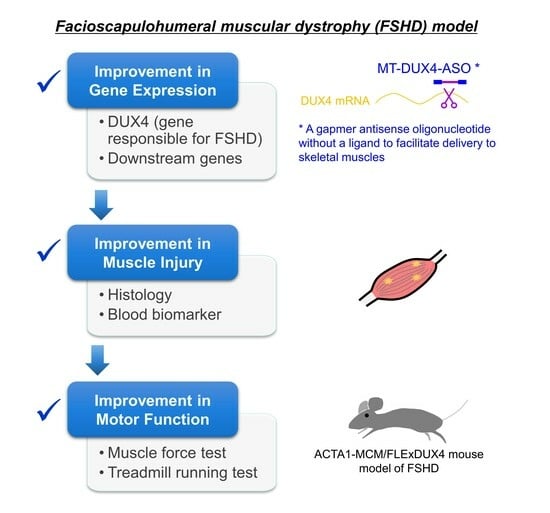
A Systemically Administered Unconjugated Antisense Oligonucleotide Targeting DUX4 Improves Muscular Injury and Motor Function in FSHD Model Mice
Abstract
1. Introduction
2. Materials and Methods
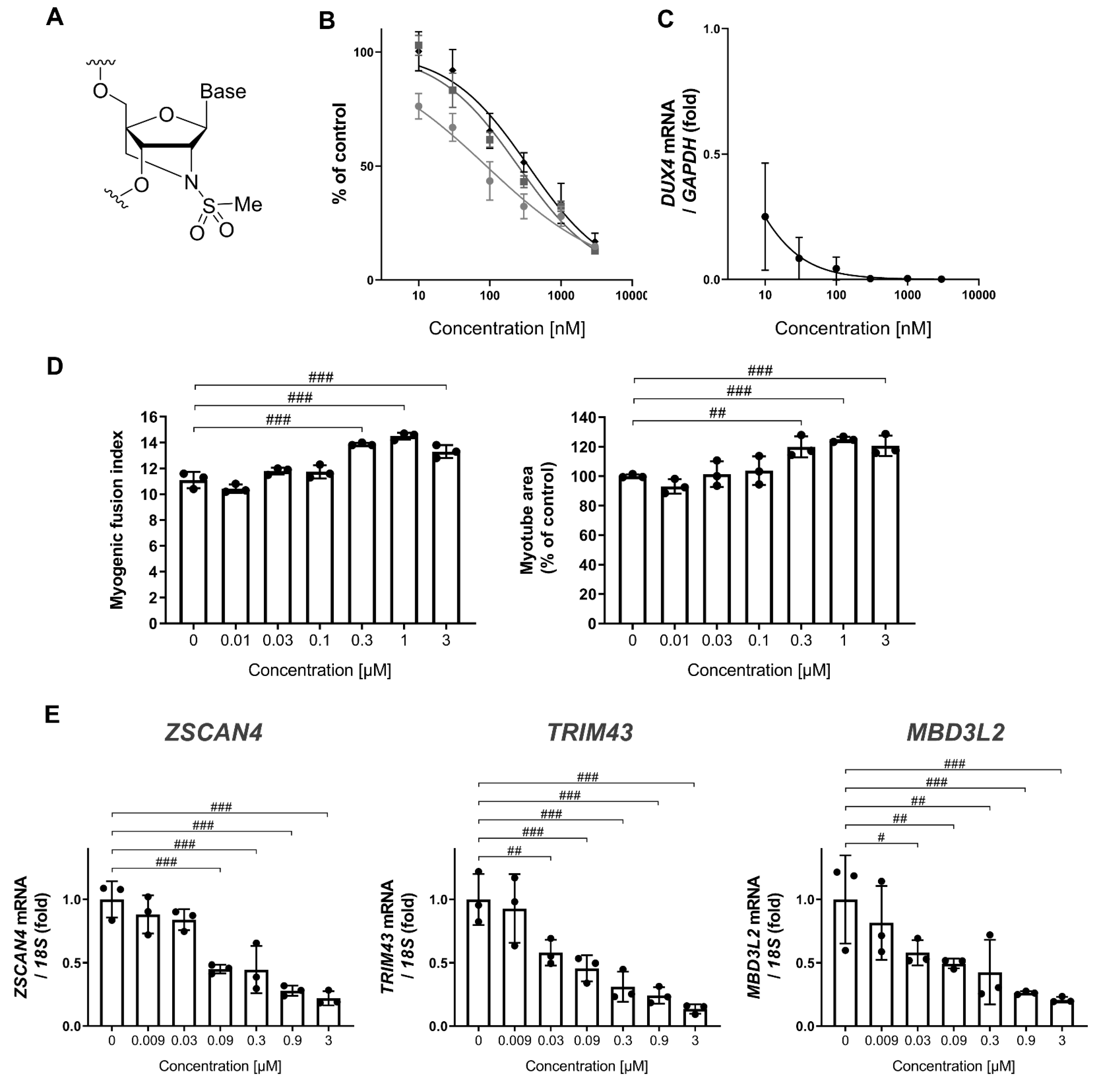
2.1. Antisense Oligonucleotides
2.2. In Vitro Luciferase Reporter Assay
2.3. In Vitro Myotube Formation and Gene Expression Analysis in Patient-Derived Myoblasts
2.4. Animals
2.5. Gene Expression Analysis of Muscles
2.6. Plasma CK Measurement
2.7. Immunohistochemistry
2.8. Treadmill Running Test
2.9. Ex Vivo Muscle Force Test
2.10. Statistical Analysis
3. Results
3.1. MT-DUX4-ASO Decreased DUX4 and Its Target Gene Expression In Vitro (In Vitro Experiments)
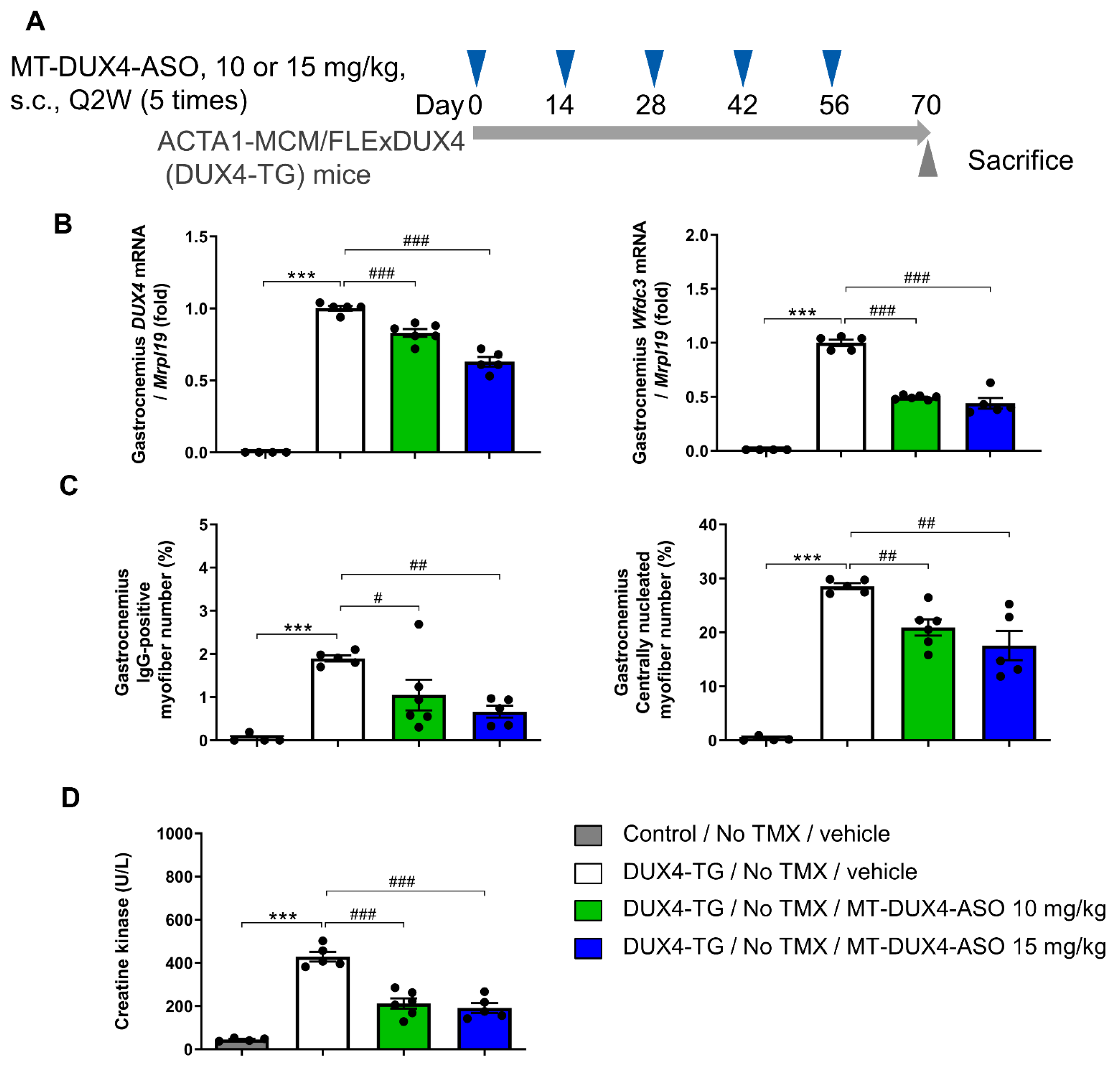
3.2. MT-DUX4-ASO Suppressed DUX4 Target Gene Expression in TMX-Untreated DUX4-TG Mice (In Vivo Experiment-1)
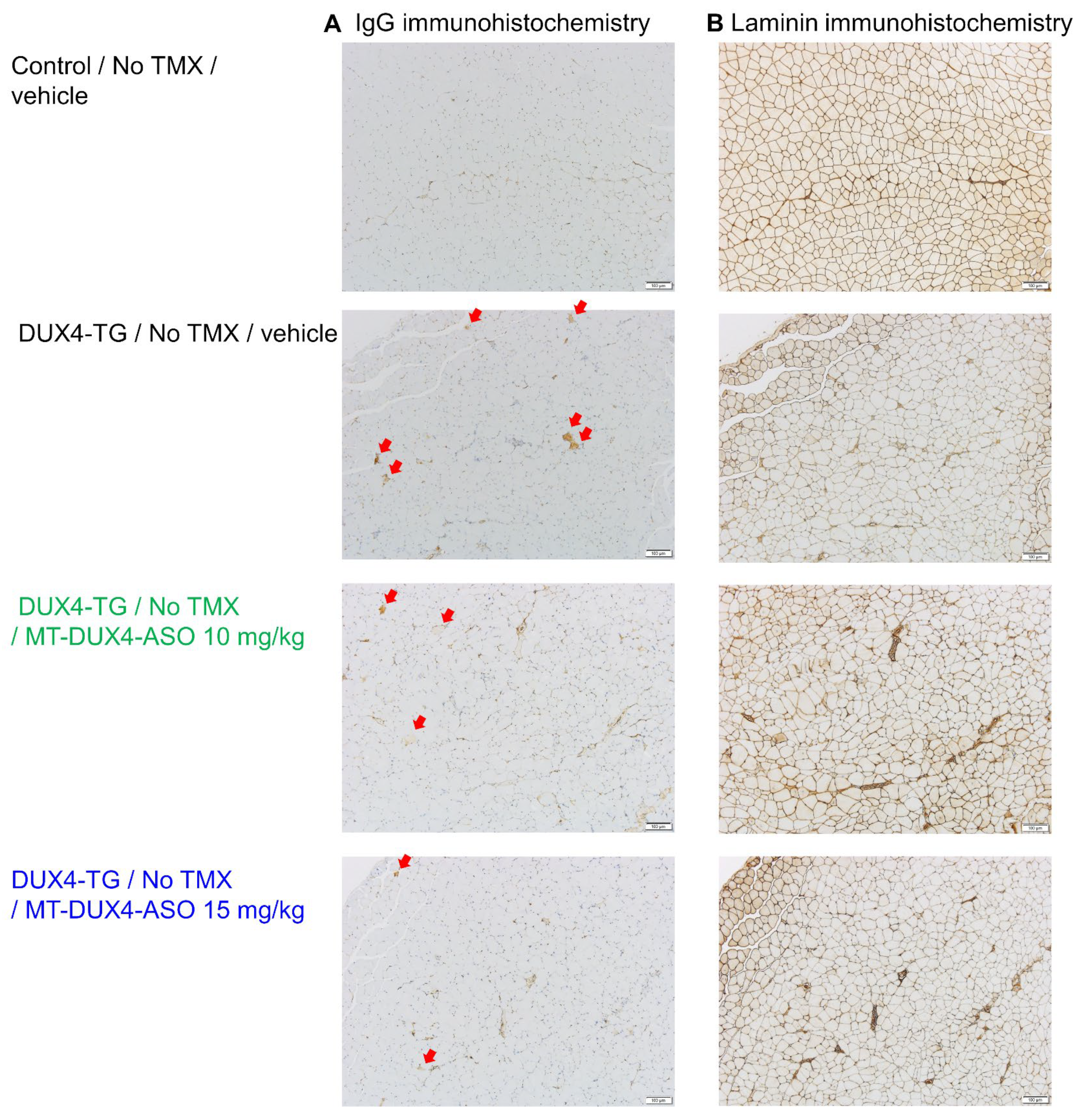
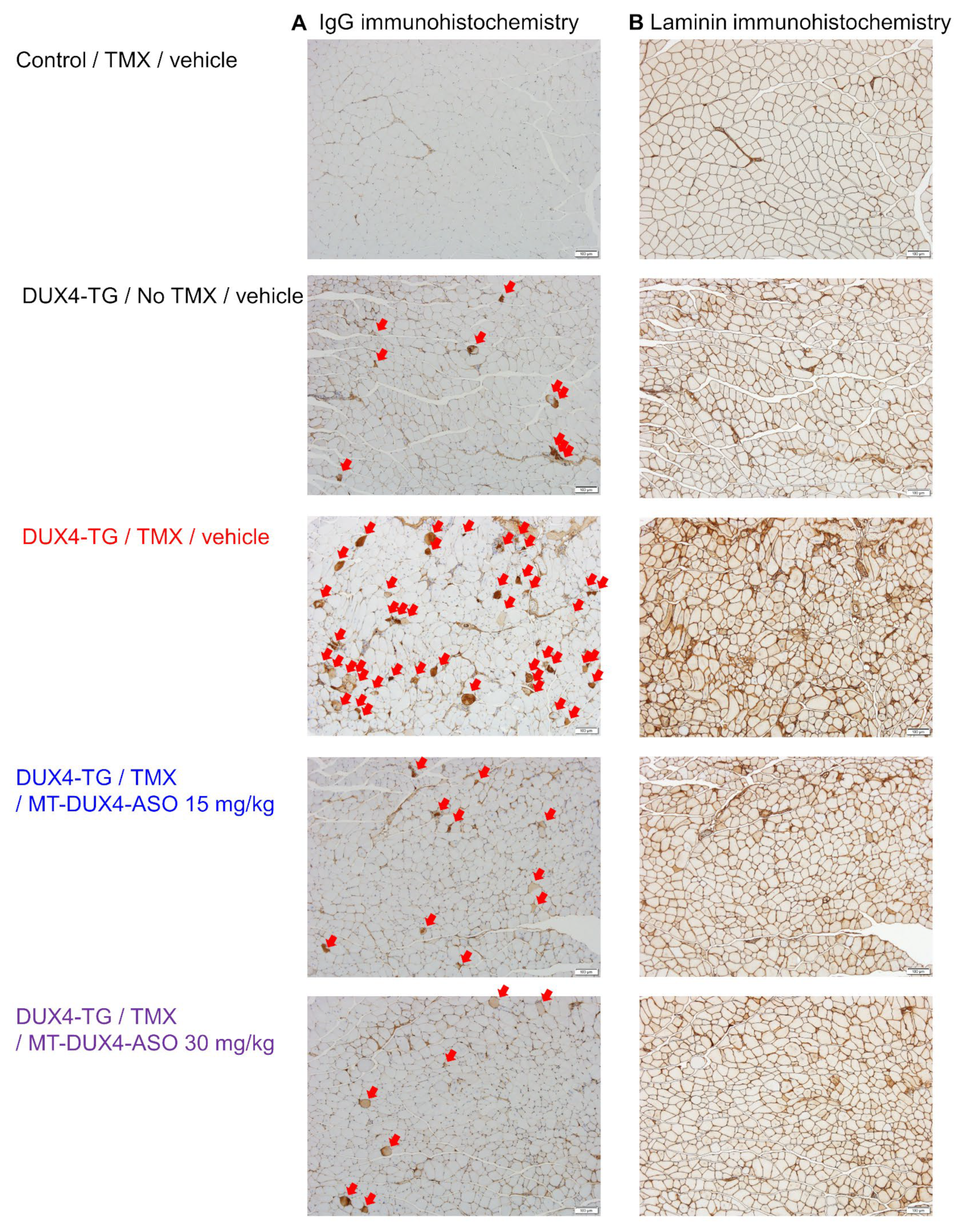
3.3. MT-DUX4-ASO Suppressed Muscle Injury in TMX-Untreated DUX4-TG Mice (In Vivo Experiment-1)
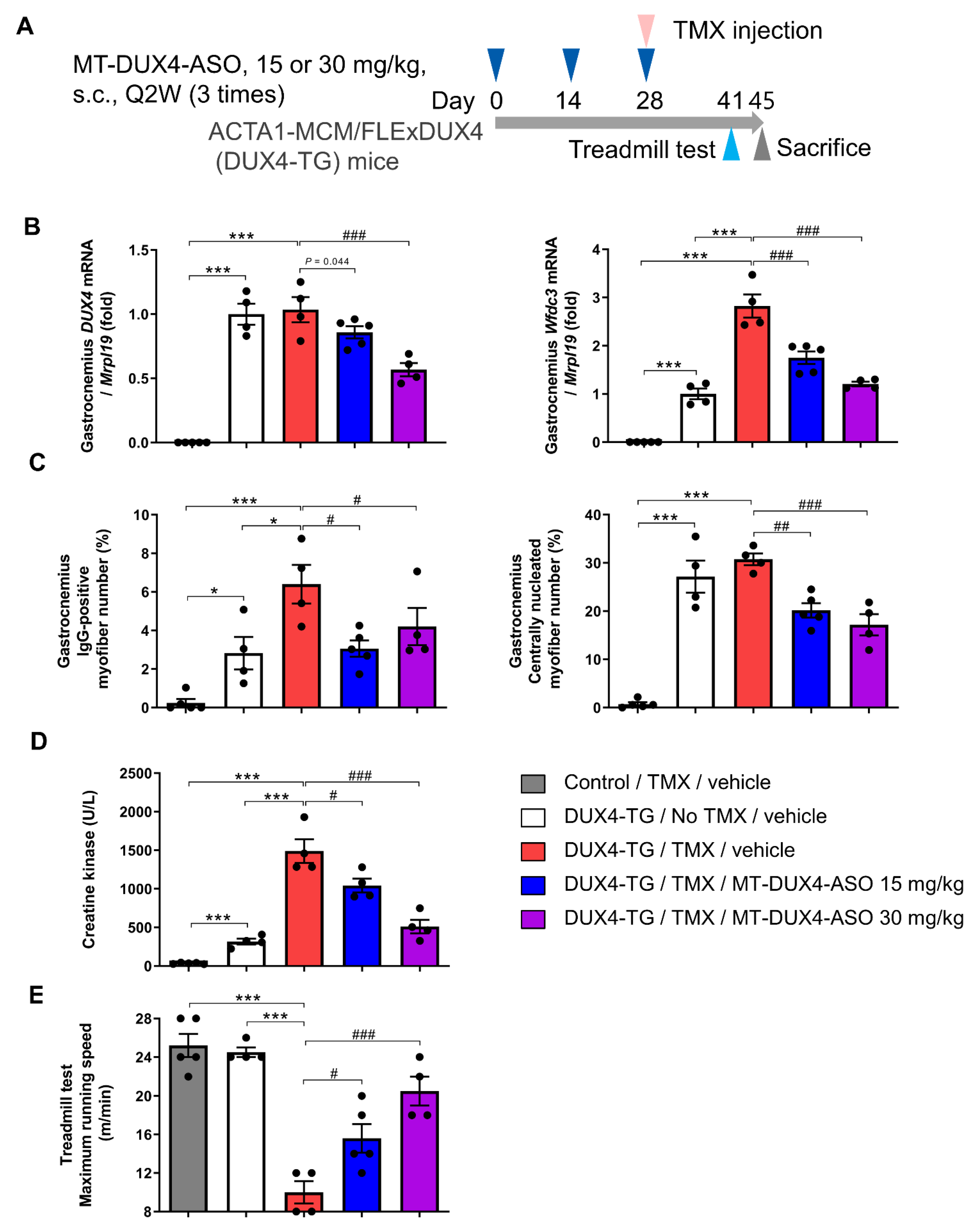
3.4. MT-DUX4-ASO Prevented TMX-Induced DUX4 Target Gene Expression and Muscle Injury in DUX4-TG Mice (In Vivo Experiment-2)
3.5. MT-DUX4-ASO Prevented TMX-Induced Motor Function Decline in DUX4-TG Mice (In Vivo Experiment-2)
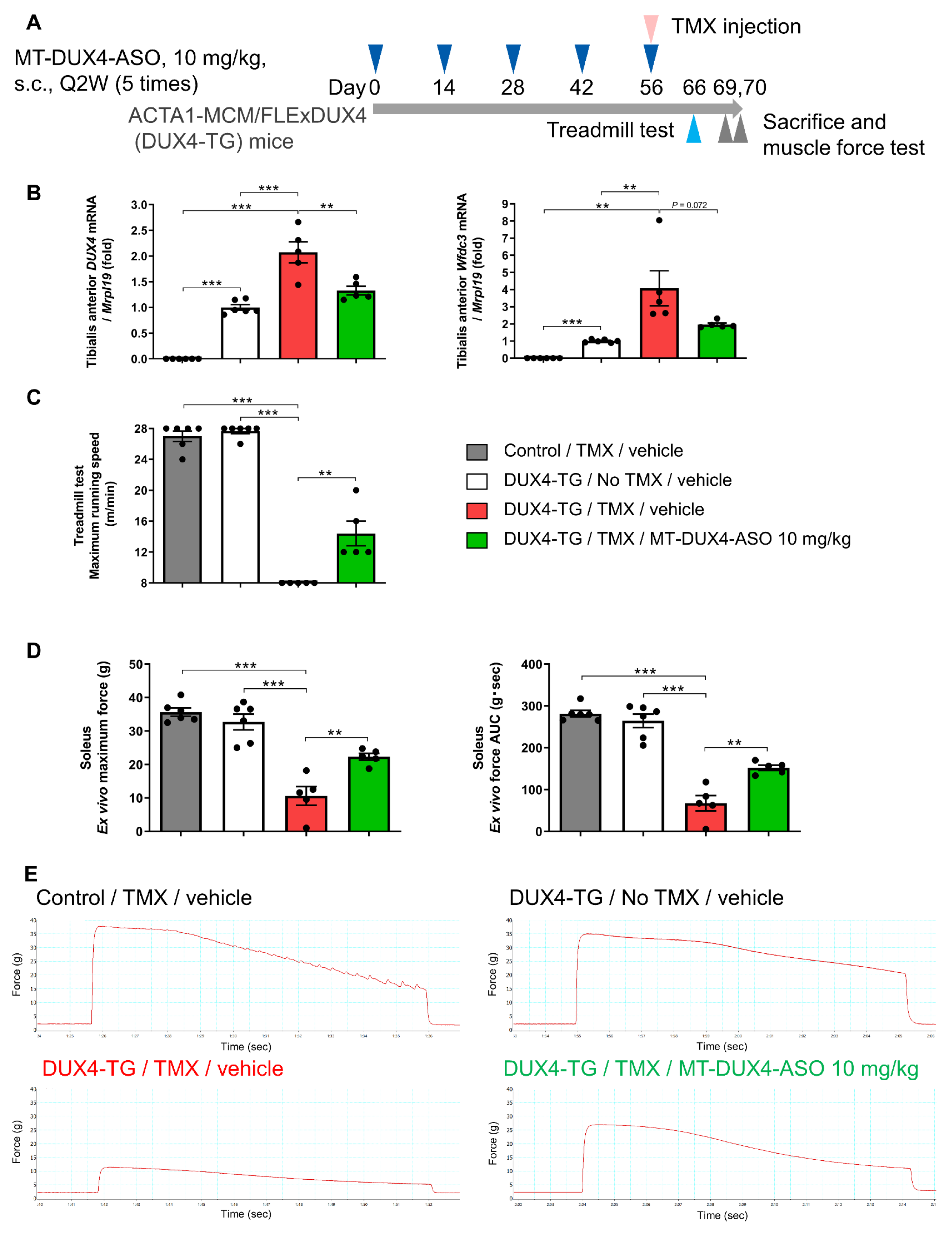
3.6. MT-DUX4-ASO Prevented TMX-Induced Decline in Muscle Force and Motor Function in DUX4-TG Mice with a Lower Dose of 10 mg/kg (In Vivo Experiment-3)
3.7. MT-DUX4-ASO at 100 mg/kg Was Well Tolerated in Mice (Tolerability Experiment)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tawil, R.; Kissel, J.T.; Heatwole, C.; Pandya, S.; Gronseth, G.; Benatar, M. Evidence-based guideline summary: Evaluation, diagnosis, and management of facioscapulohumeral muscular dystrophy: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the Practice Issues Review Panel of the American Association of Neuromuscular & Electrodiagnostic Medicine. Neurology 2015, 85, 357–364. [Google Scholar] [PubMed]
- Statland, J.M.; Tawil, R. Facioscapulohumeral Muscular Dystrophy. Continuum 2016, 22, 1916–1931. [Google Scholar] [PubMed]
- Johnson, N.E.; Quinn, C.; Eastwood, E.; Tawil, R.; Heatwole, C.R. Patient-identified disease burden in facioscapulohumeral muscular dystrophy. Muscle Nerve 2012, 46, 951–953. [Google Scholar] [CrossRef] [PubMed]
- Hamel, J.; Johnson, N.; Tawil, R.; Martens, W.B.; Dilek, N.; McDermott, M.P.; Heatwole, C. Patient-Reported Symptoms in Facioscapulohumeral Muscular Dystrophy (PRISM-FSHD). Neurology 2019, 93, e1180–e1192. [Google Scholar] [CrossRef] [PubMed]
- Statland, J.M.; Tawil, R. Risk of functional impairment in Facioscapulohumeral muscular dystrophy. Muscle Nerve 2014, 49, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.F.; Drew, A.P.; Nicholson, G.A.; Corbett, A.; Kumar, K.R. Facioscapulohumeral muscular dystrophy type 2: An update on the clinical, genetic, and molecular findings. Neuromuscul. Disord. 2021, 31, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Snider, L.; Geng, L.N.; Lemmers, R.J.; Kyba, M.; Ware, C.B.; Nelson, A.M.; Tawil, R.; Filippova, G.N.; van der Maarel, S.M.; Tapscott, S.J.; et al. Facioscapulohumeral dystrophy: Incomplete suppression of a retrotransposed gene. PLoS Genet. 2010, 6, e1001181. [Google Scholar] [CrossRef] [PubMed]
- Rickard, A.M.; Petek, L.M.; Miller, D.G. Endogenous DUX4 expression in FSHD myotubes is sufficient to cause cell death and disrupts RNA splicing and cell migration pathways. Hum. Mol. Genet. 2015, 24, 5901–5914. [Google Scholar] [CrossRef]
- Jones, T.; Jones, P.L. A cre-inducible DUX4 transgenic mouse model for investigating facioscapulohumeral muscular dystrophy. PLoS ONE 2018, 13, e0192657. [Google Scholar] [CrossRef]
- Jones, T.I.; Chew, G.L.; Barraza-Flores, P.; Schreier, S.; Ramirez, M.; Wuebbles, R.D.; Burkin, D.J.; Bradley, R.K.; Jones, P.L. Transgenic mice expressing tunable levels of DUX4 develop characteristic facioscapulohumeral muscular dystrophy-like pathophysiology ranging in severity. Skelet. Muscle 2020, 10, 8. [Google Scholar] [CrossRef]
- Wang, L.H.; Friedman, S.D.; Shaw, D.; Snider, L.; Wong, C.J.; Budech, C.B.; Poliachik, S.L.; Gove, N.E.; Lewis, L.M.; Campbell, A.E.; et al. MRI-informed muscle biopsies correlate MRI with pathology and DUX4 target gene expression in FSHD. Hum. Mol. Genet. 2019, 28, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Dahlqvist, J.R.; Poulsen, N.S.; Østergaard, S.T.; Fornander, F.; de Stricker Borch, J.; Danielsen, E.R.; Thomsen, C.; Vissing, J. Evaluation of inflammatory lesions over 2 years in facioscapulohumeral muscular dystrophy. Neurology 2020, 95, e1211–e1221. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.Q.; Maruyama, R.; Echigoya, Y.; Nguyen, Q.; Zhang, A.; Khawaja, H.; Sen Chandra, S.; Jones, T.; Jones, P.; Chen, Y.W.; et al. Inhibition of DUX4 expression with antisense LNA gapmers as a therapy for facioscapulohumeral muscular dystrophy. Proc. Natl. Acad. Sci. USA 2020, 117, 16509–16515. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.R.Q.; Bittel, A.; Maruyama, R.; Echigoya, Y.; Nguyen, Q.; Huang, Y.; Dzierlega, K.; Zhang, A.; Chen, Y.W.; Yokota, T. DUX4 transcript knockdown with antisense 2’-O-methoxyethyl gapmers for the treatment of facioscapulohumeral muscular dystrophy. Mol. Ther. 2021, 29, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, L.F.; den Hamer, B.; van den Heuvel, A.; Franken, M.; Jackson, M.; Dwyer, C.A.; Tapscott, S.J.; Rigo, F.; van der Maarel, S.M.; de Greef, J.C. Systemic delivery of a DUX4-targeting antisense oligonucleotide to treat facioscapulohumeral muscular dystrophy. Mol. Ther. Nucleic Acids 2021, 26, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Lu-Nguyen, N.; Malerba, A.; Herath, S.; Dickson, G.; Popplewell, L. Systemic antisense therapeutics inhibiting DUX4 expression ameliorates FSHD-like pathology in an FSHD mouse model. Hum. Mol. Genet. 2021, 30, 1398–1412. [Google Scholar] [CrossRef] [PubMed]
- Lu-Nguyen, N.; Dickson, G.; Malerba, A.; Popplewell, L. Long-term systemic treatment of a mouse model displaying chronic FSHD-like pathology with antisense therapeutics that inhibit DUX4 expression. Biomedicines 2022, 10, 1623. [Google Scholar] [CrossRef] [PubMed]
- Lu-Nguyen, N.; Malerba, A.; Antoni Pineda, M.; Dickson, G.; Popplewell, L. Improving molecular and histopathology in diaphragm muscle of the double transgenic ACTA1-MCM/FLExDUX4 mouse model of FSHD with systemic antisense therapy. Hum. Gene Ther. 2022, 33, 923–935. [Google Scholar] [CrossRef]
- Sawamoto, H.; Sasaki, T.; Takegawa-Araki, T.; Utsugi, M.; Furukawa, H.; Hirakawa, Y.; Yamairi, F.; Kurita, T.; Murahashi, K.; Yamada, K.; et al. Synthesis and properties of a novel modified nucleic acid, 2’-N-Methanesulfonyl-2’-Amino-Locked Nucleic Acid. Bioorg. Med. Chem. Lett. 2023, 88, 129289. [Google Scholar] [CrossRef]
- McCarthy, J.J.; Srikuea, R.; Kirby, T.J.; Peterson, C.A.; Esser, K.A. Inducible Cre transgenic mouse strain for skeletal muscle-specific gene targeting. Skelet. Muscle 2012, 2, 8. [Google Scholar] [CrossRef]
- Kakimoto, T.; Okada, K.; Hirohashi, Y.; Relator, R.; Kawai, M.; Iguchi, T.; Fujitaka, K.; Nishio, M.; Kato, T.; Fukunari, A.; et al. Automated image analysis of a glomerular injury marker desmin in spontaneously diabetic Torii rats treated with losartan. J. Endocrinol. 2014, 222, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, T.; Okada, K.; Fujitaka, K.; Nishio, M.; Kato, T.; Fukunari, A.; Utsumi, H. Quantitative analysis of markers of podocyte injury in the rat puromycin aminonucleoside nephropathy model. Exp. Toxicol. Pathol. 2015, 67, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Castro, B.; Kuang, S. Evaluation of Muscle Performance in Mice by Treadmill Exhaustion Test and Whole-limb Grip Strength Assay. Bio Protoc. 2017, 7, e2237. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, A.; Maruo, K.; Furuichi, Y.; Miyatake, S.; Tamura, K.; Fujii, N.L.; Manabe, Y. An improved glucose transport assay system for isolated mouse skeletal muscle tissues. Biosci. Biotechnol. Biochem. 2016, 80, 2224–2230. [Google Scholar] [CrossRef]
- Bosnakovski, D.; Xu, Z.; Gang, E.J.; Galindo, C.L.; Liu, M.; Simsek, T.; Garner, H.R.; Agha-Mohammadi, S.; Tassin, A.; Coppée, F.; et al. An isogenetic myoblast expression screen identifies DUX4-mediated FSHD-associated molecular pathologies. EMBO J. 2008, 27, 2766–2779. [Google Scholar] [CrossRef] [PubMed]
- Hüsken, D.; Asselbergs, F.; Kinzel, B.; Natt, F.; Weiler, J.; Martin, P.; Häner, R.; Hall, J. mRNA fusion constructs serve in a general cell-based assay to profile oligonucleotide activity. Nucleic Acids Res. 2003, 31, e102. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wallace, L.M.; Liu, J.; Domire, J.S.; Garwick-Coppens, S.E.; Guckes, S.M.; Mendell, J.R.; Flanigan, K.M.; Harper, S.Q. RNA interference inhibits DUX4-induced muscle toxicity in vivo: Implications for a targeted FSHD therapy. Mol. Ther. 2012, 20, 1417–1423. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.Y.; Al-Kharsan, M.; Garwick-Coppens, S.E.; Chermahini, G.A.; Harper, M.A.; Palo, A.; Boudreau, R.L.; Harper, S.Q. Human miRNA miR-675 inhibits DUX4 expression and may be exploited as a potential treatment for Facioscapulohumeral muscular dystrophy. Nat. Commun. 2021, 12, 7128. [Google Scholar] [CrossRef]
- Statland, J.M.; Shah, B.; Henderson, D.; Van Der Maarel, S.; Tapscott, S.J.; Tawil, R. Muscle pathology grade for facioscapulohumeral muscular dystrophy biopsies. Muscle Nerve 2015, 52, 521–526. [Google Scholar] [CrossRef]
- Straub, V.; Rafael, J.A.; Chamberlain, J.S.; Campbell, K.P. Animal models for muscular dystrophy show different patterns of sarcolemmal disruption. J. Cell Biol. 1997, 139, 375–385. [Google Scholar] [CrossRef]
- Grounds, M.D.; Radley, H.G.; Lynch, G.S.; Nagaraju, K.; De Luca, A. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol. Dis. 2008, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.R.; Stauber, W.T. Use of computer-assisted analysis for myofiber size measurements of rat soleus muscles from photographed images. J. Histochem. Cytochem. 1994, 42, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Petek, L.M.; Rickard, A.M.; Budech, C.; Poliachik, S.L.; Shaw, D.; Ferguson, M.R.; Tawil, R.; Friedman, S.D.; Miller, D.G. A cross sectional study of two independent cohorts identifies serum biomarkers for facioscapulohumeral muscular dystrophy (FSHD). Neuromuscul. Disord. 2016, 26, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk, H.; de Winter, C.; van Kuik, P.; Heuvelmans, N.; Sabatelli, P.; Rimessi, P.; Braghetta, P.; van Ommen, G.J.; de Kimpe, S.; Ferlini, A.; et al. Preclinical PK and PD studies on 2’-O-methyl-phosphorothioate RNA antisense oligonucleotides in the mdx mouse model. Mol. Ther. 2010, 18, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Malerba, A.; Thorogood, F.C.; Dickson, G.; Graham, I.R. Dosing regimen has a significant impact on the efficiency of morpholino oligomer-induced exon skipping in mdx mice. Hum. Gene Ther. 2009, 20, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Roshmi, R.R.; Yokota, T. Viltolarsen: From Preclinical Studies to FDA Approval. Methods Mol. Biol. 2023, 2587, 31–41. [Google Scholar]
| Exp. | Mouse Sex | TMX-Treated | MT-DUX4-ASO, Dosing | Investigated Changes |
|---|---|---|---|---|
| 1 | Female | Untreated | 10 or 15 mg/kg, s.c., Q2W, 5 times | Muscular gene expression, and muscle injuries (histology and plasma CK) |
| 2 | Female | 5 mg/kg, i.p. | 15 or 30 mg/kg, s.c., Q2W, 3 times | Muscular gene expression, muscle injuries (histology and plasma CK), and motor function (treadmill test) |
| 3 | Male | 7.5 mg/kg, i.p. | 10 mg/kg, s.c., Q2W, 5 times | Muscular gene expression, and motor function (treadmill test and muscle force) |
| Vehicle | MT-DUX4-ASO, 100 mg/kg, i.v., QDx4 | |
|---|---|---|
| AST (U/L) | 53.8 ± 2.5 | 60.2 ± 3.3 |
| ALT (U/L) | 30.4 ± 3.6 | 38.6 ± 3.7 |
| Urea nitrogen (mg/dL) | 20.34 ± 1.17 | 18.62 ± 1.64 |
| Creatinine (mg/dL) | 0.056 ± 0.004 | 0.054 ± 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakimoto, T.; Ogasawara, A.; Ishikawa, K.; Kurita, T.; Yoshida, K.; Harada, S.; Nonaka, T.; Inoue, Y.; Uchida, K.; Tateoka, T.; et al. A Systemically Administered Unconjugated Antisense Oligonucleotide Targeting DUX4 Improves Muscular Injury and Motor Function in FSHD Model Mice. Biomedicines 2023, 11, 2339. https://doi.org/10.3390/biomedicines11092339
Kakimoto T, Ogasawara A, Ishikawa K, Kurita T, Yoshida K, Harada S, Nonaka T, Inoue Y, Uchida K, Tateoka T, et al. A Systemically Administered Unconjugated Antisense Oligonucleotide Targeting DUX4 Improves Muscular Injury and Motor Function in FSHD Model Mice. Biomedicines. 2023; 11(9):2339. https://doi.org/10.3390/biomedicines11092339
Chicago/Turabian StyleKakimoto, Tetsuhiro, Akira Ogasawara, Kiyoshi Ishikawa, Takashi Kurita, Kumiko Yoshida, Shuichi Harada, Taeko Nonaka, Yoshimi Inoue, Keiko Uchida, Takashi Tateoka, and et al. 2023. "A Systemically Administered Unconjugated Antisense Oligonucleotide Targeting DUX4 Improves Muscular Injury and Motor Function in FSHD Model Mice" Biomedicines 11, no. 9: 2339. https://doi.org/10.3390/biomedicines11092339
APA StyleKakimoto, T., Ogasawara, A., Ishikawa, K., Kurita, T., Yoshida, K., Harada, S., Nonaka, T., Inoue, Y., Uchida, K., Tateoka, T., Ohta, T., Kumagai, S., Sasaki, T., & Aihara, H. (2023). A Systemically Administered Unconjugated Antisense Oligonucleotide Targeting DUX4 Improves Muscular Injury and Motor Function in FSHD Model Mice. Biomedicines, 11(9), 2339. https://doi.org/10.3390/biomedicines11092339