A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
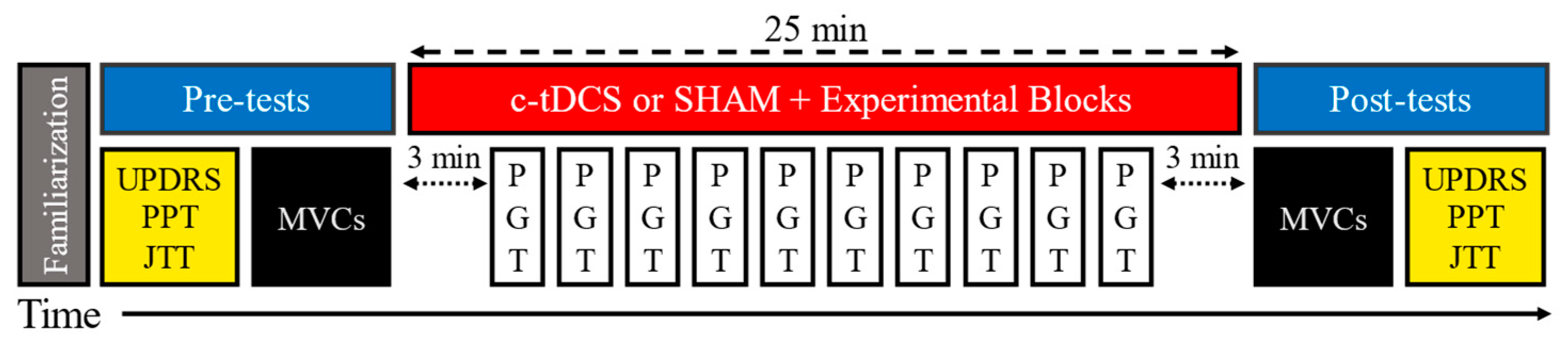
2.1. Experimental Design
2.2. Participants
2.3. Experimental Procedures
2.4. Pinch Grip Task (PGT)
2.5. Cerebellar Transcranial Direct Current Stimulation (c-tDCS)
2.6. Transfer Tasks
2.7. Data Analysis
2.8. Statistical Analysis
3. Results
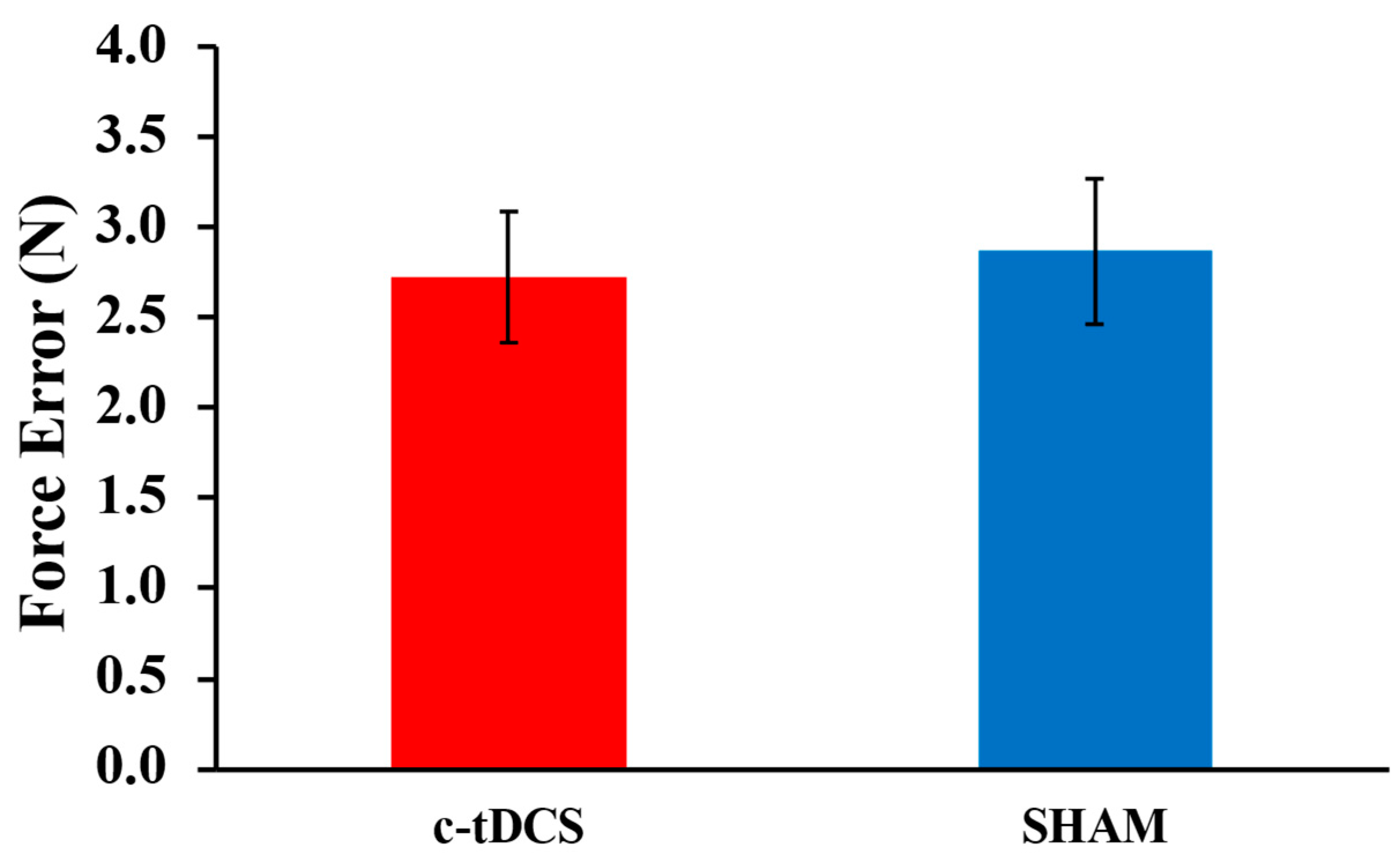
3.1. PGT
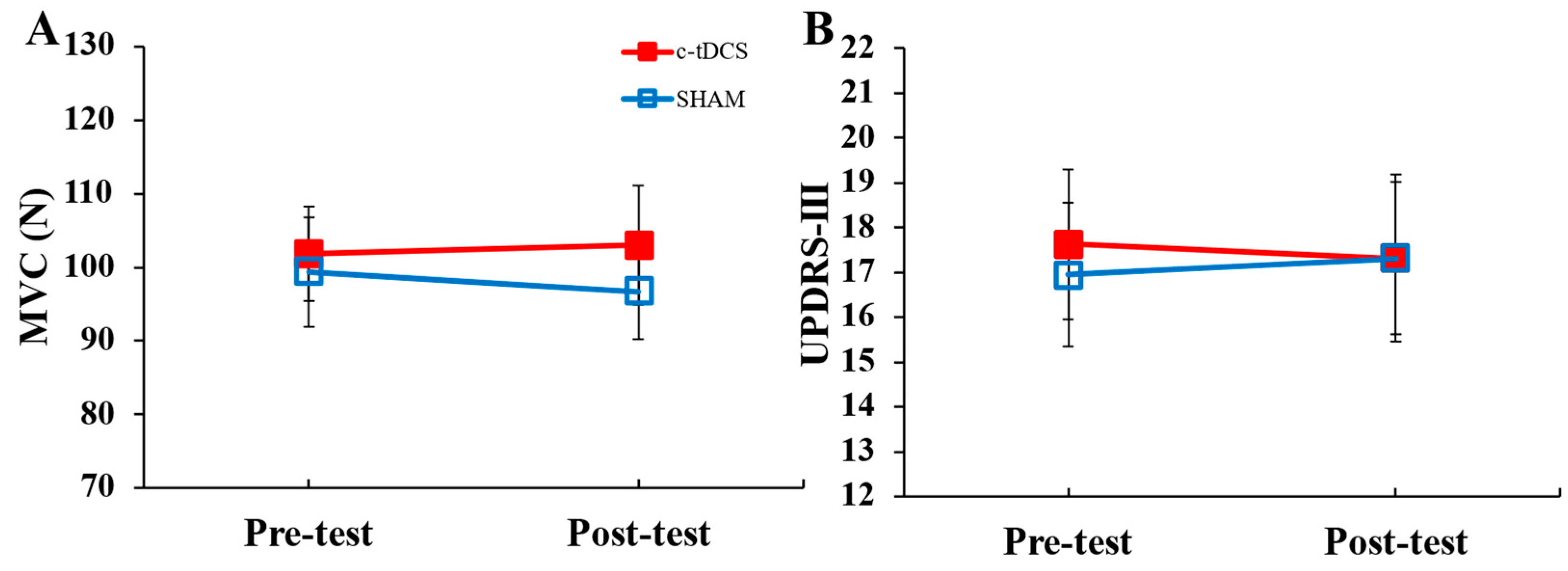
3.2. Transfer Tasks
3.3. Futility Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.J. Parkinson’s Disease: Health-Related Quality of Life, Economic Cost, and Implications of Early Treatment. Am. J. Manag. Care 2010, 16, S87–S93. [Google Scholar] [PubMed]
- Broeder, S.; Nackaerts, E.; Heremans, E.; Vervoort, G.; Meesen, R.; Verheyden, G.; Nieuwboer, A. Transcranial Direct Current Stimulation in Parkinson’s Disease: Neurophysiological Mechanisms and Behavioral Effects. Neurosci. Biobehav. Rev. 2015, 57, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Caligiore, D.; Helmich, R.C.; Hallett, M.; Moustafa, A.A.; Timmermann, L.; Toni, I.; Baldassarre, G. Parkinson’s Disease as a System-Level Disorder. NPJ Park. Dis. 2016, 2, 16025. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Schauenburg, A.; Lang, N.; Liebetanz, D.; Exner, C.; Paulus, W.; Tergau, F. Facilitation of Implicit Motor Learning by Weak Transcranial Direct Current Stimulation of the Primary Motor Cortex in the Human. J. Cogn. Neurosci. 2003, 15, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Meek, A.W.; Greenwell, D.; Poston, B.; Riley, Z.A. Anodal Tdcs Accelerates On-Line Learning of Dart Throwing. Neurosci. Lett. 2021, 764, 136211. [Google Scholar] [CrossRef]
- Wilson, M.A.; Greenwell, D.; Meek, A.W.; Poston, B.; Riley, Z.A. Neuroenhancement of a Dexterous Motor Task with Anodal Tdcs. Brain Res. 2022, 1790, 147993. [Google Scholar] [CrossRef]
- Buch, E.R.; Santarnecchi, E.; Antal, A.; Born, J.; Celnik, P.A.; Classen, J.; Gerloff, C.; Hallett, M.; Hummel, F.C.; Nitsche, M.A.; et al. Effects of Tdcs on Motor Learning and Memory Formation: A Consensus and Critical Position Paper. Clin. Neurophysiol. 2017, 128, 589–603. [Google Scholar] [CrossRef]
- Simpson, M.W.; Mak, M. The Effect of Transcranial Direct Current Stimulation on Upper Limb Motor Performance in Parkinson’s Disease: A Systematic Review. J. Neurol. 2020, 267, 3479–3488. [Google Scholar] [CrossRef]
- Oldrati, V.; Schutter, D.J.L.G. Targeting the Human Cerebellum with Transcranial Direct Current Stimulation to Modulate Behavior: A Meta-Analysis. Cerebellum 2018, 17, 228–236. [Google Scholar] [CrossRef]
- Jackson, A.K.; de Albuquerque, L.L.; Pantovic, M.; Fischer, K.M.; Guadagnoli, M.A.; Riley, Z.A.; Poston, B. Cerebellar Transcranial Direct Current Stimulation Enhances Motor Learning in a Complex Overhand Throwing Task. Cerebellum 2019, 18, 813–816. [Google Scholar] [CrossRef]
- Cantarero, G.; Spampinato, D.; Reis, J.; Ajagbe, L.; Thompson, T.; Kulkarni, K.; Celnik, P. Cerebellar Direct Current Stimulation Enhances On-Line Motor Skill Acquisition through an Effect on Accuracy. J. Neurosci. 2015, 35, 3285–3290. [Google Scholar] [CrossRef] [PubMed]
- Hardwick, R.M.; Celnik, P.A. Cerebellar Direct Current Stimulation Enhances Motor Learning in Older Adults. Neurobiol. Aging 2014, 35, 2217–2221. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hallett, M. The Cerebellum in Parkinson’s Disease. Brain 2013, 136 Pt 3, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Pinto, A.D.; Lang, A.E.; Chen, R. Involvement of the Cerebellothalamocortical Pathway in Parkinson Disease. Ann. Neurol. 2010, 68, 816–824. [Google Scholar] [CrossRef]
- Galea, J.M.; Vazquez, A.; Pasricha, N.; de Xivry, J.J.; Celnik, P. Dissociating the Roles of the Cerebellum and Motor Cortex During Adaptive Learning: The Motor Cortex Retains What the Cerebellum Learns. Cereb. Cortex. 2011, 21, 1761–1770. [Google Scholar] [CrossRef]
- Rathelot, J.A.; Strick, P.L. Subdivisions of Primary Motor Cortex Based on Cortico-Motoneuronal Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 918–923. [Google Scholar] [CrossRef]
- Reis, J.; Swayne, O.B.; Vandermeeren, Y.; Camus, M.; Dimyan, M.A.; Harris-Love, M.; Perez, M.A.; Ragert, P.; Rothwell, J.C.; Cohen, L.G. Contribution of Transcranial Magnetic Stimulation to the Understanding of Cortical Mechanisms Involved in Motor Control. J. Physiol. 2008, 586, 325–351. [Google Scholar] [CrossRef]
- Opie, G.M.; Liao, W.Y.; Semmler, J.G. Interactions between Cerebellum and the Intracortical Excitatory Circuits of Motor Cortex: A Mini-Review. Cerebellum 2022, 21, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Bostan, A.C.; Dum, R.P.; Strick, P.L. The Basal Ganglia Communicate with the Cerebellum. Proc. Natl. Acad. Sci. USA 2010, 107, 8452–8456. [Google Scholar] [CrossRef]
- Tanaka, T.; Takano, Y.; Tanaka, S.; Hironaka, N.; Kobayashi, K.; Hanakawa, T.; Watanabe, K.; Honda, M. Transcranial Direct-Current Stimulation Increases Extracellular Dopamine Levels in the Rat Striatum. Front. Syst. Neurosci. 2013, 7, 6. [Google Scholar] [CrossRef]
- Polanía, R.; Paulus, W.; Nitsche, M.A. Modulating Cortico-Striatal and Thalamo-Cortical Functional Connectivity with Transcranial Direct Current Stimulation. Hum. Brain Mapp. 2012, 33, 2499–2508. [Google Scholar] [CrossRef] [PubMed]
- Roche, N.; Lackmy, A.; Achache, V.; Bussel, B.; Katz, R. Effects of Anodal Transcranial Direct Current Stimulation over the Leg Motor Area on Lumbar Spinal Network Excitability in Healthy Subjects. J. Physiol. 2011, 589 Pt 11, 2813–2826. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Lei, X.; Yan, T.; Li, H.; Huang, B.; Li, L.; Xu, L.; Liu, L.; Chen, N.; Lü, L.; et al. The Temporary and Accumulated Effects of Transcranial Direct Current Stimulation for the Treatment of Advanced Parkinson’s Disease Monkeys. Sci. Rep. 2015, 5, 12178. [Google Scholar] [CrossRef]
- Krakauer, J.W.; Mazzoni, P. Human Sensorimotor Learning: Adaptation, Skill, and Beyond. Curr. Opin. Neurobiol. 2011, 21, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Boisgontier, M.P. Motor Aging Results from Cerebellar Neuron Death. Trends Neurosci. 2015, 38, 127–128. [Google Scholar] [CrossRef]
- Block, H.; Celnik, P. Stimulating the Cerebellum Affects Visuomotor Adaptation but not Intermanual Transfer of Learning. Cerebellum 2013, 12, 781–793. [Google Scholar] [CrossRef]
- Lima de Albuquerque, L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. An Acute Application of Cerebellar Transcranial Direct Current Stimulation does not Improve Motor Performance in Parkinson’s Disease. Brain Sci. 2020, 10, 735. [Google Scholar] [CrossRef]
- Lima de Albuquerque, L.; Pantovic, M.; Clingo, M.G.; Fischer, K.M.; Jalene, S.; Landers, M.R.; Mari, Z.; Poston, B. Long-Term Application of Cerebellar Transcranial Direct Current Stimulation does not Improve Motor Learning in Parkinson’s Disease. Cerebellum 2022, 21, 333–349. [Google Scholar] [CrossRef]
- Workman, C.D.; Fietsam, A.C.; Uc, E.Y.; Rudroff, T. Cerebellar Transcranial Direct Current Stimulation in People with Parkinson’s Disease: A Pilot Study. Brain Sci. 2020, 10, 96. [Google Scholar] [CrossRef]
- Wong, P.L.; Yang, Y.R.; Huang, S.F.; Fuh, J.L.; Chiang, H.L.; Wang, R.Y. Transcranial Direct Current Stimulation on Different Targets to Modulate Cortical Activity and Dual-Task Walking in Individuals with Parkinson’s Disease: A Double Blinded Randomized Controlled Trial. Front. Aging Neurosci. 2022, 14, 807151. [Google Scholar] [CrossRef]
- Ferrucci, R.; Cortese, F.; Bianchi, M.; Pittera, D.; Turrone, R.; Bocci, T.; Borroni, B.; Vergari, M.; Cogiamanian, F.; Ardolino, G.; et al. Cerebellar and Motor Cortical Transcranial Stimulation Decrease Levodopa-Induced Dyskinesias in Parkinson’s Disease. Cerebellum 2016, 15, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Uehara, K.; Hanakawa, T. The Contribution of Interindividual Factors to Variability of Response in Transcranial Direct Current Stimulation Studies. Front. Cell Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Zoghi, M.; Jaberzadeh, S. Biological and Anatomical Factors Influencing Interindividual Variability to Noninvasive Brain Stimulation of the Primary Motor Cortex: A Systematic Review and Meta-Analysis. Rev. Neurosci. 2018, 29, 199–222. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Screening Questionnaire before Tms: An Update. Clin. Neurophysiol. 2011, 122, 1686. [Google Scholar] [CrossRef]
- Miterko, L.N.; Baker, K.B.; Beckinghausen, J.; Bradnam, L.V.; Cheng, M.Y.; Cooperrider, J.; DeLong, M.R.; Gornati, S.V.; Hallett, M.; Heck, D.H.; et al. Consensus Paper: Experimental Neurostimulation of the Cerebellum. Cerebellum 2019, 18, 1064–1097. [Google Scholar] [CrossRef] [PubMed]
- MacInnis, M.J.; McGlory, C.; Gibala, M.J.; Phillips, S.M. Investigating Human Skeletal Muscle Physiology with Unilateral Exercise Models: When One Limb Is More Powerful than Two. Appl. Physiol. Nutr. Metab. 2017, 42, 563–570. [Google Scholar] [CrossRef]
- Defer, G.L.; Widner, H.; Marie, R.M.; Remy, P.; Levivier, M. Core Assessment Program for Surgical Interventional Therapies in Parkinson’s Disease (Capsit-Pd). Mov. Disord. 1999, 14, 572–584. [Google Scholar] [CrossRef]
- Lidstone, D.E.; Miah, F.Z.; Poston, B.; Beasley, J.F.; Mostofsky, S.H.; Dufek, J.S. Children with Autism Spectrum Disorder Show Impairments during Dynamic Versus Static Grip-Force Tracking. Autism. Res. 2020, 13, 2177–2189. [Google Scholar] [CrossRef]
- Reis, J.; Fischer, J.T.; Prichard, G.; Weiller, C.; Cohen, L.G.; Fritsch, B. Time- but not Sleep-Dependent Consolidation of Tdcs-Enhanced Visuomotor Skills. Cereb. Cortex. 2015, 25, 109–117. [Google Scholar] [CrossRef]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive Cortical Stimulation Enhances Motor Skill Acquisition over Multiple Days through an Effect on Consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef]
- Spraker, M.B.; Corcos, D.M.; Kurani, A.S.; Prodoehl, J.; Swinnen, S.P.; Vaillancourt, D.E. Specific Cerebellar Regions Are Related to Force Amplitude and Rate of Force Development. Neuroimage 2012, 59, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.W.; Diedrichsen, J.; Krakauer, J.W.; Shadmehr, R.; Bastian, A.J. Sensory Prediction Errors Drive Cerebellum-Dependent Adaptation of Reaching. J. Neurophysiol. 2007, 98, 54–62. [Google Scholar] [CrossRef]
- Vaillancourt, D.E.; Thulborn, K.R.; Corcos, D.M. Neural Basis for the Processes That Underlie Visually Guided and Internally Guided Force Control in Humans. J. Neurophysiol. 2003, 90, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Burciu, R.G.; Ofori, E.; Shukla, P.; Planetta, P.J.; Snyder, A.F.; Li, H.; Hass, C.J.; Okun, M.S.; McFarland, N.R.; Vaillancourt, D.E. Distinct Patterns of Brain Activity in Progressive Supranuclear Palsy and Parkinson’s Disease. Mov. Disord. 2015, 30, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.W.; Burciu, R.G.; Ofori, E.; Coombes, S.A.; Christou, E.A.; Okun, M.S.; Hess, C.W.; Vaillancourt, D.E. Beta-Band Oscillations in the Supplementary Motor Cortex Are Modulated by Levodopa and Associated with Functional Activity in the Basal Ganglia. Neuroimage Clin. 2018, 19, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Neely, K.A.; Planetta, P.J.; Prodoehl, J.; Corcos, D.M.; Comella, C.L.; Goetz, C.G.; Shannon, K.L.; Vaillancourt, D.E. Vaillancourt. Force Control Deficits in Individuals with Parkinson’s Disease, Multiple Systems Atrophy, and Progressive Supranuclear Palsy. PLoS ONE 2013, 8, e58403. [Google Scholar] [CrossRef]
- Planetta, P.J.; McFarland, N.R.; Okun, M.S.; Vaillancourt, D.E. Mri Reveals Brain Abnormalities in Drug-Naive Parkinson’s Disease. Exerc. Sport Sci. Rev. 2014, 42, 12–22. [Google Scholar] [CrossRef]
- Prodoehl, J.; Corcos, D.M.; Vaillancourt, D.E. Basal Ganglia Mechanisms Underlying Precision Grip Force Control. Neurosci. Biobehav. Rev. 2009, 33, 900–908. [Google Scholar] [CrossRef]
- Prodoehl, J.; Planetta, P.J.; Kurani, A.S.; Comella, C.L.; Corcos, D.M.; Vaillancourt, D.E. Differences in Brain Activation between Tremor- and Nontremor-Dominant Parkinson Disease. JAMA Neurol. 2013, 70, 100–106. [Google Scholar] [CrossRef]
- Spraker, M.B.; Prodoehl, J.; Corcos, D.M.; Comella, C.L.; Vaillancourt, D.E. Basal Ganglia Hypoactivity During Grip Force in Drug Naive Parkinson’s Disease. Hum. Brain Mapp. 2010, 31, 1928–1941. [Google Scholar] [CrossRef]
- Vaillancourt, D.E.; Slifkin, A.B.; Newell, K.M. Intermittency in the Visual Control of Force in Parkinson’s Disease. Exp. Brain Res. 2001, 138, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, L.L.; Fischer, K.M.; Pauls, A.L.; Pantovic, M.; Guadagnoli, M.A.; Riley, Z.A.; Poston, B. An Acute Application of Transcranial Random Noise Stimulation does not Enhance Motor Skill Acquisition or Retention in a Golf Putting Task. Hum. Mov. Sci. 2019, 66, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Poston, B.; Christou, E.A.; Enoka, J.A.; Enoka, R.M. Timing Variability and not Force Variability Predicts the Endpoint Accuracy of Fast and Slow Isometric Contractions. Exp. Brain Res. 2010, 202, 189–202. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kenville, R.; Maudrich, T.; Maudrich, D.; Villringer, A.; Ragert, P. Cerebellar Transcranial Direct Current Stimulation Improves Maximum Isometric Force Production during Isometric Barbell Squats. Brain Sci. 2020, 10, 235. [Google Scholar] [CrossRef]
- Steiner, K.M.; Enders, A.; Thier, W.; Batsikadze, G.; Ludolph, N.; Ilg, W.; Timmann, D. Cerebellar Tdcs does not Improve Learning in a Complex Whole Body Dynamic Balance Task in Young Healthy Subjects. PLoS ONE 2016, 11, e0163598. [Google Scholar] [CrossRef]
- Sadnicka, A.; Hamada, M.; Bhatia, K.P.; Rothwell, J.C.; Edwards, M.J. Cerebellar Stimulation Fails to Modulate Motor Cortex Plasticity in Writing Dystonia. Mov. Disord. 2014, 29, 1304–1307. [Google Scholar] [CrossRef]
- Krakauer, J.W.; Mazzoni, P.; Ghazizadeh, A.; Ravindran, R.; Shadmehr, R. Generalization of Motor Learning Depends on the History of Prior Action. PLoS Biol. 2006, 4, e316. [Google Scholar] [CrossRef]
- Hamoudi, M.; Schambra, H.M.; Fritsch, B.; Schoechlin-Marx, A.; Weiller, C.; Cohen, L.G.; Reis, J. Transcranial Direct Current Stimulation Enhances Motor Skill Learning but not Generalization in Chronic Stroke. Neurorehabil. Neural. Repair 2018, 32, 295–308. [Google Scholar] [CrossRef]
- Orban de Xivry, J.J.; Marko, M.K.; Pekny, S.E.; Pastor, D.; Izawa, J.; Celnik, P.; Shadmehr, R. Stimulation of the Human Motor Cortex Alters Generalization Patterns of Motor Learning. J. Neurosc. 2011, 31, 7102–7110. [Google Scholar] [CrossRef][Green Version]
- Parikh, P.J.; Cole, K.J. Effects of Transcranial Direct Current Stimulation in Combination with Motor Practice on Dexterous Grasping and Manipulation in Healthy Older Adults. Physiol. Rep. 2014, 2, e00255. [Google Scholar] [CrossRef]
- Spampinato, D.A.; Celnik, P.A.; Rothwell, J.C. Cerebellar-Motor Cortex Connectivity: One or Two Different Networks? J. Neurosci. 2020, 40, 4230–4239. [Google Scholar] [CrossRef]
- Hamada, M.; Galea, J.M.; Di Lazzaro, V.; Mazzone, P.; Ziemann, U.; Rothwell, J.C. Two Distinct Interneuron Circuits in Human Motor Cortex Are Linked to Different Subsets of Physiological and Behavioral Plasticity. J. Neurosci. 2014, 34, 12837–12849. [Google Scholar] [CrossRef] [PubMed]
- Galea, J.M.; Jayaram, G.; Ajagbe, L.; Celnik, P. Modulation of Cerebellar Excitability by Polarity-Specific Noninvasive Direct Current Stimulation. J. Neurosci. 2009, 29, 9115–9122. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, G.; Tang, B.; Pallegadda, R.; Vasudevan, E.V.; Celnik, P.; Bastian, A. Modulating Locomotor Adaptation with Cerebellar Stimulation. J. Neurophysiol. 2012, 107, 2950–2957. [Google Scholar] [CrossRef] [PubMed]
- Rezaee, Z.; Dutta, A. Lobule-Specific Dosage Considerations for Cerebellar Transcranial Direct Current Stimulation During Healthy Aging: A Computational Modeling Study Using Age-Specific Magnetic Resonance Imaging Templates. Neuromodulation 2020, 23, 341–365. [Google Scholar] [CrossRef]
- Consideration of Sample Size in Neuroscience Studies. J. Neurosci. 2020, 40, 4076–4077. [CrossRef]
- Szucs, D.; Ioannidis, J.P. Sample Size Evolution in Neuroimaging Research: An Evaluation of Highly-Cited Studies (1990–2012) and of Latest Practices (2017–2018) in High-Impact Journals. Neuroimage 2020, 221, 117164. [Google Scholar] [CrossRef]
- Siew-Pin Leuk, J.; Yow, K.E.; Zi-Xin Tan, C.; Hendy, A.M.; Kar-Wing Tan, M.; Hock-Beng Ng, T.; Teo, W.P. A Meta-Analytical Review of Transcranial Direct Current Stimulation Parameters on Upper Limb Motor Learning in Healthy Older Adults and People with Parkinson’s Disease. Rev. Neurosci. 2023, 34, 325–348. [Google Scholar] [CrossRef]
- Samaei, A.; Ehsani, F.; Zoghi, M.; Yosephi, M.H.; Jaberzadeh, S. Online and Offline Effects of Cerebellar Transcranial Direct Current Stimulation on Motor Learning in Healthy Older Adults: A Randomized Double-Blind Sham-Controlled Study. Eur. J. Neurosci. 2017, 45, 1177–1185. [Google Scholar] [CrossRef]
- Ruggiero, F.; Dini, M.; Cortese, F.; Vergari, M.; Nigro, M.; Poletti, B.; Priori, A.; Ferrucci, R. Anodal Transcranial Direct Current Stimulation over the Cerebellum Enhances Sadness Recognition in Parkinson’s Disease Patients: A Pilot Study. Cerebellum 2022, 21, 234–243. [Google Scholar] [CrossRef]
- Naro, A.; Bramanti, A.; Leo, A.; Manuli, A.; Sciarrone, F.; Russo, M.; Bramanti, P.; Calabro, R.S. Effects of Cerebellar Transcranial Alternating Current Stimulation on Motor Cortex Excitability and Motor Function. Brain Struct. Funct. 2017, 222, 2891–2906. [Google Scholar] [CrossRef] [PubMed]
- Miyaguchi, S.; Inukai, Y.; Matsumoto, Y.; Miyashita, M.; Takahashi, R.; Otsuru, N.; Onishi, H. Effects on Motor Learning of Transcranial Alternating Current Stimulation Applied over the Primary Motor Cortex and Cerebellar Hemisphere. J. Clin. Neurosci. 2020, 78, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Miyaguchi, S.; Otsuru, N.; Kojima, S.; Saito, K.; Inukai, Y.; Masaki, M.; Onishi, H. Transcranial Alternating Current Stimulation with Gamma Oscillations over the Primary Motor Cortex and Cerebellar Hemisphere Improved Visuomotor Performance. Front. Behav. Neurosci. 2018, 12, 132. [Google Scholar] [CrossRef] [PubMed]
- Miyaguchi, S.; Otsuru, N.; Kojima, S.; Yokota, H.; Saito, K.; Inukai, Y.; Onishi, H. Gamma Tacs over M1 and Cerebellar Hemisphere Improves Motor Performance in a Phase-Specific Manner. Neurosci. Lett. 2019, 694, 64–68. [Google Scholar] [CrossRef]

| Dependent Variable | Statistical Test | p | ηp2 | |
|---|---|---|---|---|
| PGT (N) | Paired t-test | 0.322 | ||
| MVC (N) | 2 × 2 within-subjects ANOVA | condition | 0.224 | 0.158 |
| test | 0.749 | 0.007 | ||
| condition × test | 0.446 | 0.036 | ||
| UPDRS-III (Score) | 2 × 2 within-subjects ANOVA | condition | 0.709 | 0.010 |
| test | 0.920 | 0.001 | ||
| condition × test | 0.341 | 0.061 | ||
| Purdue Pegboard (pegs) | 2 × 2 within-subjects ANOVA | condition | 0.412 | 0.045 |
| test | 0.268 | 0.081 | ||
| condition × test | 0.222 | 0.098 | ||
| JTT (sec) | 2 × 2 within-subjects ANOVA | condition | 0.607 | 0.018 |
| test | 0.004 | 0.427 | ||
| condition × test | 0.872 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Albuquerque, L.L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study. Biomedicines 2023, 11, 2219. https://doi.org/10.3390/biomedicines11082219
de Albuquerque LL, Pantovic M, Clingo M, Fischer K, Jalene S, Landers M, Mari Z, Poston B. A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study. Biomedicines. 2023; 11(8):2219. https://doi.org/10.3390/biomedicines11082219
Chicago/Turabian Stylede Albuquerque, Lidio Lima, Milan Pantovic, Mitchell Clingo, Katherine Fischer, Sharon Jalene, Merrill Landers, Zoltan Mari, and Brach Poston. 2023. "A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study" Biomedicines 11, no. 8: 2219. https://doi.org/10.3390/biomedicines11082219
APA Stylede Albuquerque, L. L., Pantovic, M., Clingo, M., Fischer, K., Jalene, S., Landers, M., Mari, Z., & Poston, B. (2023). A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study. Biomedicines, 11(8), 2219. https://doi.org/10.3390/biomedicines11082219










