Specific Cellular and Humoral Response after the Third Dose of Anti-SARS-CoV-2 RNA Vaccine in Patients with Immune-Mediated Rheumatic Diseases on Immunosuppressive Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Evaluation of SARS-CoV-2 Humoral Response
2.3. Evaluation of SARS-CoV-2 Cellular Response
2.4. Statistical Analysis
3. Results
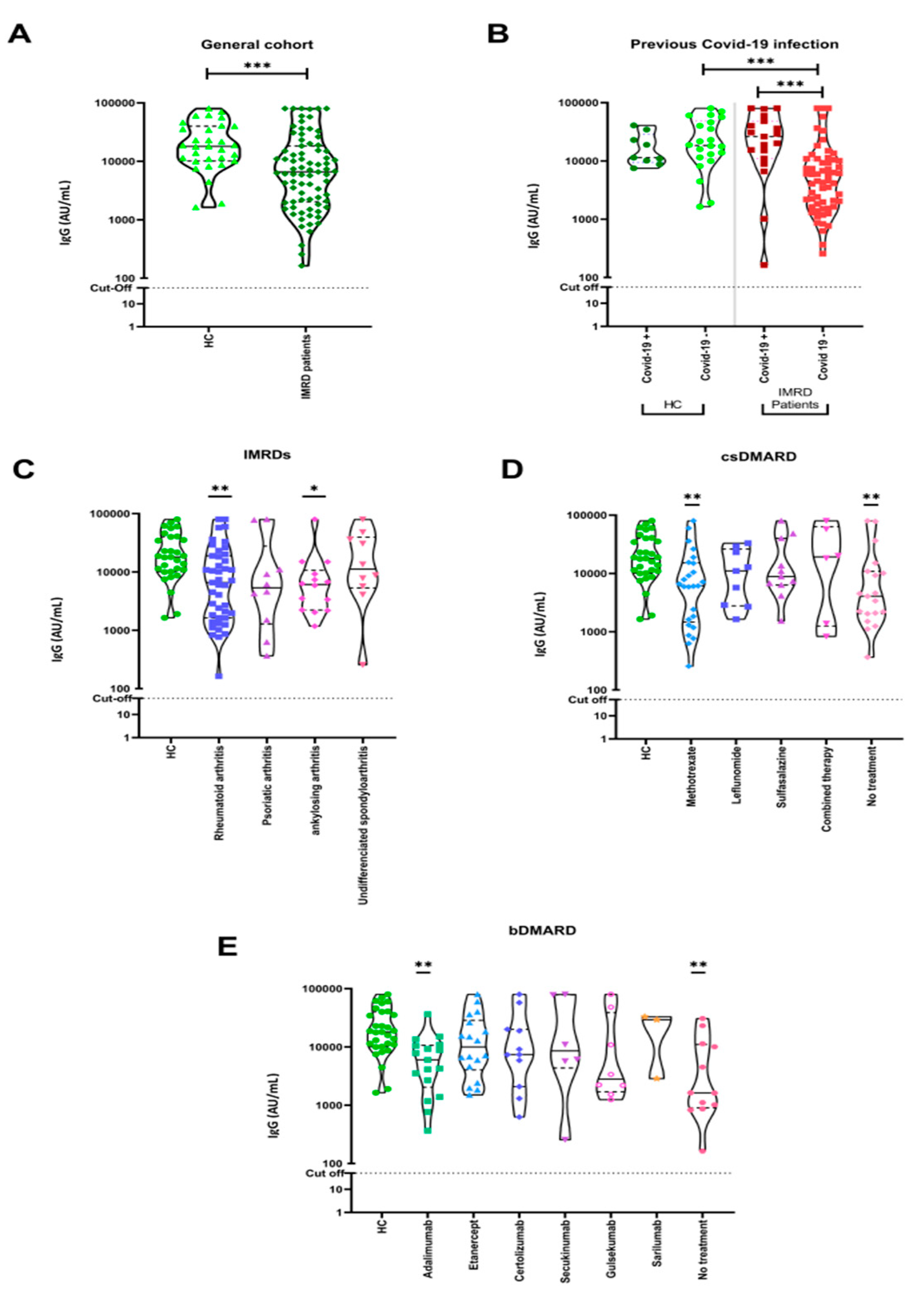
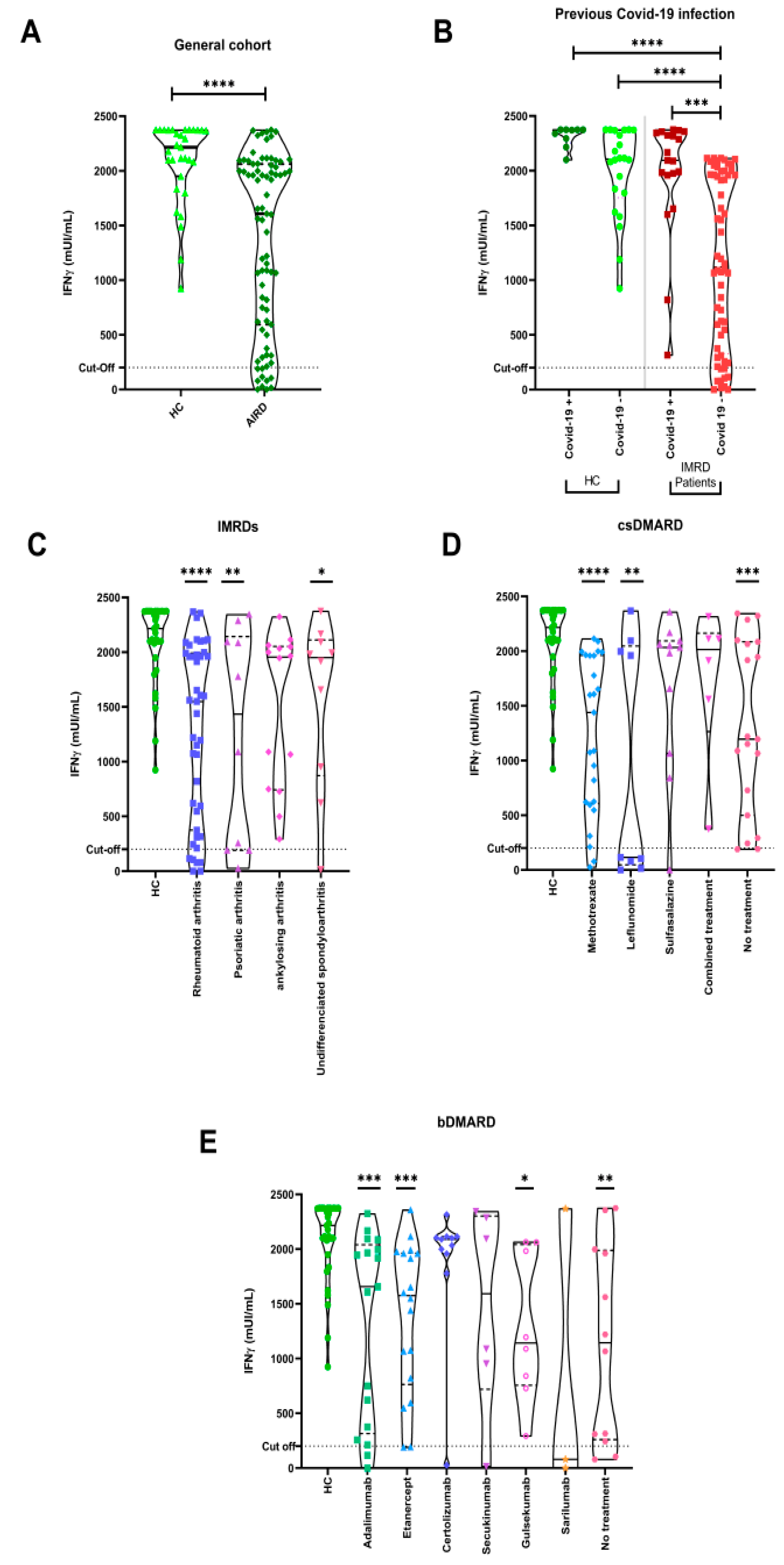
3.1. Epidemiological and Immunological Characteristics of the Study Population
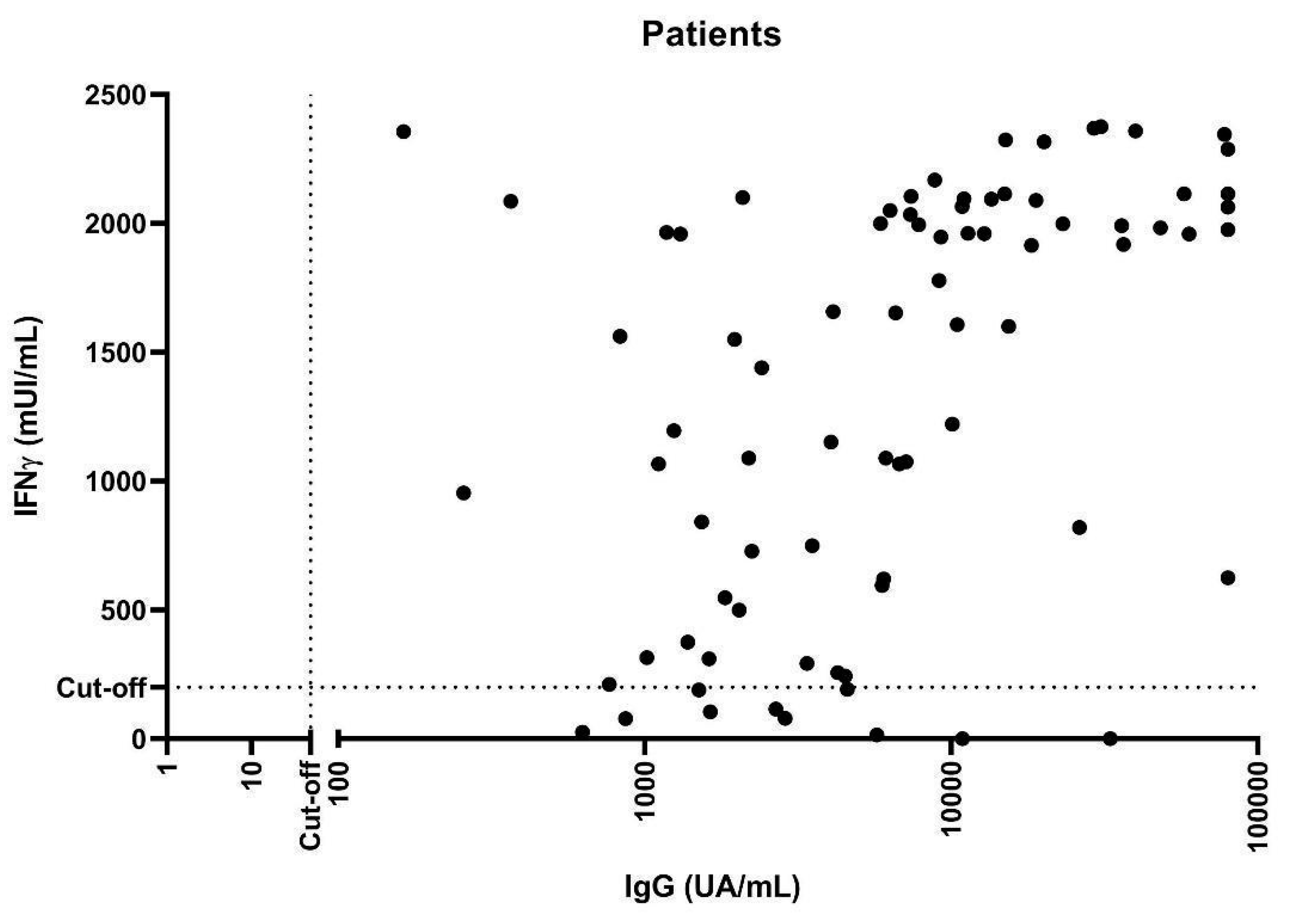
3.2. Humoral Immune Responses to COVID-19 Vaccination
3.3. Cellular Immune Responses to COVID-19 Vaccination
3.4. SARS-CoV-2 Infection Follow-Up
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors Associated with COVID-19-Related Death Using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Rutherford, M.A.; Scott, J.; Karabayas, M.; Antonelou, M.; Gopaluni, S.; Gray, D.; Barrett, J.; Brix, S.R.; Dhaun, N.; McAdoo, S.P.; et al. Risk Factors for Severe Outcomes in Patients with Systemic Vasculitis and COVID-19: A Binational, Registry-Based Cohort Study. Arthritis Rheumatol. 2021, 73, 1713–1719. [Google Scholar] [CrossRef]
- Fernandez-Gutierrez, B.; Leon, L.; Madrid, A.; Rodriguez-Rodriguez, L.; Freites, D.; Font, J.; Mucientes, A.; Culebras, E.; Colome, J.I.; Jover, J.A.; et al. Hospital Admissions in Inflammatory Rheumatic Diseases during the Peak of COVID-19 Pandemic: Incidence and Role of Disease-Modifying Agents. Ther. Adv. Musculoskelet. Dis. 2021, 13, 1759720X20962692. [Google Scholar] [CrossRef]
- Nuñez, D.F.; Leon, L.; Garcia, A.M.; Arce, J.I.C.; Mucientes, A.; Gutierrez-Fernandez, B.; Rodriguez, L.; Cristóbal, I.P.S.; Álvarez, P.; Prada, C.M.; et al. Mortality Related to COVID-19 in Patients with Rheumatic and Musculoskeletal Diseases, First Wave of the Outbreak: A Single-Center Study. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221090296. [Google Scholar] [CrossRef]
- Strangfeld, A.; Schäfer, M.; Gianfrancesco, M.A.; Lawson-Tovey, S.; Liew, J.W.; Ljung, L.; Mateus, E.F.; Richez, C.; Santos, M.J.; Schmajuk, G.; et al. Factors Associated with COVID-19-Related Death in People with Rheumatic Diseases: Results from the COVID-19 Global Rheumatology Alliance Physician-Reported Registry. Ann. Rheum. Dis. 2021, 80, 930–942. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-Dose Administration and the Influence of the Timing of the Booster Dose on Immunogenicity and Efficacy of ChAdOx1 NCoV-19 (AZD1222) Vaccine: A Pooled Analysis of Four Randomised Trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the MRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Logunov, D.Y.; Dolzhikova, I.V.; Shcheblyakov, D.V.; Tukhvatulin, A.I.; Zubkova, O.V.; Dzharullaeva, A.S.; Kovyrshina, A.V.; Lubenets, N.L.; Grousova, D.M.; Erokhova, A.S.; et al. Safety and Efficacy of an RAd26 and RAd5 Vector-Based Heterologous Prime-Boost COVID-19 Vaccine: An Interim Analysis of a Randomised Controlled Phase 3 Trial in Russia. Lancet 2021, 397, 671–681. [Google Scholar] [CrossRef]
- Oren, S.; Mandelboim, M.; Braun-Moscovici, Y.; Paran, D.; Ablin, J.; Litinsky, I.; Comaneshter, D.; Levartovsky, D.; Mendelson, E.; Azar, R.; et al. Vaccination against Influenza in Patients with Rheumatoid Arthritis: The Effect of Rituximab on the Humoral Response. Ann. Rheum. Dis. 2008, 67, 937–941. [Google Scholar] [CrossRef]
- Hua, C.; Barnetche, T.; Combe, B.; Morel, J. Effect of Methotrexate, Anti-Tumor Necrosis Factor α, and Rituximab on the Immune Response to Influenza and Pneumococcal Vaccines in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Arthritis Care Res. 2014, 66, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Arad, U.; Tzadok, S.; Amir, S.; Mandelboim, M.; Mendelson, E.; Wigler, I.; Sarbagil-Maman, H.; Paran, D.; Caspi, D.; Elkayam, O. The Cellular Immune Response to Influenza Vaccination Is Preserved in Rheumatoid Arthritis Patients Treated with Rituximab. Vaccine 2011, 29, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- van Assen, S.; Holvast, A.; Benne, C.A.; Posthumus, M.D.; van Leeuwen, M.A.; Voskuyl, A.E.; Blom, M.; Risselada, A.P.; de Haan, A.; Westra, J.; et al. Humoral Responses after Influenza Vaccination Are Severely Reduced in Patients with Rheumatoid Arthritis Treated with Rituximab. Arthritis Rheum. 2010, 62, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Mauro, D.; Ciancio, A.; Di Vico, C.; Passariello, L.; Rozza, G.; Pasquale, M.D.; Pantano, I.; Cannistrà, C.; Bucci, L.; Scriffignano, S.; et al. Serological Response to BNT162b2 Anti-SARS-CoV-2 Vaccination in Patients with Inflammatory Rheumatic Diseases: Results From the RHEUVAX Cohort. Front. Immunol. 2022, 13, 901055. [Google Scholar] [CrossRef]
- Furer, V.; Eviatar, T.; Zisman, D.; Peleg, H.; Paran, D.; Levartovsky, D.; Zisapel, M.; Elalouf, O.; Kaufman, I.; Meidan, R.; et al. Immunogenicity and Safety of the BNT162b2 MRNA COVID-19 Vaccine in Adult Patients with Autoimmune Inflammatory Rheumatic Diseases and in the General Population: A Multicentre Study. Ann. Rheum. Dis. 2021, 80, 1330–1338. [Google Scholar] [CrossRef]
- Boekel, L.; Steenhuis, M.; Hooijberg, F.; Besten, Y.R.; van Kempen, Z.L.E.; Kummer, L.Y.; van Dam, K.P.J.; Stalman, E.W.; Vogelzang, E.H.; Cristianawati, O.; et al. Antibody Development after COVID-19 Vaccination in Patients with Autoimmune Diseases in the Netherlands: A Substudy of Data from Two Prospective Cohort Studies. Lancet Rheumatol. 2021, 3, e778–e788. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Li, H.J.; Chen, L.; Lin, S.P. Immunogenicity of Inactivated COVID-19 Vaccine in Patients with Autoimmune Inflammatory Rheumatic Diseases. Sci. Rep. 2022, 12, 17955. [Google Scholar] [CrossRef]
- Raptis, C.E.; Berger, C.T.; Ciurea, A.; Andrey, D.O.; Polysopoulos, C.; Lescuyer, P.; Maletic, T.; Riek, M.; Scherer, A.; von Loga, I.; et al. Type of MRNA COVID-19 Vaccine and Immunomodulatory Treatment Influence Humoral Immunogenicity in Patients with Inflammatory Rheumatic Diseases. Front. Immunol. 2022, 13, 1016927. [Google Scholar] [CrossRef]
- Sugihara, K.; Wakiya, R.; Shimada, H.; Kameda, T.; Nakashima, S.; Kato, M.; Miyagi, T.; Mizusaki, M.; Mino, R.; Nomura, Y.; et al. Immunogenicity against the BNT162b2 MRNA COVID-19 Vaccine in Rheumatic Disease Patients Receiving Immunosuppressive Therapy. Intern. Med. 2022, 61, 1953–1958. [Google Scholar] [CrossRef]
- Pri-Paz Basson, Y.; Tayer-Shifman, O.E.; Naser, R.; Tartakover Matalon, S.; Kimhi, O.; Gepstein, R.; Halperin, T.; Ziv-Baran, T.; Ziv, A.; Parikh, R.; et al. Immunogenicity and Safety of the MRNA-Based BNT162b2 Vaccine in Systemic Autoimmune Rheumatic Diseases Patients. Clin. Rheumatol. 2022, 41, 3879–3885. [Google Scholar] [CrossRef]
- Mueller, S.N.; Rouse, B.T. Immune Responses to Viruses. In Clinical Immunology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008; Volume 31, p. 1578. ISBN 0323044042. [Google Scholar]
- Almendro-Vázquez, P.; Laguna-Goya, R.; Paz-Artal, E. Defending against SARS-CoV-2: The T cell perspective. Front. Immunol. 2023, 14, 1107803. [Google Scholar] [CrossRef] [PubMed]
- Kared, H.; Redd, A.D.; Bloch, E.M.; Bonny, T.S.; Sumatoh, H.; Kairi, F.; Carbajo, D.; Abel, B.; Newell, E.W.; Bettinotti, M.P.; et al. SARS-CoV-2-specific CD8+ T cell responses in convalescent COVID-19 individuals. J. Clin. Investig. 2021, 131, e145476. [Google Scholar] [CrossRef] [PubMed]
- McMahan, K.; Yu, J.; Mercado, N.B.; Loos, C.; Tostanoski, L.H.; Chandrashekar, A.; Liu, J.; Peter, L.; Atyeo, C.; Zhu, A.; et al. Correlates of Protection against SARS-CoV-2 in Rhesus Macaques. Nature 2021, 590, 630–634. [Google Scholar] [CrossRef]
- Prendecki, M.; Clarke, C.; Edwards, H.; McIntyre, S.; Mortimer, P.; Gleeson, S.; Martin, P.; Thomson, T.; Randell, P.; Shah, A.; et al. Humoral and T-Cell Responses to SARS-CoV-2 Vaccination in Patients Receiving Immunosuppression. Ann. Rheum. Dis. 2021, 80, 1322–1329. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Aiello, A.; Laganà, B.; Agrati, C.; Castilletti, C.; Meschi, S.; Farroni, C.; Lapa, D.; Najafi Fard, S.; Cuzzi, G.; et al. ImmunosuppressiveTherapies Differently Modulate Humoral- and T-Cell-Specific Responses to COVID-19 MRNA Vaccine in Rheumatoid Arthritis Patients. Front. Immunol. 2021, 12, 740249. [Google Scholar] [CrossRef] [PubMed]
- Izmirly, P.M.; Kim, M.Y.; Samanovic, M.; Fernandez-Ruiz, R.; Ohana, S.; Deonaraine, K.K.; Engel, A.J.; Masson, M.; Xie, X.; Cornelius, A.R.; et al. Evaluation of Immune Response and Disease Status in Systemic Lupus Erythematosus Patients Following SARS-CoV-2 Vaccination. Arthritis Rheumatol. 2022, 74, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Sieiro Santos, C.; Calleja Antolin, S.; Moriano Morales, C.; Garcia Herrero, J.; Diez Alvarez, E.; Ramos Ortega, F.; Ruiz De Morales, J.G. Immune Responses to MRNA Vaccines against SARS-CoV-2 in Patients with Immune-Mediated Inflammatory Rheumatic Diseases. RMD Open 2022, 8, e001898. [Google Scholar] [CrossRef]
- Moor, M.B.; Suter-Riniker, F.; Horn, M.P.; Aeberli, D.; Amsler, J.; Möller, B.; Njue, L.M.; Medri, C.; Angelillo-Scherrer, A.; Borradori, L.; et al. Humoral and Cellular Responses to MRNA Vaccines against SARS-CoV-2 in Patients with a History of CD20 B-Cell-Depleting Therapy (RituxiVac): An Investigator-Initiated, Single-Centre, Open-Label Study. Lancet Rheumatol. 2021, 3, e789–e797. [Google Scholar] [CrossRef] [PubMed]
- Mrak, D.; Simader, E.; Sieghart, D.; Mandl, P.; Radner, H.; Perkmann, T.; Haslacher, H.; Mayer, M.; Koblischke, M.; Hofer, P.; et al. Immunogenicity and Safety of a Fourth COVID-19 Vaccination in Rituximab-Treated Patients: An Open-Label Extension Study. Ann. Rheum. Dis. 2022, 81, 1750–1756. [Google Scholar] [CrossRef]
- Schumacher, F.; Mrdenovic, N.; Scheicht, D.; Pons-Kühnemann, J.; Scheibelhut, C.; Strunk, J. Humoral Immunogenicity of COVID-19 Vaccines in Patients with Inflammatory Rheumatic Diseases under Treatment with Rituximab: A Case-Control Study (COVID-19VacRTX). Rheumatology 2022, 61, 3912–3918. [Google Scholar] [CrossRef]
- Mrak, D.; Tobudic, S.; Koblischke, M.; Graninger, M.; Radner, H.; Sieghart, D.; Hofer, P.; Perkmann, T.; Haslacher, H.; Thalhammer, R.; et al. SARS-CoV-2 Vaccination in Rituximab-Treated Patients: B Cells Promote Humoral Immune Responses in the Presence of T-Cell-Mediated Immunity. Ann. Rheum. Dis. 2021, 80, 1345–1350. [Google Scholar] [CrossRef]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef]
- Sparks, J.A. Rheumatoid Arthritis. Ann. Intern. Med. 2019, 170, ITC1–ITC16. [Google Scholar] [CrossRef]
- Sepriano, A.; Ramiro, S.; FitzGerald, O.; Østergaard, M.; Homik, J.; van der Heijde, D.; Elkayam, O.; Thorne, J.C.; Larché, M.J.; Ferraccioli, G.; et al. Adherence to Treat-to-Target Management in Rheumatoid Arthritis and Associated Factors: Data from the International RA BIODAM Cohort. J. Rheumatol. 2019, 47, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Assawasaksakul, T.; Sathitratanacheewin, S.; Vichaiwattana, P.; Wanlapakorn, N.; Poovorawan, Y.; Avihingsanon, Y.; Assawasaksakul, N.; Kittanamongkolchai, W. Immunogenicity of the Third and Fourth BNT162b2 MRNA COVID-19 Boosters and Factors Associated with Immune Response in Patients with SLE and Rheumatoid Arthritis. Lupus Sci. Med. 2022, 9, e000726. [Google Scholar] [CrossRef] [PubMed]
- Pilz, S.; Theiler-Schwetz, V.; Trummer, C.; Krause, R.; Ioannidis, J.P.A. SARS-CoV-2 Reinfections: Overview of Efficacy and Duration of Natural and Hybrid Immunity. Environ. Res. 2022, 209, 112911. [Google Scholar] [CrossRef]
- Haberman, R.H.; Herati, R.S.; Sedaghat Herati, R.; Simon, D.; Samanovic, M.; Blank, R.B.; Tuen, M.; Koralov, S.B.; Atreya, R.; Tascilar, K.; et al. Methotrexate Hampers Immunogenicity to BNT162b2 MRNA COVID-19 Vaccine in Immune-Mediated Inflammatory Disease (Equal Contribution). medRxiv 2021, 80, 1339–1344. [Google Scholar] [CrossRef]
- Liu, W.; Long, X.; Wan, K.; Yin, M.; Yin, Y.; Zhang, B.; Li, L.; Song, Y. The endogenous factors affecting the detection of serum SARS-CoV-2 IgG/IgM antibodies by ELISA. J. Med. Virol. 2022, 94, 1976–1982. [Google Scholar] [CrossRef]
- Benucci, M.; Damiani, A.; Gobbi, F.L.; Lari, B.; Grossi, V.; Infantino, M.; Manfredi, M. Role of Booster with BNT162b2 MRNA in SARS-CoV-2 Vaccination in Patients with Rheumatoid Arthritis. Immunol. Res. 2022, 70, 493–500. [Google Scholar] [CrossRef]
- Park, J.K.; Lee, Y.J.; Shin, K.; Ha, Y.-J.; Lee, E.Y.; Song, Y.W.; Choi, Y.; Winthrop, K.L.; Lee, E.B. Impact of Temporary Methotrexate Discontinuation for 2 Weeks on Immunogenicity of Seasonal Influenza Vaccination in Patients with Rheumatoid Arthritis: A Randomised Clinical Trial. Ann. Rheum. Dis. 2018, 77, 898–904. [Google Scholar] [CrossRef]
- Bechmann, N.; Barthel, A.; Schedl, A.; Herzig, S.; Varga, Z.; Gebhard, C.; Mayr, M.; Hantel, C.; Beuschlein, F.; Wolfrum, C.; et al. Sexual Dimorphism in COVID-19: Potential Clinical and Public Health Implications. Lancet Diabetes Endocrinol. 2022, 10, 221–230. [Google Scholar] [CrossRef]
- Bienvenu, L.A.; Noonan, J.; Wang, X.; Peter, K. Higher Mortality of COVID-19 in Males: Sex Differences in Immune Response and Cardiovascular Comorbidities. Cardiovasc. Res. 2020, 116, 2197–2206. [Google Scholar] [CrossRef]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K.; Philippot, Q.; Rosain, J.; Béziat, V.; et al. Autoantibodies against Type I IFNs in Patients with Life-Threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef]
- Benitez Fuentes, J.D.; Mohamed Mohamed, K.; de Luna Aguilar, A.; Jiménez García, C.; Guevara-Hoyer, K.; Fernandez-Arquero, M.; Rodríguez de la Peña, M.A.; Garciía Bravo, L.; Jiménez Ortega, A.F.; Flores Navarro, P.; et al. Evidence of Exhausted Lymphocytes after the Third Anti-SARS-CoV-2 Vaccine Dose in Cancer Patients. Front. Oncol. 2022, 12, 975980. [Google Scholar] [CrossRef]
- Frenz, T.; Grabski, E.; Buschjäger, D.; Vaas, L.A.I.; Burgdorf, N.; Schmidt, R.E.; Witte, T.; Kalinke, U. CD4(+) T Cells in Patients with Chronic Inflammatory Rheumatic Disorders Show Distinct Levels of Exhaustion. J. Allergy Clin. Immunol. 2016, 138, 586–589.e10. [Google Scholar] [CrossRef]
- Koerber, N.; Priller, A.; Yazici, S.; Bauer, T.; Cheng, C.-C.; Mijočević, H.; Wintersteller, H.; Jeske, S.; Vogel, E.; Feuerherd, M.; et al. Dynamics of Spike-and Nucleocapsid Specific Immunity during Long-Term Follow-up and Vaccination of SARS-CoV-2 Convalescents. Nat. Commun. 2022, 13, 153. [Google Scholar] [CrossRef]
- Oyaert, M.; De Scheerder, M.A.; Van Herrewege, S.; Laureys, G.; Van Assche, S.; Cambron, M.; Naesens, L.; Hoste, L.; Claes, K.; Haerynck, F.; et al. Evaluation of Humoral and Cellular Responses in SARS-CoV-2 mRNA Vaccinated Immunocompromised Patients. Front. Immunol. 2022, 13, 858399. [Google Scholar] [CrossRef]
- Sattui, S.E.; Liew, J.W.; Kennedy, K.; Sirotich, E.; Putman, M.; Moni, T.T.; Akpabio, A.; Alpízar-Rodríguez, D.; Berenbaum, F.; Bulina, I.; et al. Early Experience of COVID-19 Vaccination in Adults with Systemic Rheumatic Diseases: Results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open 2021, 7, e001814. [Google Scholar] [CrossRef]
- Watad, A.; De Marco, G.; Mahajna, H.; Druyan, A.; Eltity, M.; Hijazi, N.; Haddad, A.; Elias, M.; Zisman, D.; Naffaa, M.E.; et al. Immune-Mediated Disease Flares or New-Onset Disease in 27 Subjects Following MRNA/DNA SARS-CoV-2 Vaccination. Vaccines 2021, 9, 435. [Google Scholar] [CrossRef]
- Geisen, U.M.; Berner, D.K.; Tran, F.; Sümbül, M.; Vullriede, L.; Ciripoi, M.; Reid, H.M.; Schaffarzyk, A.; Longardt, A.C.; Franzenburg, J.; et al. Immunogenicity and Safety of Anti-SARS-CoV-2 MRNA Vaccines in Patients with Chronic Inflammatory Conditions and Immunosuppressive Therapy in a Monocentric Cohort. Ann. Rheum. Dis. 2021, 80, 1306–1311. [Google Scholar] [CrossRef]
- Braun-Moscovici, Y.; Kaplan, M.; Braun, M.; Markovits, D.; Giryes, S.; Toledano, K.; Tavor, Y.; Dolnikov, K.; Balbir-Gurman, A. Disease Activity and Humoral Response in Patients with Inflammatory Rheumatic Diseases after Two Doses of the Pfizer MRNA Vaccine against SARS-CoV-2. Ann. Rheum. Dis. 2021, 80, 1317–1321. [Google Scholar] [CrossRef]
| Healthy Controls (n = 31) | IMRDs Patients (n = 79) | ||
|---|---|---|---|
| Age | Mean ± SD years | 50.9 ± 13.1 | 58.2 ± 11.4 |
| Sex n (%) | Female | 21 (60.8) | 48 (67.7) |
| Male | 10 (39.2) | 31 (32.2) | |
| Diagnosis n (%) | Rheumatoid arthritis | N/A | 43 (54.4) |
| Psoriatic arthritis | N/A | 10 (12.7) | |
| Ankylosing spondylitis | N/A | 14 (17.7) | |
| Undifferentiated spondyloarthritis | N/A | 10 (12.7) | |
| IBD-associated spondyloarthritis | N/A | 2 (2.5) | |
| Medication n (%) | Glucocorticoids | N/A | 32 (40.5) |
| Dose, mean ± SD (mg) | N/A | 5.3 ±1.7 | |
| Conventional synthetic DMARDs | |||
| Methotrexate | N/A | 25 (31.6) | |
| Sulfasalazine | N/A | 11 (13.9) | |
| Leflunomide | N/A | 9 (11.4) | |
| Mycophenolate | N/A | 1 (1.3) | |
| Hydroxychloroquine | N/A | 2 (2.5) | |
| Biologicals DMARDs | |||
| Etanercept | N/A | 18 (22.8) | |
| Adalimumab | N/A | 17 (21.5) | |
| Certolizumab | N/A | 11 (13.9) | |
| Gulsekumab | N/A | 8 (10.1) | |
| Secukinumab | N/A | 6 (7.6) | |
| Sarilumab | N/A | 3 (3.8) | |
| Infliximab | N/A | 2 (2.5) | |
| Ixekizumab | N/A | 1 (1.3) | |
| Abatacept | N/A | 1 (1.3) | |
| Targeted Synthetic DMARDs | |||
| Baricitinib | N/A | 5(6.3) | |
| Upadacitinib | N/A | 4(5.1) | |
| Combination of conventional and biological DMARDs | N/A | 50(63.3) | |
| 3rd COVID-19 vaccine n (%) | BNT162b2 (Pfizer) | 5 (16.1) | 63 (79.7) |
| mRNA-1273 (Moderna) | 26 (83.9) | 16 (20.3) | |
| Prior history of COVID-19 n (%) | PCR or IgG antigenic test | 5 (16.1) | 14 (17.7) |
| Responders | Non-Responders | p-Value | |
|---|---|---|---|
| (n = 69) (%) | (n = 10) (%) | ||
| Age (Median) | 58 | 62 | 0.4433 |
| Female | 45 (65.2) | 3 (30.0) | 0.0429 |
| Diagnosis | 0.2672 | ||
| Rheumatoid arthritis | 37 (53.6) | 6 (60) | 0.7481 |
| Psoriatic arthritis | 7 (10.1) | 3 (30) | 0.1094 |
| Ankylosing spondylitis | 14 (20.3) | 0 | 0 |
| Undifferentiated spondyloarthritis | 9 (13.1) | 1 (10) | 1 |
| IBD-associated spondyloarthritis | 2 (2.9) | 0 | 0 |
| Comorbidity | |||
| Arterial hypertension | 25 (36.2) | 3 (30) | 1 |
| Diabetes mellitus | 5 (7.2) | 1 (10) | 0.5687 |
| Dyslipidemia | 27 (39.1) | 2 (20) | 0.31 |
| Cardiovascular disease | 0 | 1 (10) | 0.1266 |
| COPD | 3 (4.3) | 1 (10) | 0.4246 |
| Interstitial lung disease | 0 | 0 | - |
| Oncologic disease | 0 | 0 | - |
| Treatment | |||
| Glucocorticoids (mean) (mg/day) | 1.79 | 3.35 | 0.2171 |
| Conventional synthetic DMARDs | 0.1249 | ||
| Methotrexate | 31 (45) | 2 (20) | 0.18 |
| Leflunomide | 6 (8.7) | 5 (50) | 0.0036 |
| Sulfasalazine | 12 (17.4) | 1 (10) | 1 |
| Mycophenolate | 1 (1.4) | 0 | 1 |
| Hydroxychloroquine | 10 (14.5) | 0 | 1 |
| Biologicals DMARDs | 0.4556 | ||
| Adalimumab | 15 (21.7) | 2 (20) | 1 |
| Infliximab | 8 (11.6) | 0 | 0.5865 |
| Etanercept | 2 (2.9) | 0 | 1 |
| Certolizumab | 16 (23.2) | 2 (20) | 1 |
| Secukinumab | 10 (14.5) | 1 (10) | 1 |
| Ixekizumab | 5 (7.24) | 1 (10) | 0.5687 |
| Gulsekumab | 1 (1.4) | 0 | 1 |
| Abatacept | 1 (1.4) | 0 | 1 |
| Sarilumab | 1 (1.4) | 2 (20) | 0.0407 |
| Targeted synthetic DMARDs | |||
| Baricitinib | 4 (5.8) | 1 (10) | 0.5013 |
| Upadacitinib | 4 (5.8) | 0 | 1 |
| Prior history of COVID-19 | 19 (27.5) | 0 | 0.1069 |
| 3rd COVID-19 vaccine | 1 | ||
| Pfizer | 55 (79.7) | 8 (80) | |
| Moderna | 14 (20.8) | 2 (20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed Mohamed, K.; Álvarez-Hernández, M.P.; Jiménez García, C.; Guevara-Hoyer, K.; Freites, D.; Martínez Prada, C.; Pérez-Sancristóbal, I.; Fernández Gutiérrez, B.; Mato Chaín, G.; Rodero, M.; et al. Specific Cellular and Humoral Response after the Third Dose of Anti-SARS-CoV-2 RNA Vaccine in Patients with Immune-Mediated Rheumatic Diseases on Immunosuppressive Therapy. Biomedicines 2023, 11, 2418. https://doi.org/10.3390/biomedicines11092418
Mohamed Mohamed K, Álvarez-Hernández MP, Jiménez García C, Guevara-Hoyer K, Freites D, Martínez Prada C, Pérez-Sancristóbal I, Fernández Gutiérrez B, Mato Chaín G, Rodero M, et al. Specific Cellular and Humoral Response after the Third Dose of Anti-SARS-CoV-2 RNA Vaccine in Patients with Immune-Mediated Rheumatic Diseases on Immunosuppressive Therapy. Biomedicines. 2023; 11(9):2418. https://doi.org/10.3390/biomedicines11092418
Chicago/Turabian StyleMohamed Mohamed, Kauzar, María Paula Álvarez-Hernández, Carlos Jiménez García, Kissy Guevara-Hoyer, Dalifer Freites, Cristina Martínez Prada, Inés Pérez-Sancristóbal, Benjamín Fernández Gutiérrez, Gloria Mato Chaín, Maria Rodero, and et al. 2023. "Specific Cellular and Humoral Response after the Third Dose of Anti-SARS-CoV-2 RNA Vaccine in Patients with Immune-Mediated Rheumatic Diseases on Immunosuppressive Therapy" Biomedicines 11, no. 9: 2418. https://doi.org/10.3390/biomedicines11092418
APA StyleMohamed Mohamed, K., Álvarez-Hernández, M. P., Jiménez García, C., Guevara-Hoyer, K., Freites, D., Martínez Prada, C., Pérez-Sancristóbal, I., Fernández Gutiérrez, B., Mato Chaín, G., Rodero, M., Rodríguez de la Peña, A., Mulero, T., Bravo, C., Toledano, E., Culebras López, E., Mediero Valeros, B., Pérez Segura, P., Sánchez-Ramón, S., & Candelas Rodríguez, G. (2023). Specific Cellular and Humoral Response after the Third Dose of Anti-SARS-CoV-2 RNA Vaccine in Patients with Immune-Mediated Rheumatic Diseases on Immunosuppressive Therapy. Biomedicines, 11(9), 2418. https://doi.org/10.3390/biomedicines11092418





