. Eur. Arch. Psychiatry Clin. Neurosci. 2010, 260, 583–600. [Google Scholar] [CrossRef]
- Morrison, T.R.; Melloni, R.H., Jr. The role of serotonin, vasopressin, and serotonin/vasopressin interactions in aggressive behavior. Curr. Top. Behav. Neurosci. 2014, 17, 189–228. [Google Scholar]
- Zhou, B.; Zheng, X.; Chen, Y.; Yan, X.; Peng, J.; Liu, Y.; Zhang, Y.; Tang, L.; Wen, M. The Changes of Amygdala Transcriptome in Autism Rat Model After Arginine Vasopressin Treatment. Front. Neurosci. 2022, 16, 838942. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Shang, S.; Su, Y. The arginine vasopressin V1b receptor gene and prosociality: Mediation role of emotional empathy. Psych. J. 2015, 4, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.Y.; Gu, Y.Y.; Li, M.J.; Song, T.J.; Zhai, F.J.; Zhang, Y.; Zhan, J.S.; Bockers, T.M.; Yue, X.N.; Wang, J.N.; et al. Poly(I:C)-induced maternal immune activation causes elevated self-grooming in male rat offspring: Involvement of abnormal postpartum static nursing in dam. Front. Cell Dev. Biol. 2023, 11, 1054381. [Google Scholar] [CrossRef] [PubMed]
- Morais, L.H.; Felice, D.; Golubeva, A.V.; Moloney, G.; Dinan, T.G.; Cryan, J.F. Strain differences in the susceptibility to the gut-brain axis and neurobehavioural alterations induced by maternal immune activation in mice. Behav. Pharmacol. 2018, 29, 181–198. [Google Scholar] [CrossRef]
- Whylings, J.; Rigney, N.; Peters, N.V.; de Vries, G.J.; Petrulis, A. Sexually dimorphic role of BNST vasopressin cells in sickness and social behavior in male and female mice. Brain Behav. Immun. 2020, 83, 68–77. [Google Scholar] [CrossRef]
- Taylor, P.V.; Veenema, A.H.; Paul, M.J.; Bredewold, R.; Isaacs, S.; de Vries, G.J. Sexually dimorphic effects of a prenatal immune challenge on social play and vasopressin expression in juvenile rats. Biol. Sex. Differ. 2012, 3, 15. [Google Scholar] [CrossRef]
- Fetter-Pruneda, I.; Hart, T.; Ulrich, Y.; Gal, A.; Oxley, P.R.; Olivos-Cisneros, L.; Ebert, M.S.; Kazmi, M.A.; Garrison, J.L.; Bargmann, C.I.; et al. An oxytocin/vasopressin-related neuropeptide modulates social foraging behavior in the clonal raider ant. PLoS Biol. 2021, 19, e3001305. [Google Scholar] [CrossRef]
- Gemmer, A.; Mirkes, K.; Anneser, L.; Eilers, T.; Kibat, C.; Mathuru, A.; Ryu, S.; Schuman, E. Oxytocin receptors influence the development and maintenance of social behavior in zebrafish (Danio rerio). Sci. Rep. 2022, 12, 4322. [Google Scholar] [CrossRef]
- Tomaszycki, M.L.; Atchley, D. Pairing Increases Activation of V1aR, but not OTR, in Auditory Regions of Zebra Finches: The Importance of Signal Modality in Nonapeptide-Social Behavior Relationships. Integr. Comp. Biol. 2017, 57, 878–890. [Google Scholar] [CrossRef]
- Albers, H.E.; Pollock, J.; Simmons, W.H.; Ferris, C.F. A V1-like receptor mediates vasopressin-induced flank marking behavior in hamster hypothalamus. J. Neurosci. 1986, 6, 2085–2089. [Google Scholar] [CrossRef]
- Irvin, R.W.; Szot, P.; Dorsa, D.M.; Potegal, M.; Ferris, C.F. Vasopressin in the septal area of the golden hamster controls scent marking and grooming. Physiol. Behav. 1990, 48, 693–699. [Google Scholar] [CrossRef]
- Wang, Z.; Ferris, C.F.; De Vries, G.J. Role of septal vasopressin innervation in paternal behavior in prairie voles (Microtus ochrogaster). Proc. Natl. Acad. Sci. USA 1994, 91, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Tan, O.; Musullulu, H.; Raymond, J.S.; Wilson, B.; Langguth, M.; Bowen, M.T. Oxytocin and vasopressin inhibit hyper-aggressive behaviour in socially isolated mice. Neuropharmacology 2019, 156, 107573. [Google Scholar] [CrossRef] [PubMed]
- Friebe, A.; Douglas, A.J.; Solano, E.; Blois, S.M.; Hagen, E.; Klapp, B.F.; Clark, D.A.; Arck, P.C. Neutralization of LPS or blockage of TLR4 signaling prevents stress-triggered fetal loss in murine pregnancy. J. Mol. Med. 2011, 89, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Bosch, O.J.; Neumann, I.D. Vasopressin released within the central amygdala promotes maternal aggression. Eur. J. Neurosci. 2010, 31, 883–891. [Google Scholar] [CrossRef]
- Torok, B.; Csikota, P.; Fodor, A.; Balazsfi, D.; Ferenczi, S.; Demeter, K.; Toth, Z.E.; Konczol, K.; Perna, J.C.; Farkas, I.; et al. Rescue of Vasopressin Synthesis in Magnocellular Neurons of the Supraoptic Nucleus Normalises Acute Stress-Induced Adrenocorticotropin Secretion and Unmasks an Effect on Social Behaviour in Male Vasopressin-Deficient Brattleboro Rats. Int. J. Mol. Sci. 2022, 23, 1357. [Google Scholar] [CrossRef]
- Zelena, D.; Demeter, K.; Haller, J.; Balazsfi, D. Considerations for the use of virally delivered genetic tools for in-vivo circuit analysis and behavior in mutant mice: A practical guide to optogenetics. Behav. Pharmacol. 2017, 28, 598–609. [Google Scholar] [CrossRef]
- Csikota, P.; Fodor, A.; Balazsfi, D.; Pinter, O.; Mizukami, H.; Weger, S.; Heilbronn, R.; Engelmann, M.; Zelena, D. Vasopressinergic control of stress-related behavior: Studies in Brattleboro rats. Stress 2016, 19, 349–361. [Google Scholar] [CrossRef]
- Sipos, E.; Torok, B.; Barna, I.; Engelmann, M.; Zelena, D. Vasopressin and post-traumatic stress disorder. Stress 2020, 23, 732–745. [Google Scholar] [CrossRef]
- Zelena, D.; Pinter, O.; Balazsfi, D.G.; Langnaese, K.; Richter, K.; Landgraf, R.; Makara, G.B.; Engelmann, M. Vasopressin signaling at brain level controls stress hormone release: The vasopressin-deficient Brattleboro rat as a model. Amino Acids 2015, 47, 2245–2253. [Google Scholar] [CrossRef]
- Feifel, D.; Mexal, S.; Melendez, G.; Liu, P.Y.; Goldenberg, J.R.; Shilling, P.D. The brattleboro rat displays a natural deficit in social discrimination that is restored by clozapine and a neurotensin analog. Neuropsychopharmacology 2009, 34, 2011–2018. [Google Scholar] [CrossRef] [PubMed]
- Torok, B.; Fazekas, C.L.; Szabo, A.; Zelena, D. Epigenetic Modulation of Vasopressin Expression in Health and Disease. Int. J. Mol. Sci. 2021, 22, 9415. [Google Scholar] [CrossRef] [PubMed]
- Torok, B.; Fodor, A.; Klausz, B.; Varga, J.; Zelena, D. Ameliorating schizophrenia-like symptoms in vasopressin deficient male Brattleboro rat by chronic antipsychotic treatment. Eur. J. Pharmacol. 2021, 909, 174383. [Google Scholar] [CrossRef] [PubMed]
- Varga, J.; Fodor, A.; Klausz, B.; Zelena, D. Anxiogenic role of vasopressin during the early postnatal period: Maternal separation-induced ultrasound vocalization in vasopressin-deficient Brattleboro rats. Amino Acids 2015, 47, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Scattoni, M.L.; McFarlane, H.G.; Zhodzishsky, V.; Caldwell, H.K.; Young, W.S.; Ricceri, L.; Crawley, J.N. Reduced ultrasonic vocalizations in vasopressin 1b knockout mice. Behav. Brain Res. 2008, 187, 371–378. [Google Scholar] [CrossRef]
- Torok, B.; Fodor, A.; Zsebok, S.; Sipos, E.; Zelena, D. The Effect of Vasopressin Antagonists on Maternal-Separation-Induced Ultrasonic Vocalization and Stress-Hormone Level Increase during the Early Postnatal Period. Brain Sci. 2021, 11, 444. [Google Scholar] [CrossRef]
- Fodor, A.; Klausz, B.; Pinter, O.; Daviu, N.; Rabasa, C.; Rotllant, D.; Balazsfi, D.; Kovacs, K.B.; Nadal, R.; Zelena, D. Maternal neglect with reduced depressive-like behavior and blunted c-fos activation in Brattleboro mothers, the role of central vasopressin. Horm. Behav. 2012, 62, 539–551. [Google Scholar] [CrossRef]
- Levy, G.; Oppenheim, D.; Koren-Karie, N.; Ariav-Paraira, I.; Gal, N.; Yirmiya, N. Disrupted maternal communication and attachment disorganization in children with autism spectrum disorder. Attach. Hum. Dev. 2020, 22, 568–581. [Google Scholar] [CrossRef]
- Avinun, R.; Ebstein, R.P.; Knafo, A. Human maternal behaviour is associated with arginine vasopressin receptor 1A gene. Biol. Lett. 2012, 8, 894–896. [Google Scholar] [CrossRef]
- Wu, J.; Dai, Y.C.; Lan, X.Y.; Zhang, H.F.; Bai, S.Z.; Hu, Y.; Han, S.P.; Han, J.S.; Zhang, R. Postnatal AVP treatments prevent social deficit in adolescence of valproic acid-induced rat autism model. Peptides 2021, 137, 170493. [Google Scholar] [CrossRef]
- Freeman, A.R.; Hare, J.F.; Anderson, W.G.; Caldwell, H.K. Effects of arginine vasopressin on Richardson’s ground squirrel social and vocal behavior. Behav. Neurosci. 2018, 132, 34–50. [Google Scholar] [CrossRef] [PubMed]
- Dobolyi, A.; Kekesi, K.A.; Juhasz, G.; Szekely, A.D.; Lovas, G.; Kovacs, Z. Receptors of peptides as therapeutic targets in epilepsy research. Curr. Med. Chem. 2014, 21, 764–787. [Google Scholar] [CrossRef] [PubMed]
- Madlon-Kay, S.; Montague, M.J.; Brent, L.J.N.; Ellis, S.; Zhong, B.; Snyder-Mackler, N.; Horvath, J.E.; Skene, J.H.P.; Platt, M.L. Weak effects of common genetic variation in oxytocin and vasopressin receptor genes on rhesus macaque social behavior. Am. J. Primatol. 2018, 80, e22873. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.H.; Walton, J.C.; McCann, K.E.; Norvelle, A.; Liu, Q.; Vander Velden, J.W.; Borland, J.M.; Hart, M.; Jin, C.; Huhman, K.L.; et al. CRISPR-Cas9 editing of the arginine-vasopressin V1a receptor produces paradoxical changes in social behavior in Syrian hamsters. Proc. Natl. Acad. Sci. USA 2022, 119, e2121037119. [Google Scholar] [CrossRef] [PubMed]
- Bayerl, D.S.; Kaczmarek, V.; Jurek, B.; van den Burg, E.H.; Neumann, I.D.; Gassner, B.M.; Klampfl, S.M.; Bosch, O.J. Antagonism of V1b receptors promotes maternal motivation to retrieve pups in the MPOA and impairs pup-directed behavior during maternal defense in the mpBNST of lactating rats. Horm. Behav. 2016, 79, 18–27. [Google Scholar] [CrossRef]
- Caldwell, H.K.; Aulino, E.A.; Rodriguez, K.M.; Witchey, S.K.; Yaw, A.M. Social Context, Stress, Neuropsychiatric Disorders, and the Vasopressin 1b Receptor. Front. Neurosci. 2017, 11, 567. [Google Scholar] [CrossRef]
- Cataldo, I.; Azhari, A.; Esposito, G. A Review of Oxytocin and Arginine-Vasopressin Receptors and Their Modulation of Autism Spectrum Disorder. Front. Mol. Neurosci. 2018, 11, 27. [Google Scholar] [CrossRef]
- Tansey, K.E.; Hill, M.J.; Cochrane, L.E.; Gill, M.; Anney, R.J.; Gallagher, L. Functionality of promoter microsatellites of arginine vasopressin receptor 1A (AVPR1A): Implications for autism. Mol. Autism 2011, 2, 3. [Google Scholar] [CrossRef]
- Yang, S.Y.; Cho, S.C.; Yoo, H.J.; Cho, I.H.; Park, M.; Yoe, J.; Kim, S.A. Family-based association study of microsatellites in the 5′ flanking region of AVPR1A with autism spectrum disorder in the Korean population. Psychiatry Res. 2010, 178, 199–201. [Google Scholar] [CrossRef]
- Meisenberg, G. Short-term behavioral effects of posterior pituitary peptides in mice. Peptides 1981, 2, 1–8. [Google Scholar] [CrossRef]
- Meisenberg, G. Short-term behavioural effects of neurohypophyseal hormones: Pharmacological characteristics. Neuropharmacology 1982, 21, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Meisenberg, G.; Simmons, W.H. Behavioral effects of intracerebroventricularly administered neurohypophyseal hormone analogs in mice. Pharmacol. Biochem. Behav. 1982, 16, 819–825. [Google Scholar] [CrossRef]
- Boakes, R.J.; Ednie, J.M.; Edwardson, J.A.; Keith, A.B.; Sahgal, A.; Wright, C. Abnormal behavioural changes associated with vasopressin-induced barrel rotations. Brain Res. 1985, 326, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Van Wimersma Greidanus, T.B.; Kroodsma, J.M.; Pot, M.L.; Stevens, M.; Maigret, C. Neurohypophyseal hormones and excessive grooming behaviour. Eur. J. Pharmacol. 1990, 187, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cormier, H.C.; Della-Maggiore, V.; Karatsoreos, I.N.; Koletar, M.M.; Ralph, M.R. Suprachiasmatic vasopressin and the circadian regulation of voluntary locomotor behavior. Eur. J. Neurosci. 2015, 41, 79–88. [Google Scholar] [CrossRef]
- Mlynarik, M.; Zelena, D.; Bagdy, G.; Makara, G.B.; Jezova, D. Signs of attenuated depression-like behavior in vasopressin deficient Brattleboro rats. Horm. Behav. 2007, 51, 395–405. [Google Scholar] [CrossRef]
- Fodor, A.; Kovacs, K.B.; Balazsfi, D.; Klausz, B.; Pinter, O.; Demeter, K.; Daviu, N.; Rabasa, C.; Rotllant, D.; Nadal, R.; et al. Depressive- and anxiety-like behaviors and stress-related neuronal activation in vasopressin-deficient female Brattleboro rats. Physiol. Behav. 2016, 158, 100–111. [Google Scholar] [CrossRef]
- Winslow, J.; Insel, T.R. Vasopressin modulates male squirrel monkeys′ behavior during social separation. Eur. J. Pharmacol. 1991, 200, 95–101. [Google Scholar] [CrossRef]
- Caldwell, J.D.; Drago, F.; Prange, A.J., Jr.; Pedersen, C.A. A comparison of grooming behavior potencies of neurohypophyseal nonapeptides. Regul. Pept. 1986, 14, 261–271. [Google Scholar] [CrossRef]
- Gaffori, O.; de Wied, D. Further evidence for a dissociation of peripheral and central effects of vasopressin. Psychoneuroendocrinology 1985, 10, 439–444. [Google Scholar] [CrossRef]
- Tendis, S.; Klingberg, F.; Schaeker, W. Arginine-vasopressin and lysine-vasopressin have different effects on spontaneous behaviour of rats. Biomed. Biochim. Acta 1987, 46, 719–725. [Google Scholar] [PubMed]
- Taylor, G.T.; Lerch, S.; Chourbaji, S. Marble burying as compulsive behaviors in male and female mice. Acta Neurobiol. Exp. 2017, 77, 254–260. [Google Scholar] [CrossRef]
- Egashira, N.; Tanoue, A.; Matsuda, T.; Koushi, E.; Harada, S.; Takano, Y.; Tsujimoto, G.; Mishima, K.; Iwasaki, K.; Fujiwara, M. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav. Brain Res. 2007, 178, 123–127. [Google Scholar] [CrossRef]
- Balazsfi, D.; Pinter, O.; Klausz, B.; Kovacs, K.B.; Fodor, A.; Torok, B.; Engelmann, M.; Zelena, D. Restoration of peripheral V2 receptor vasopressin signaling fails to correct behavioral changes in Brattleboro rats. Psychoneuroendocrinology 2015, 51, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Schatz, K.C.; Kyne, R.F.; Parmeter, S.L.; Paul, M.J. Investigation of social, affective, and locomotor behavior of adolescent Brattleboro rats reveals a link between vasopressin’s actions on arousal and social behavior. Horm. Behav. 2018, 106, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Assuah, F.B.; Emanuel, B.; Lacasse, B.M.; Beggs, J.; Lou, J.; Motta, F.C.; Nemzer, L.R.; Worth, R.; Cravens, G.D. A Literature Review of Similarities Between and Among Patients with Autism Spectrum Disorder and Epilepsy. Cureus 2023, 15, e33946. [Google Scholar] [CrossRef]
- Brang, D.; Ramachandran, V.S. Olfactory bulb dysgenesis, mirror neuron system dysfunction, and autonomic dysregulation as the neural basis for autism. Med. Hypotheses 2010, 74, 919–921. [Google Scholar] [CrossRef]
- Croiset, G.; De Wied, D. Proconvulsive effect of vasopressin; mediation by a putative V2 receptor subtype in the central nervous system. Brain Res. 1997, 759, 18–23. [Google Scholar] [CrossRef]
- Clynen, E.; Swijsen, A.; Raijmakers, M.; Hoogland, G.; Rigo, J.M. Neuropeptides as targets for the development of anticonvulsant drugs. Mol. Neurobiol. 2014, 50, 626–646. [Google Scholar] [CrossRef]
- Wurpel, J.N.; Dundore, R.L.; Barbella, Y.R.; Balaban, C.D.; Keil, L.C.; Severs, W.B. Barrel rotation evoked by intracerebroventricular vasopressin injections in conscious rats. II. Visual/vestibular interactions and efficacy of antiseizure drugs. Brain Res. 1986, 365, 30–41. [Google Scholar] [CrossRef]
- Krause, K.H.; Rascher, W.; Berlit, P. Plasma arginine vasopressin concentrations in epileptics under monotherapy. J. Neurol. 1983, 230, 193–196. [Google Scholar] [CrossRef]
- Javadian, N.; Rahimi, N.; Javadi-Paydar, M.; Doustimotlagh, A.H.; Dehpour, A.R. The modulatory effect of nitric oxide in pro- and anti-convulsive effects of vasopressin in PTZ-induced seizures threshold in mice. Epilepsy Res. 2016, 126, 134–140. [Google Scholar] [CrossRef]
- Fehr, F.S.; Stern, J.A. Peripheral physiological variables and emotion: The James-Lange theory revisited. Psychol. Bull. 1970, 74, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Villar de Araujo, T.; Ruesch, A.; Bankwitz, A.; Rufer, M.; Kleim, B.; Olbrich, S. Autism spectrum disorders in adults and the autonomic nervous system: Heart rate variability markers in the diagnostic procedure. J. Psychiatr. Res. 2023, 164, 235–242. [Google Scholar] [CrossRef]
- Chiu, H.T.; Ip, I.N.; Ching, F.N.Y.; Wong, B.P.; Lui, W.H.; Tse, C.S.; Wong, S.W.H. Resting Heart Rate Variability and Emotion Dysregulation in Adolescents with Autism Spectrum Disorder. J. Autism Dev. Disord. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- de Vries, L.M.; Amelynck, S.; Nystrom, P.; van Esch, L.; Van Lierde, T.; Warreyn, P.; Roeyers, H.; Noens, I.; Naulaers, G.; Boets, B.; et al. Investigating the development of the autonomic nervous system in infancy through pupillometry. J. Neural Transm. 2023, 130, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Szczepanska-Sadowska, E. The Heart as a Target of Vasopressin and Other Cardiovascular Peptides in Health and Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 14414. [Google Scholar] [CrossRef]
- Goldstein, D.S. Stress and the “extended” autonomic system. Auton. Neurosci. 2021, 236, 102889. [Google Scholar] [CrossRef]
- Barbier, A.; Chen, J.H.; Huizinga, J.D. Autism Spectrum Disorder in Children Is Not Associated with Abnormal Autonomic Nervous System Function: Hypothesis and Theory. Front. Psychiatry 2022, 13, 830234. [Google Scholar] [CrossRef]
- Schlader, Z.J.; Vargas, N.T. Regulation of Body Temperature by Autonomic and Behavioral Thermoeffectors. Exerc. Sport. Sci. Rev. 2019, 47, 116–126. [Google Scholar] [CrossRef]
- Yuan, H.L.; Lai, C.Y.Y.; Wong, M.N.K.; Kwong, T.C.; Choy, Y.S.; Mung, S.W.Y.; Chan, C.C.H. Interventions for Sensory Over-Responsivity in Individuals with Autism Spectrum Disorder: A Narrative Review. Children 2022, 9, 1584. [Google Scholar] [CrossRef] [PubMed]
- Curran, L.K.; Newschaffer, C.J.; Lee, L.C.; Crawford, S.O.; Johnston, M.V.; Zimmerman, A.W. Behaviors associated with fever in children with autism spectrum disorders. Pediatrics 2007, 120, e1386–e1392. [Google Scholar] [CrossRef] [PubMed]
- Good, P. Simplifying study of fever’s dramatic relief of autistic behavior. Clin. Nutr. ESPEN 2017, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kasting, N.W.; Carr, D.B.; Martin, J.B.; Blume, H.; Bergland, R. Changes in cerebrospinal fluid and plasma vasopressin in the febrile sheep. Can. J. Physiol. Pharmacol. 1983, 61, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Sharples, P.M.; Seckl, J.R.; Human, D.; Lightman, S.L.; Dunger, D.B. Plasma and cerebrospinal fluid arginine vasopressin in patients with and without fever. Arch. Dis. Child. 1992, 67, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Richmond, C.A. The role of arginine vasopressin in thermoregulation during fever. J. Neurosci. Nurs. 2003, 35, 281–286. [Google Scholar] [CrossRef]
- Zelena, D. Vasopressin in health and disease with a focus on affective disorders. Cent. Nerv. Syst. Agents Med. Chem. 2012, 12, 286–303. [Google Scholar] [CrossRef]
- Cooper, K.E.; Blahser, S.; Malkinson, T.J.; Merker, G.; Roth, J.; Zeisberger, E. Changes in body temperature and vasopressin content of brain neurons, in pregnant and non-pregnant guinea pigs, during fevers produced by Poly I:Poly C. Pflug. Arch. 1988, 412, 292–296. [Google Scholar] [CrossRef]
- Milton, N.G.; Hillhouse, E.W.; Milton, A.S. Does endogenous peripheral arginine vasopressin have a role in the febrile responses of conscious rabbits? J. Physiol. 1993, 469, 525–534. [Google Scholar] [CrossRef]
- Marinovic-Curin, J.; Marinovic-Terzic, I.; Bujas-Petkovic, Z.; Zekan, L.; Skrabic, V.; Dogas, Z.; Terzic, J. Slower cortisol response during ACTH stimulation test in autistic children. Eur. Child. Adolesc. Psychiatry 2008, 17, 39–43. [Google Scholar] [CrossRef]
- Corbett, B.A.; Simon, D. Adolescence, Stress and Cortisol in Autism Spectrum Disorders. OA Autism 2014, 1, 2. [Google Scholar] [PubMed]
- Jacobson, L. Hypothalamic-pituitary-adrenocortical axis: Neuropsychiatric aspects. Compr. Physiol. 2014, 4, 715–738. [Google Scholar] [PubMed]
- Hamza, R.T.; Hewedi, D.H.; Ismail, M.A. Basal and adrenocorticotropic hormone stimulated plasma cortisol levels among Egyptian autistic children: Relation to disease severity. Ital. J. Pediatr. 2010, 36, 71. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Abdeen, A.; Ibrahim, S.F.; Mani, V.; Iqbal, M.S.; Bhatia, S.; et al. Exploring the role of neuropeptides in depression and anxiety. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 114, 110478. [Google Scholar] [CrossRef]
- Landgraf, R.; Kessler, M.S.; Bunck, M.; Murgatroyd, C.; Spengler, D.; Zimbelmann, M.; Nussbaumer, M.; Czibere, L.; Turck, C.W.; Singewald, N.; et al. Candidate genes of anxiety-related behavior in HAB/LAB rats and mice: Focus on vasopressin and glyoxalase-I. Neurosci. Biobehav. Rev. 2007, 31, 89–102. [Google Scholar] [CrossRef]
- Bielsky, I.F.; Hu, S.B.; Szegda, K.L.; Westphal, H.; Young, L.J. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology 2004, 29, 483–493. [Google Scholar] [CrossRef]
- Bleickardt, C.J.; Mullins, D.E.; Macsweeney, C.P.; Werner, B.J.; Pond, A.J.; Guzzi, M.F.; Martin, F.D.; Varty, G.B.; Hodgson, R.A. Characterization of the V1a antagonist, JNJ-17308616, in rodent models of anxiety-like behavior. Psychopharmacology 2009, 202, 711–718. [Google Scholar] [CrossRef]
- Lago, T.R.; Brownstein, M.J.; Page, E.; Beydler, E.; Manbeck, A.; Beale, A.; Roberts, C.; Balderston, N.; Damiano, E.; Pineles, S.L.; et al. The novel vasopressin receptor (V1aR) antagonist SRX246 reduces anxiety in an experimental model in humans: A randomized proof-of-concept study. Psychopharmacology 2021, 238, 2393–2403. [Google Scholar] [CrossRef]
- Simon, N.G.; Guillon, C.; Fabio, K.; Heindel, N.D.; Lu, S.F.; Miller, M.; Ferris, C.F.; Brownstein, M.J.; Garripa, C.; Koppel, G.A. Vasopressin antagonists as anxiolytics and antidepressants: Recent developments. Recent. Pat. CNS Drug Discov. 2008, 3, 77–93. [Google Scholar] [CrossRef]
- Griebel, G.; Beeske, S.; Stahl, S.M. The vasopressin V(1b) receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: Results from 4 randomized, double-blind, placebo-controlled studies. J. Clin. Psychiatry 2012, 73, 1403–1411. [Google Scholar] [CrossRef]
- Griebel, G.; Holsboer, F. Neuropeptide receptor ligands as drugs for psychiatric diseases: The end of the beginning? Nat. Rev. Drug Discov. 2012, 11, 462–478. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.S.; Aman, M.G.; Arnold, L.E. Neurochemical correlates of autistic disorder: A review of the literature. Res. Dev. Disabil. 2006, 27, 254–289. [Google Scholar] [CrossRef] [PubMed]
- Rodnyy, A.Y.; Kondaurova, E.M.; Tsybko, A.S.; Popova, N.K.; Kudlay, D.A.; Naumenko, V.S. The brain serotonin system in autism. Rev. Neurosci. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.F.; Delville, Y. Vasopressin and serotonin interactions in the control of agonistic behavior. Psychoneuroendocrinology 1994, 19, 593–601. [Google Scholar] [CrossRef]
- Landry, M.; Frasier, M.; Chen, Z.; Van De Kar, L.D.; Zhang, Y.; Garcia, F.; Battaglia, G. Fluoxetine treatment of prepubescent rats produces a selective functional reduction in the 5-HT2A receptor-mediated stimulation of oxytocin. Synapse 2005, 58, 102–109. [Google Scholar] [CrossRef]
- Ferris, C.F.; Stolberg, T.; Delville, Y. Serotonin regulation of aggressive behavior in male golden hamsters (Mesocricetus auratus). Behav. Neurosci. 1999, 113, 804–815. [Google Scholar] [CrossRef]
- Couturier, J.L.; Speechley, K.N.; Steele, M.; Norman, R.; Stringer, B.; Nicolson, R. Parental perception of sleep problems in children of normal intelligence with pervasive developmental disorders: Prevalence, severity, and pattern. J. Am. Acad. Child. Adolesc. Psychiatry 2005, 44, 815–822. [Google Scholar] [CrossRef]
- Krakowiak, P.; Goodlin-Jones, B.; Hertz-Picciotto, I.; Croen, L.A.; Hansen, R.L. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population-based study. J. Sleep. Res. 2008, 17, 197–206. [Google Scholar] [CrossRef]
- Delahaye, J.; Kovacs, E.; Sikora, D.; Hall, T.A.; Orlich, F.; Clemons, T.E.; van der Weerd, E.; Glick, L.; Kuhlthau, K. The relationship between Health-Related Quality of Life and sleep problems in children with Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2014, 8, 292–303. [Google Scholar] [CrossRef]
- Hoshino, K. Problems in the Development of the Sleep-Wake Rhythm Influence Neurodevelopmental Disorders in Children. Diagnostics 2023, 13, 1859. [Google Scholar] [CrossRef]
- Reghunandanan, V. Vasopressin in circadian function of SCN. J. Biosci. 2020, 45, 1–11. [Google Scholar] [CrossRef]
- Dell’Osso, L.; Massoni, L.; Battaglini, S.; Cremone, I.M.; Carmassi, C.; Carpita, B. Biological correlates of altered circadian rhythms, autonomic functions and sleep problems in autism spectrum disorder. Ann. Gen. Psychiatry 2022, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Barassin, S.; Kalsbeek, A.; Saboureau, M.; Bothorel, B.; Vivien-Roels, B.; Malan, A.; Buijs, R.M.; Pevet, P. Potentiation effect of vasopressin on melatonin secretion as determined by trans-pineal microdialysis in the Rat. J. Neuroendocrinol. 2000, 12, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Catrina, S.B.; Rotarus, R.; Wivall, I.L.; Coculescu, M.; Brismar, K. The influence of vasopressin deficiency and acute desmopressin administration on melatonin secretion in patients with central diabetes insipidus. J. Endocrinol. Investig. 2004, 27, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Failla, M.D.; Gerdes, M.B.; Williams, Z.J.; Moore, D.J.; Cascio, C.J. Increased pain sensitivity and pain-related anxiety in individuals with autism. Pain. Rep. 2020, 5, e861. [Google Scholar] [CrossRef]
- Zheng, H.; Lim, J.Y.; Kim, Y.; Jung, S.T.; Hwang, S.W. The role of oxytocin, vasopressin, and their receptors at nociceptors in peripheral pain modulation. Front. Neuroendocrinol. 2021, 63, 100942. [Google Scholar] [CrossRef]
- Yang, F.J.; Ma, L.; Yang, J.; Zhu, Z.L.; Wang, C.H. Intranasal Vasopressin Relieves Orthopedic Pain After Surgery. Pain. Manag. Nurs. 2019, 20, 126–132. [Google Scholar] [CrossRef]
- Schorscher-Petcu, A.; Sotocinal, S.; Ciura, S.; Dupre, A.; Ritchie, J.; Sorge, R.E.; Crawley, J.N.; Hu, S.B.; Nishimori, K.; Young, L.J.; et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J. Neurosci. 2010, 30, 8274–8284. [Google Scholar] [CrossRef]
- Legrain, V.; Iannetti, G.D.; Plaghki, L.; Mouraux, A. The pain matrix reloaded: A salience detection system for the body. Prog. Neurobiol. 2011, 93, 111–124. [Google Scholar] [CrossRef]
- Yang, J.; Song, C.Y.; Liu, W.Y.; Lin, B.C. Only through the brain nuclei, arginine vasopressin regulates antinociception in the rat. Peptides 2006, 27, 3341–3346. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.M.; Liu, W.Y.; Song, C.Y.; Lin, B.C. Through V2, not V1 receptor relating to endogenous opiate peptides, arginine vasopressin in periaqueductal gray regulates antinociception in the rat. Regul. Pept. 2006, 137, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Oztan, O.; Garner, J.P.; Partap, S.; Sherr, E.H.; Hardan, A.Y.; Farmer, C.; Thurm, A.; Swedo, S.E.; Parker, K.J. Cerebrospinal fluid vasopressin and symptom severity in children with autism. Ann. Neurol. 2018, 84, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Newman, S.W. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann. N. Y. Acad. Sci. 1999, 877, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, H.K.; Albers, H.E. Oxytocin, Vasopressin, and the Motivational Forces that Drive Social Behaviors. Curr. Top. Behav. Neurosci. 2016, 27, 51–103. [Google Scholar] [PubMed]
- Bamshad, M.; Albers, H.E. Neural circuitry controlling vasopressin-stimulated scent marking in Syrian hamsters (Mesocricetus auratus). J. Comp. Neurol. 1996, 369, 252–263. [Google Scholar] [CrossRef]
- Keller, D.; Láng, T.; Cservenák, M.; Puska, G.; Barna, J.; Csillag, V.; Farkas, I.; Zelena, D.; Dóra, F.; Barteczko, L.; et al. A thalamo-preoptic pathway promoting social touch. bioRxiv 2022. [Google Scholar] [CrossRef]
- Bosch, O.J.; Pfortsch, J.; Beiderbeck, D.I.; Landgraf, R.; Neumann, I.D. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J. Neuroendocrinol. 2010, 22, 420–429. [Google Scholar] [CrossRef]
- Bielsky, I.F.; Hu, S.B.; Ren, X.; Terwilliger, E.F.; Young, L.J. The V1a vasopressin receptor is necessary and sufficient for normal social recognition: A gene replacement study. Neuron 2005, 47, 503–513. [Google Scholar] [CrossRef]
- Gabor, C.S.; Phan, A.; Clipperton-Allen, A.E.; Kavaliers, M.; Choleris, E. Interplay of oxytocin, vasopressin, and sex hormones in the regulation of social recognition. Behav. Neurosci. 2012, 126, 97–109. [Google Scholar] [CrossRef]
- Liu, Y.; Curtis, J.T.; Wang, Z. Vasopressin in the lateral septum regulates pair bond formation in male prairie voles (Microtus ochrogaster). Behav. Neurosci. 2001, 115, 910–919. [Google Scholar] [CrossRef]
- Wang, Z.; Young, L.J.; De Vries, G.J.; Insel, T.R. Voles and vasopressin: A review of molecular, cellular, and behavioral studies of pair bonding and paternal behaviors. Prog. Brain Res. 1998, 119, 483–499. [Google Scholar] [PubMed]
- Insel, T.R.; Gelhard, R.; Shapiro, L.E. The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice. Neuroscience 1991, 43, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Borie, A.M.; Dromard, Y.; Guillon, G.; Olma, A.; Manning, M.; Muscatelli, F.; Desarmenien, M.G.; Jeanneteau, F. Correction of vasopressin deficit in the lateral septum ameliorates social deficits of mouse autism model. J. Clin. Investig. 2021, 131, e144450. [Google Scholar] [CrossRef] [PubMed]
- Rilling, J.K.; Li, T.; Chen, X.; Gautam, P.; Haroon, E.; Thompson, R.R. Arginine Vasopressin Effects on Subjective Judgments and Neural Responses to Same and Other-Sex Faces in Men and Women. Front. Endocrinol. 2017, 8, 200. [Google Scholar] [CrossRef]
- Rood, B.D.; De Vries, G.J. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord. J. Comp. Neurol. 2011, 519, 2434–2474. [Google Scholar] [CrossRef]
- Li, Y.; Mathis, A.; Grewe, B.F.; Osterhout, J.A.; Ahanonu, B.; Schnitzer, M.J.; Murthy, V.N.; Dulac, C. Neuronal Representation of Social Information in the Medial Amygdala of Awake Behaving Mice. Cell 2017, 171, 1176–1190 e17. [Google Scholar] [CrossRef]
- Zink, C.F.; Stein, J.L.; Kempf, L.; Hakimi, S.; Meyer-Lindenberg, A. Vasopressin modulates medial prefrontal cortex-amygdala circuitry during emotion processing in humans. J. Neurosci. 2010, 30, 7017–7022. [Google Scholar] [CrossRef]
- Shou, X.J.; Xu, X.J.; Zeng, X.Z.; Liu, Y.; Yuan, H.S.; Xing, Y.; Jia, M.X.; Wei, Q.Y.; Han, S.P.; Zhang, R.; et al. A Volumetric and Functional Connectivity MRI Study of Brain Arginine-Vasopressin Pathways in Autistic Children. Neurosci. Bull. 2017, 33, 130–142. [Google Scholar] [CrossRef]
- Avino, T.A.; Barger, N.; Vargas, M.V.; Carlson, E.L.; Amaral, D.G.; Bauman, M.D.; Schumann, C.M. Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc. Natl. Acad. Sci. USA 2018, 115, 3710–3715. [Google Scholar] [CrossRef]
- Walker, D.L.; Davis, M. Role of the extended amygdala in short-duration versus sustained fear: A tribute to Dr. Lennart Heimer. Brain Struct. Funct. 2008, 213, 29–42. [Google Scholar] [CrossRef]
- Paul, M.J.; Terranova, J.I.; Probst, C.K.; Murray, E.K.; Ismail, N.I.; de Vries, G.J. Sexually dimorphic role for vasopressin in the development of social play. Front. Behav. Neurosci. 2014, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.N.; Clauss, J.A.; Blackford, J.U. The Human BNST: Functional Role in Anxiety and Addiction. Neuropsychopharmacology 2016, 41, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Bruzsik, B.; Biro, L.; Sarosdi, K.R.; Zelena, D.; Sipos, E.; Szebik, H.; Torok, B.; Mikics, E.; Toth, M. Neurochemically distinct populations of the bed nucleus of stria terminalis modulate innate fear response to weak threat evoked by predator odor stimuli. Neurobiol. Stress 2021, 15, 100415. [Google Scholar] [CrossRef]
- Clauss, J.A.; Avery, S.N.; Benningfield, M.M.; Blackford, J.U. Social anxiety is associated with BNST response to unpredictability. Depress. Anxiety 2019, 36, 666–675. [Google Scholar] [CrossRef]
- Murray, E.K.; Varnum, M.M.; Fernandez, J.L.; de Vries, G.J.; Forger, N.G. Effects of neonatal treatment with valproic acid on vasopressin immunoreactivity and olfactory behaviour in mice. J. Neuroendocrinol. 2011, 23, 906–914. [Google Scholar] [CrossRef][Green Version]
- Mohapatra, A.N.; Wagner, S. The role of the prefrontal cortex in social interactions of animal models and the implications for autism spectrum disorder. Front. Psychiatry 2023, 14, 1205199. [Google Scholar] [CrossRef] [PubMed]
- Sailer, L.; Duclot, F.; Wang, Z.; Kabbaj, M. Consequences of prenatal exposure to valproic acid in the socially monogamous prairie voles. Sci. Rep. 2019, 9, 2453. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Yan, X.; Yang, L.; Zheng, X.; Chen, Y.; Liu, Y.; Ren, Y.; Peng, J.; Zhang, Y.; Huang, J.; et al. Effects of arginine vasopressin on the transcriptome of prefrontal cortex in autistic rat model. J. Cell. Mol. Med. 2022, 26, 5493–5505. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; Koob, G.F.; Bluthe, R.M.; Le Moal, M. Septal vasopressin modulates social memory in male rats. Brain Res. 1988, 457, 143–147. [Google Scholar] [CrossRef]
- de Vries, G.J.; Buijs, R.M.; Swaab, D.F. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain--presence of a sex difference in the lateral septum. Brain Res. 1981, 218, 67–78. [Google Scholar] [CrossRef]
- Engelmann, M.; Landgraf, R. Microdialysis administration of vasopressin into the septum improves social recognition in Brattleboro rats. Physiol. Behav. 1994, 55, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Veenema, A.H.; Beiderbeck, D.I.; Lukas, M.; Neumann, I.D. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm. Behav. 2010, 58, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Compaan, J.C.; Buijs, R.M.; Pool, C.W.; De Ruiter, A.J.; Koolhaas, J.M. Differential lateral septal vasopressin innervation in aggressive and nonaggressive male mice. Brain Res. Bull. 1993, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Curley, J.P.; Jensen, C.L.; Franks, B.; Champagne, F.A. Variation in maternal and anxiety-like behavior associated with discrete patterns of oxytocin and vasopressin 1a receptor density in the lateral septum. Horm. Behav. 2012, 61, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Ferris, C.F.; Albers, H.E.; Wesolowski, S.M.; Goldman, B.D.; Luman, S.E. Vasopressin injected into the hypothalamus triggers a stereotypic behavior in golden hamsters. Science 1984, 224, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, A.C.; Whitman, D.C.; Albers, H.E. Microinjection of arginine-vasopressin into the periaqueductal gray stimulates flank marking in Syrian hamsters (Mesocricetus auratus). Brain Res. 1992, 569, 136–140. [Google Scholar] [CrossRef]
- Islam, M.T.; Maejima, T.; Matsui, A.; Mieda, M. Paraventricular hypothalamic vasopressin neurons induce self-grooming in mice. Mol. Brain 2022, 15, 47. [Google Scholar] [CrossRef]
- Verevkina, S.V. Some mechanisms of action of vasopressin on animal behavior. Neurosci. Behav. Physiol. 1988, 18, 306–310. [Google Scholar] [CrossRef]
- Elkabir, D.R.; Wyatt, M.E.; Vellucci, S.V.; Herbert, J. The effects of separate or combined infusions of corticotrophin-releasing factor and vasopressin either intraventricularly or into the amygdala on aggressive and investigative behaviour in the rat. Regul. Pept. 1990, 28, 199–214. [Google Scholar] [CrossRef]
- Willcox, B.J.; Poulin, P.; Veale, W.L.; Pittman, Q.J. Vasopressin-induced motor effects: Localization of a sensitive site in the amygdala. Brain Res. 1992, 596, 58–64. [Google Scholar] [CrossRef]
- Maiti, A.; Shahid Salles, K.; Grassi, S.; Abood, L.G. Barrel rotation and prostration by vasopressin and nicotine in the vestibular cerebellum. Pharmacol. Biochem. Behav. 1986, 25, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.H.; Aldinger, K.A.; Ashwood, P.; Bauman, M.L.; Blaha, C.D.; Blatt, G.J.; Chauhan, A.; Chauhan, V.; Dager, S.R.; Dickson, P.E.; et al. Consensus paper: Pathological role of the cerebellum in autism. Cerebellum 2012, 11, 777–807. [Google Scholar] [CrossRef] [PubMed]
- Pierce, K.; Courchesne, E. Evidence for a cerebellar role in reduced exploration and stereotyped behavior in autism. Biol. Psychiatry 2001, 49, 655–664. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, A.M.; Stoodley, C.J. Cerebro-cerebellar circuits in autism spectrum disorder. Front. Neurosci. 2015, 9, 408. [Google Scholar] [CrossRef] [PubMed]
- Reith, R.M.; McKenna, J.; Wu, H.; Hashmi, S.S.; Cho, S.H.; Dash, P.K.; Gambello, M.J. Loss of Tsc2 in Purkinje cells is associated with autistic-like behavior in a mouse model of tuberous sclerosis complex. Neurobiol. Dis. 2013, 51, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, S.; Van ‘t Veer, A.E.; Witteman, J.; Meijer, W.M.; Van, I.M.H.; Bakermans-Kranenburg, M.J. Effects of vasopressin on neural processing of infant crying in expectant fathers. Horm. Behav. 2018, 103, 19–27. [Google Scholar] [CrossRef]
- Zhang, L.; Hernandez, V.S. Synaptic innervation to rat hippocampus by vasopressin-immuno-positive fibres from the hypothalamic supraoptic and paraventricular nuclei. Neuroscience 2013, 228, 139–162. [Google Scholar] [CrossRef]
- Rood, B.D.; Beck, S.G. Vasopressin indirectly excites dorsal raphe serotonin neurons through activation of the vasopressin1A receptor. Neuroscience 2014, 260, 205–216. [Google Scholar] [CrossRef]
- Keck, M.E.; Welt, T.; Muller, M.B.; Uhr, M.; Ohl, F.; Wigger, A.; Toschi, N.; Holsboer, F.; Landgraf, R. Reduction of hypothalamic vasopressinergic hyperdrive contributes to clinically relevant behavioral and neuroendocrine effects of chronic paroxetine treatment in a psychopathological rat model. Neuropsychopharmacology 2003, 28, 235–243. [Google Scholar] [CrossRef]
- Bao, A.M.; Swaab, D.F. Corticotropin-releasing hormone and arginine vasopressin in depression focus on the human postmortem hypothalamus. Vitam. Horm. 2010, 82, 339–365. [Google Scholar]
- Wang, S.S.; Kamphuis, W.; Huitinga, I.; Zhou, J.N.; Swaab, D.F. Gene expression analysis in the human hypothalamus in depression by laser microdissection and real-time PCR: The presence of multiple receptor imbalances. Mol. Psychiatry 2008, 13, 786–799, 741. [Google Scholar] [CrossRef]
- Grzeda, E.; Ziarniak, K.; Sliwowska, J.H. The paraventricular nucleus of the hypothalamus—The concertmaster of autonomic control. Focus on blood pressure regulation. Acta Neurobiol. Exp. 2023, 83, 34–44. [Google Scholar] [CrossRef]
- Devnani, P.A.; Hegde, A.U. Autism and sleep disorders. J. Pediatr. Neurosci. 2015, 10, 304–307. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, S.; de Zavalia, N.; Belforte, N.; Amir, S. In utero Exposure to Valproic-Acid Alters Circadian Organisation and Clock-Gene Expression: Implications for Autism Spectrum Disorders. Front. Behav. Neurosci. 2021, 15, 711549. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Fliers, E.; Hofman, M.A.; Swaab, D.F.; Buijs, R.M. Vasopressin and the output of the hypothalamic biological clock. J. Neuroendocrinol. 2010, 22, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.D.; Burton, K.J.; Zhang, C.; Hu, S.B.; Zhou, Q.Y. Vasopressin receptor V1a regulates circadian rhythms of locomotor activity and expression of clock-controlled genes in the suprachiasmatic nuclei. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R824–R830. [Google Scholar] [CrossRef]
- Song, C.K.; Bartness, T.J.; Petersen, S.L.; Bittman, E.L. Co-expression of melatonin (MEL1a) receptor and arginine vasopressin mRNAs in the Siberian hamster suprachiasmatic nucleus. J. Neuroendocrinol. 2000, 12, 627–634. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Rikkers, M.; Vivien-Roels, B.; Pevet, P. Vasopressin and vasoactive intestinal peptide infused in the paraventricular nucleus of the hypothalamus elevate plasma melatonin levels. J. Pineal Res. 1993, 15, 46–52. [Google Scholar] [CrossRef]
- Tordjman, S.; Anderson, G.M.; Bellissant, E.; Botbol, M.; Charbuy, H.; Camus, F.; Graignic, R.; Kermarrec, S.; Fougerou, C.; Cohen, D.; et al. Day and nighttime excretion of 6-sulphatoxymelatonin in adolescents and young adults with autistic disorder. Psychoneuroendocrinology 2012, 37, 1990–1997. [Google Scholar] [CrossRef]
- Christensen, D.L.; Baio, J.; Van Naarden Braun, K.; Bilder, D.; Charles, J.; Constantino, J.N.; Daniels, J.; Durkin, M.S.; Fitzgerald, R.T.; Kurzius-Spencer, M.; et al. Prevention, Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years--Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2012. MMWR Surveill. Summ. 2016, 65, 1–23. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Lombardo, M.V.; Auyeung, B.; Ashwin, E.; Chakrabarti, B.; Knickmeyer, R. Why are autism spectrum conditions more prevalent in males? PLoS Biol. 2011, 9, e1001081. [Google Scholar] [CrossRef] [PubMed]
- Dworzynski, K.; Ronald, A.; Bolton, P.; Happe, F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J. Am. Acad. Child. Adolesc. Psychiatry 2012, 51, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.; Jacquemont, S. The effects of sex on prevalence and mechanisms underlying neurodevelopmental disorders. Handb. Clin. Neurol. 2020, 173, 327–339. [Google Scholar] [PubMed]
- Aulino, E.A.; Caldwell, H.K. Subtle sex differences in vasopressin mRNA expression in the embryonic mouse brain. J. Neuroendocrinol. 2020, 32, e12835. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S. Sex differences in oxytocin and vasopressin: Implications for autism spectrum disorders? Behav. Brain Res. 2007, 176, 170–186. [Google Scholar] [CrossRef]
- van Leeuwen, F.W.; Caffe, A.R.; De Vries, G.J. Vasopressin cells in the bed nucleus of the stria terminalis of the rat: Sex differences and the influence of androgens. Brain Res. 1985, 325, 391–394. [Google Scholar] [CrossRef]
- DiBenedictis, B.T.; Nussbaum, E.R.; Cheung, H.K.; Veenema, A.H. Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J. Comp. Neurol. 2017, 525, 2549–2570. [Google Scholar] [CrossRef]
- Veenema, A.H.; Bredewold, R.; De Vries, G.J. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm. Behav. 2012, 61, 50–56. [Google Scholar] [CrossRef]
- Walsh, M.J.M.; Wallace, G.L.; Gallegos, S.M.; Braden, B.B. Brain-based sex differences in autism spectrum disorder across the lifespan: A systematic review of structural MRI, fMRI, and DTI findings. Neuroimage Clin. 2021, 31, 102719. [Google Scholar] [CrossRef]
- Smith, R.E.W.; Avery, J.A.; Wallace, G.L.; Kenworthy, L.; Gotts, S.J.; Martin, A. Sex Differences in Resting-State Functional Connectivity of the Cerebellum in Autism Spectrum Disorder. Front. Hum. Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef]
- Dong, H.W.; Swanson, L.W. Projections from bed nuclei of the stria terminalis, anteromedial area: Cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J. Comp. Neurol. 2006, 494, 142–178. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.F.; Chung, W.C.; Kruijver, F.P.; Hofman, M.A.; Hestiantoro, A. Sex differences in the hypothalamus in the different stages of human life. Neurobiol. Aging 2003, 24 (Suppl. S1), S1–S16; discussion S17–S19. [Google Scholar] [CrossRef] [PubMed]
- De Vries, G.J.; Best, W.; Sluiter, A.A. The influence of androgens on the development of a sex difference in the vasopressinergic innervation of the rat lateral septum. Brain Res. 1983, 284, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Auger, C.J.; Vanzo, R.J. Progesterone treatment of adult male rats suppresses arginine vasopressin expression in the bed nucleus of the stria terminalis and the centromedial amygdala. J. Neuroendocrinol. 2006, 18, 187–194. [Google Scholar] [CrossRef]
- Miller, M.; Bales, K.L.; Taylor, S.L.; Yoon, J.; Hostetler, C.M.; Carter, C.S.; Solomon, M. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: Sex differences and associations with symptoms. Autism Res. 2013, 6, 91–102. [Google Scholar] [CrossRef]
- Khan, A.; Brodhead, A.E.; Schwartz, K.A.; Kolts, R.L.; Brown, W.A. Sex differences in antidepressant response in recent antidepressant clinical trials. J. Clin. Psychopharmacol. 2005, 25, 318–324. [Google Scholar] [CrossRef]
- Winslow, J.T.; Hastings, N.; Carter, C.S.; Harbaugh, C.R.; Insel, T.R. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 1993, 365, 545–548. [Google Scholar] [CrossRef]
- Insel, T.R.; Hulihan, T.J. A gender-specific mechanism for pair bonding: Oxytocin and partner preference formation in monogamous voles. Behav. Neurosci. 1995, 109, 782–789. [Google Scholar] [CrossRef]
- Cho, M.M.; DeVries, A.C.; Williams, J.R.; Carter, C.S. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behav. Neurosci. 1999, 113, 1071–1079. [Google Scholar] [CrossRef]
- Jacob, D. Types of Medication for Autism. 2022. Available online: https://www.rxlist.com/types_of_medication_for_autism/drugs-condition.htm (accessed on 1 August 2023).
- Semple, B.D.; Raghupathi, R. A Pro-social Pill? The Potential of Pharmacological Treatments to Improve Social Outcomes After Pediatric Traumatic Brain Injury. Front. Neurol. 2021, 12, 714253. [Google Scholar] [CrossRef]
- Yeung, P.P.; Johnson, K.A.; Riesenberg, R.; Orejudos, A.; Riccobene, T.; Kalluri, H.V.; Malik, P.R.; Varughese, S.; Findling, R.L. Cariprazine in Pediatric Patients with Autism Spectrum Disorder: Results of a Pharmacokinetic, Safety and Tolerability Study. J. Child. Adolesc. Psychopharmacol. 2023, 33, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Appenrodt, E.; Bojanowska, E.; Janus, J.; Stempniak, B.; Guzek, J.W.; Schwarzberg, H. Effects of methylphenidate on oxytocin and vasopressin levels in pinealectomized rats during light-dark cycle. Pharmacol. Biochem. Behav. 1997, 58, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Appenrodt, E.; Schwarzberg, H. Methylphenidate-induced motor activity in rats: Modulation by melatonin and vasopressin. Pharmacol. Biochem. Behav. 2003, 75, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Kawasaki, H.; Takasaki, K. Intracerebroventricular treatment with vasopressin V1-receptor antagonist inhibits centrally-mediated pressor response to clonidine in conscious rats. Jpn. J. Pharmacol. 1993, 63, 447–453. [Google Scholar] [CrossRef]
- Brown, G.M.; Mazurek, M.; Allen, D.; Szechtman, B.; Cleghorn, J.M. Dose-response profiles of plasma growth hormone and vasopressin after clonidine challenge in man. Psychiatry Res. 1990, 31, 311–320. [Google Scholar] [CrossRef]
- Alexander, S.L.; Irvine, C.H. The effect of the alpha-2-adrenergic agonist, clonidine, on secretion patterns and rates of adrenocorticotropic hormone and its secretagogues in the horse. J. Neuroendocrinol. 2000, 12, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Bailey, A.R.; Clarke, G.; Wakerley, J.B. Inhibition of supraoptic vasopressin neurones following systemic clonidine. Neuropharmacology 1994, 33, 211–214. [Google Scholar] [CrossRef]
- Pujol, C.N.; Pellissier, L.P.; Clement, C.; Becker, J.A.J.; Le Merrer, J. Back-translating behavioral intervention for autism spectrum disorders to mice with blunted reward restores social abilities. Transl. Psychiatry 2018, 8, 197. [Google Scholar] [CrossRef]
- Zhang, R.; Jia, M.X.; Zhang, J.S.; Xu, X.J.; Shou, X.J.; Zhang, X.T.; Li, L.; Li, N.; Han, S.P.; Han, J.S. Transcutaneous electrical acupoint stimulation in children with autism and its impact on plasma levels of arginine-vasopressin and oxytocin: A prospective single-blinded controlled study. Res. Dev. Disabil. 2012, 33, 1136–1146. [Google Scholar] [CrossRef]
- Arnold, L.E.; Luna, R.A.; Williams, K.; Chan, J.; Parker, R.A.; Wu, Q.; Hollway, J.A.; Jeffs, A.; Lu, F.; Coury, D.L.; et al. Probiotics for Gastrointestinal Symptoms and Quality of Life in Autism: A Placebo-Controlled Pilot Trial. J. Child. Adolesc. Psychopharmacol. 2019, 29, 659–669. [Google Scholar] [CrossRef]
- Donovan, M.; Mackey, C.S.; Lynch, M.D.J.; Platt, G.N.; Brown, A.N.; Washburn, B.K.; Trickey, D.J.; Curtis, J.T.; Liu, Y.; Charles, T.C.; et al. Limosilactobacillus reuteri administration alters the gut-brain-behavior axis in a sex-dependent manner in socially monogamous prairie voles. Front. Microbiol. 2023, 14, 1015666. [Google Scholar] [CrossRef] [PubMed]
- Felix-Ortiz, A.C.; Febo, M. Gestational valproate alters BOLD activation in response to complex social and primary sensory stimuli. PLoS ONE 2012, 7, e37313. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.H.; Intorre, A.A.; French, J.A. Vasopressin and Oxytocin Reduce Food Sharing Behavior in Male, but Not Female Marmosets in Family Groups. Front. Endocrinol. 2017, 8, 181. [Google Scholar] [CrossRef] [PubMed]
- Simmons, T.C.; Balland, J.F.; Dhauna, J.; Yang, S.Y.; Traina, J.L.; Vazquez, J.; Bales, K.L. Early Intranasal Vasopressin Administration Impairs Partner Preference in Adult Male Prairie Voles (Microtus ochrogaster). Front. Endocrinol. 2017, 8, 145. [Google Scholar] [CrossRef]
- Jarcho, M.R.; Mendoza, S.P.; Mason, W.A.; Yang, X.; Bales, K.L. Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes. Brain Behav. 2011, 10, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.J.; Oztan, O.; Libove, R.A.; Mohsin, N.; Karhson, D.S.; Sumiyoshi, R.D.; Summers, J.E.; Hinman, K.E.; Motonaga, K.S.; Phillips, J.M.; et al. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci. Transl. Med. 2019, 11, eaau7356. [Google Scholar] [CrossRef] [PubMed]
- Tabak, B.A.; Teed, A.R.; Castle, E.; Dutcher, J.M.; Meyer, M.L.; Bryan, R.; Irwin, M.R.; Lieberman, M.D.; Eisenberger, N.I. Null results of oxytocin and vasopressin administration across a range of social cognitive and behavioral paradigms: Evidence from a randomized controlled trial. Psychoneuroendocrinology 2019, 107, 124–132. [Google Scholar] [CrossRef]
- Ludwig, M.; Tobin, V.A.; Callahan, M.F.; Papadaki, E.; Becker, A.; Engelmann, M.; Leng, G. Intranasal application of vasopressin fails to elicit changes in brain immediate early gene expression, neural activity and behavioural performance of rats. J. Neuroendocrinol. 2013, 25, 655–667. [Google Scholar] [CrossRef]
- Zhuang, Q.; Zheng, X.; Becker, B.; Lei, W.; Xu, X.; Kendrick, K.M. Intranasal vasopressin like oxytocin increases social attention by influencing top-down control, but additionally enhances bottom-up control. Psychoneuroendocrinology 2021, 133, 105412. [Google Scholar] [CrossRef]
- Price, D.; Burris, D.; Cloutier, A.; Thompson, C.B.; Rilling, J.K.; Thompson, R.R. Dose-Dependent and Lasting Influences of Intranasal Vasopressin on Face Processing in Men. Front. Endocrinol. 2017, 8, 220. [Google Scholar] [CrossRef]
- Wu, X.; Xu, P.; Luo, Y.J.; Feng, C. Differential Effects of Intranasal Vasopressin on the Processing of Adult and Infant Cues: An ERP Study. Front. Hum. Neurosci. 2018, 12, 329. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Coccaro, E.F.; Cremers, H.; McCarron, R.; Lu, S.F.; Brownstein, M.J.; Simon, N.G. A novel V1a receptor antagonist blocks vasopressin-induced changes in the CNS response to emotional stimuli: An fMRI study. Front. Syst. Neurosci. 2013, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Brunnlieb, C.; Munte, T.F.; Tempelmann, C.; Heldmann, M. Vasopressin modulates neural responses related to emotional stimuli in the right amygdala. Brain Res. 2013, 1499, 29–42. [Google Scholar] [CrossRef]
- Silver, J.M.; Anderson, K. Vasopressin treats the persistent feeling of coldness after brain injury. J. Neuropsychiatry Clin. Neurosci. 1999, 11, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Pitman, R.K.; Orr, S.P.; Lasko, N.B. Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1993, 48, 107–117. [Google Scholar] [CrossRef]
- Perras, B.; Pannenborg, H.; Marshall, L.; Pietrowsky, R.; Born, J.; Lorenz Fehm, H. Beneficial treatment of age-related sleep disturbances with prolonged intranasal vasopressin. J. Clin. Psychopharmacol. 1999, 19, 28–36. [Google Scholar] [CrossRef]
- Perras, B.; Droste, C.; Born, J.; Fehm, H.L.; Pietrowsky, R. Verbal memory after three months of intranasal vasopressin in healthy old humans. Psychoneuroendocrinology 1997, 22, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Baska, F.; Bozo, E.; Patocs, T. Vasopressin receptor antagonists: A patent summary (2018–2022). Expert. Opin. Ther. Pat. 2023, 33, 385–395. [Google Scholar] [CrossRef]
- Umbricht, D.; Del Valle Rubido, M.; Hollander, E.; McCracken, J.T.; Shic, F.; Scahill, L.; Noeldeke, J.; Boak, L.; Khwaja, O.; Squassante, L.; et al. A Single Dose, Randomized, Controlled Proof-of-Mechanism Study of a Novel Vasopressin 1a Receptor Antagonist (RG7713) in High-Functioning Adults with Autism Spectrum Disorder. Neuropsychopharmacology 2017, 42, 1914–1923. [Google Scholar] [CrossRef]
- Bolognani, F.; Rubido, M.d.V.; Squassante, L.; Wandel, C.; Derks, M.; Murtagh, L.; Sevigny, J.; Khwaja, O.; Umbricht, D.; Fontoura, P. A phase 2 clinical trial of a vasopressin V1a receptor antagonist shows improved adaptive behaviors in men with autism spectrum disorder. Sci. Transl. Med. 2019, 11, eaat7838. [Google Scholar] [CrossRef]
- Schnider, P.; Bissantz, C.; Bruns, A.; Dolente, C.; Goetschi, E.; Jakob-Roetne, R.; Kunnecke, B.; Mueggler, T.; Muster, W.; Parrott, N.; et al. Discovery of Balovaptan, a Vasopressin 1a Receptor Antagonist for the Treatment of Autism Spectrum Disorder. J. Med. Chem. 2020, 63, 1511–1525. [Google Scholar] [CrossRef] [PubMed]
- Hollander, E.; Jacob, S.; Jou, R.; McNamara, N.; Sikich, L.; Tobe, R.; Smith, J.; Sanders, K.; Squassante, L.; Murtagh, L.; et al. Balovaptan vs. Placebo for Social Communication in Childhood Autism Spectrum Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2022, 79, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Veenstra-VanderWeele, J.; Murphy, D.; McCracken, J.; Smith, J.; Sanders, K.; Meyenberg, C.; Wiese, T.; Deol-Bhullar, G.; Wandel, C.; et al. Efficacy and safety of balovaptan for socialisation and communication difficulties in autistic adults in North America and Europe: A phase 3, randomised, placebo-controlled trial. Lancet Psychiatry 2022, 9, 199–210. [Google Scholar] [CrossRef]
- Ding, Y.; Wei, Z.; Yan, H.; Guo, W. Efficacy of Treatments Targeting Hypothalamic-Pituitary-Adrenal Systems for Major Depressive Disorder: A Meta-Analysis. Front. Pharmacol. 2021, 12, 732157. [Google Scholar] [CrossRef]
- Chaki, S. Vasopressin V1B Receptor Antagonists as Potential Antidepressants. Int. J. Neuropsychopharmacol. 2021, 24, 450–463. [Google Scholar] [CrossRef]
- Inatani, S.; Mizuno-Yasuhira, A.; Kamiya, M.; Nishino, I.; Sabia, H.D.; Endo, H. Prediction of a clinically effective dose of THY1773, a novel V(1B) receptor antagonist, based on preclinical data. Biopharm. Drug Dispos. 2021, 42, 204–217. [Google Scholar] [CrossRef]
- Kamiya, M.; Sabia, H.D.; Marella, J.; Fava, M.; Nemeroff, C.B.; Umeuchi, H.; Iijima, M.; Chaki, S.; Nishino, I. Efficacy and safety of TS-121, a novel vasopressin V(1B) receptor antagonist, as adjunctive treatment for patients with major depressive disorder: A randomized, double-blind, placebo-controlled study. J. Psychiatr. Res. 2020, 128, 43–51. [Google Scholar] [CrossRef]
- Katz, D.A.; Locke, C.; Greco, N.; Liu, W.; Tracy, K.A. Hypothalamic-pituitary-adrenal axis and depression symptom effects of an arginine vasopressin type 1B receptor antagonist in a one-week randomized Phase 1b trial. Brain Behav. 2017, 7, e00628. [Google Scholar] [CrossRef]
- Watanabe, T.; Abe, O.; Kuwabara, H.; Yahata, N.; Takano, Y.; Iwashiro, N.; Natsubori, T.; Aoki, Y.; Takao, H.; Kawakubo, Y.; et al. Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: A randomized trial. JAMA Psychiatry 2014, 71, 166–175. [Google Scholar] [CrossRef]
- Hu, L.; Du, X.; Jiang, Z.; Song, C.; Liu, D. Oxytocin treatment for core symptoms in children with autism spectrum disorder: A systematic review and meta-analysis. Eur. J. Clin. Pharmacol. 2023, 79, 1357–1363. [Google Scholar] [CrossRef]
- Carson, D.S.; Howerton, C.L.; Garner, J.P.; Hyde, S.A.; Clark, C.L.; Hardan, A.Y.; Penn, A.A.; Parker, K.J. Plasma vasopressin concentrations positively predict cerebrospinal fluid vasopressin concentrations in human neonates. Peptides 2014, 61, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Zelena, D. Adenoviruses for better rain function. Integr. Physiol. 2021, 2, 254–260. [Google Scholar] [CrossRef]
- Chen, X.; Xu, Y.; Li, B.; Wu, X.; Li, T.; Wang, L.; Zhang, Y.; Lin, W.; Qu, C.; Feng, C. Intranasal vasopressin modulates resting state brain activity across multiple neural systems: Evidence from a brain imaging machine learning study. Neuropharmacology 2021, 190, 108561. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.W.; Hadizadeh, M.; Cheong, J.P.G. Global Trends in Physical-Activity Research of Autism: Bibliometric Analysis Based on the Web of Science Database (1980–2021). Int. J. Environ. Res. Public Health 2022, 19, 7278. [Google Scholar] [CrossRef]
- Zuvekas, S.H.; Grosse, S.D.; Lavelle, T.A.; Maenner, M.J.; Dietz, P.; Ji, X. Healthcare Costs of Pediatric Autism Spectrum Disorder in the United States, 2003–2015. J. Autism. Dev. Disord. 2021, 51, 2950–2958. [Google Scholar] [CrossRef]
- Liptak, G.S.; Stuart, T.; Auinger, P. Health care utilization and expenditures for children with autism: Data from U.S. national samples. J. Autism. Dev. Disord. 2006, 36, 871–879. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).