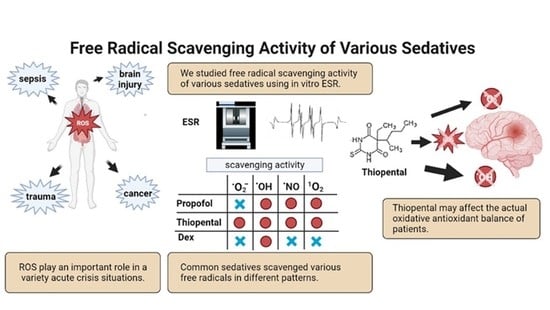
Sedation Therapy in Intensive Care Units: Harnessing the Power of Antioxidants to Combat Oxidative Stress
Abstract
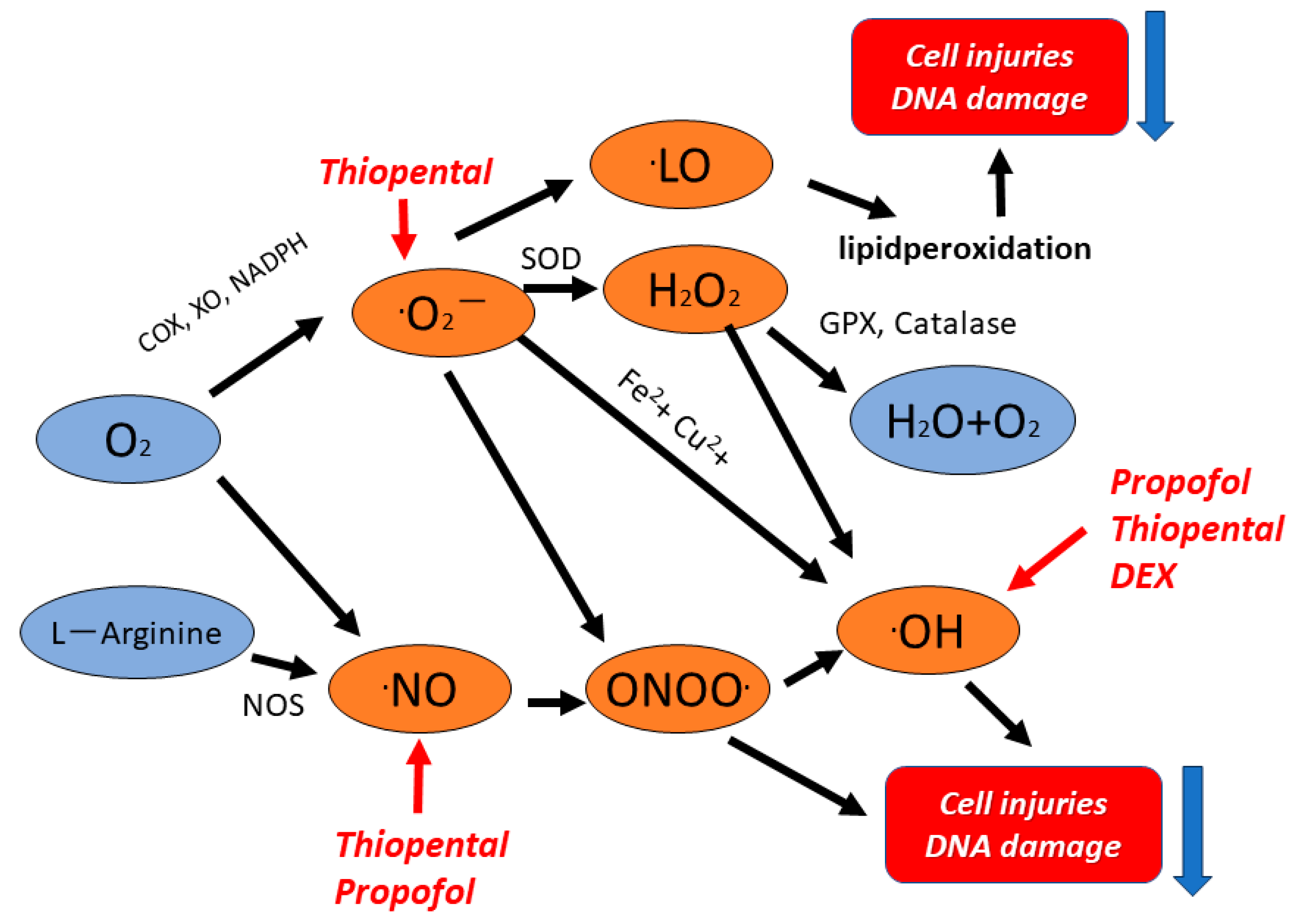
1. Introduction
2. Materials and Methods
2.1. Reagents
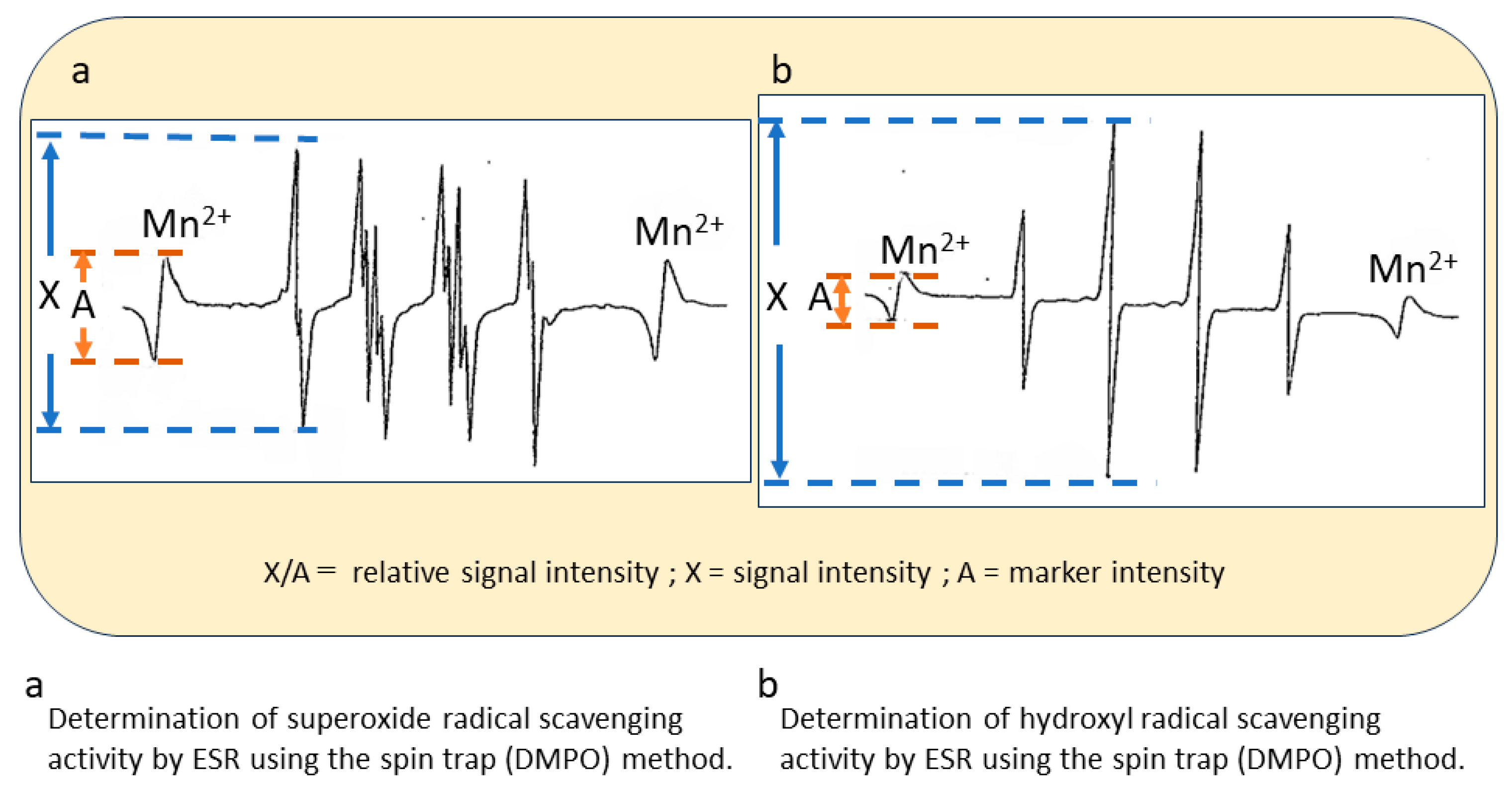
2.2. In Vitro ESR Method
2.3. Statistical Analysis
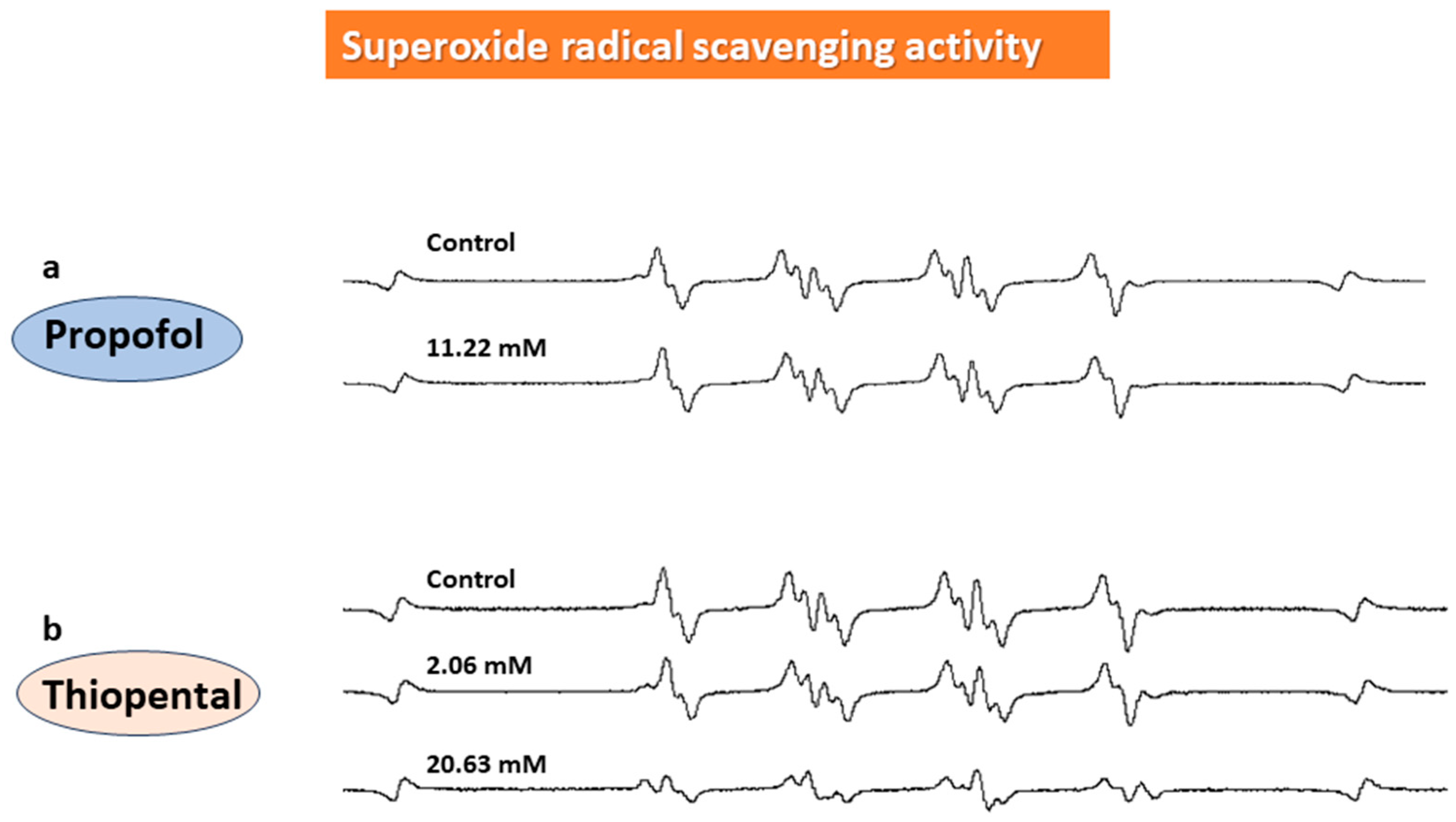
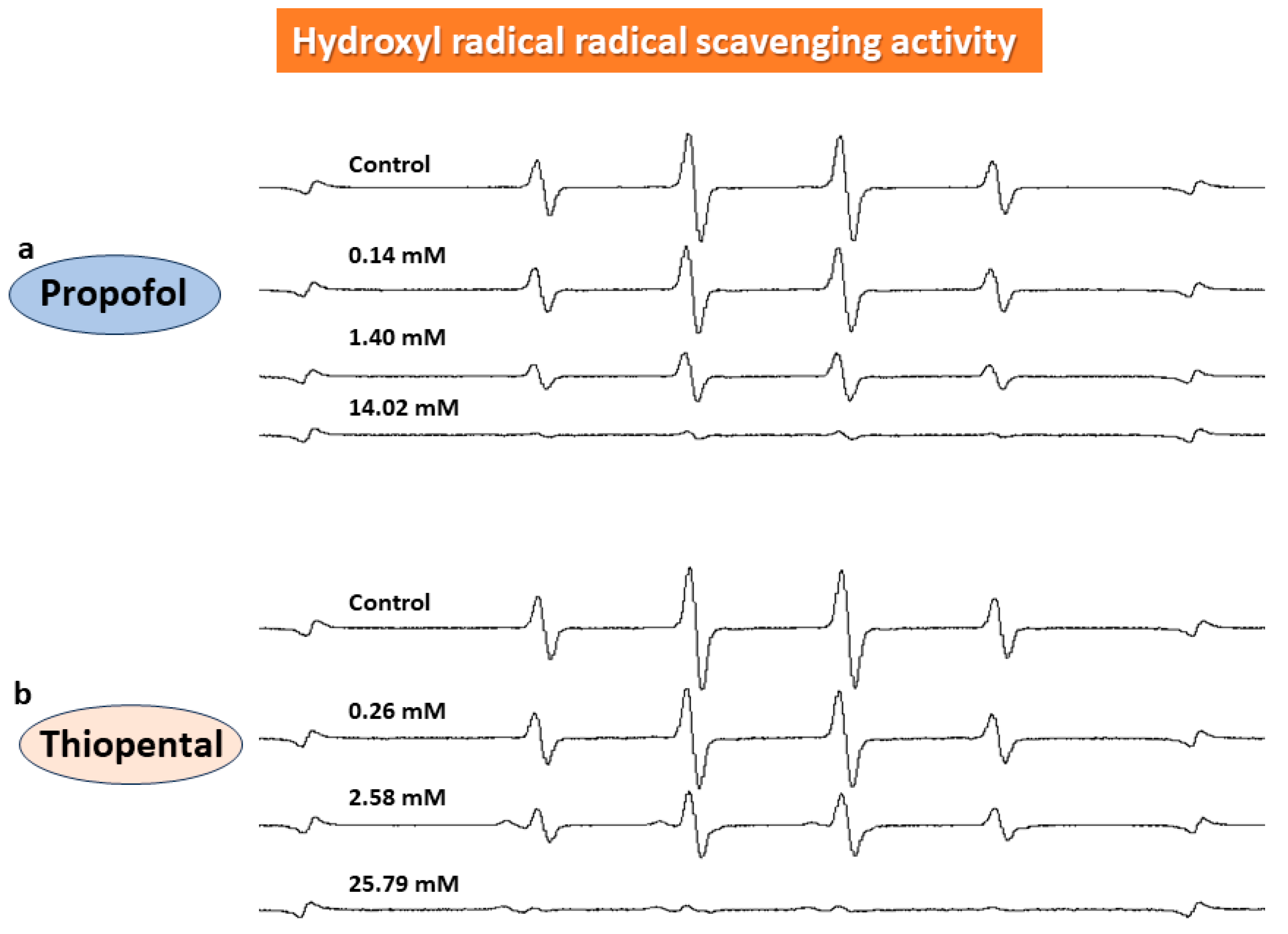
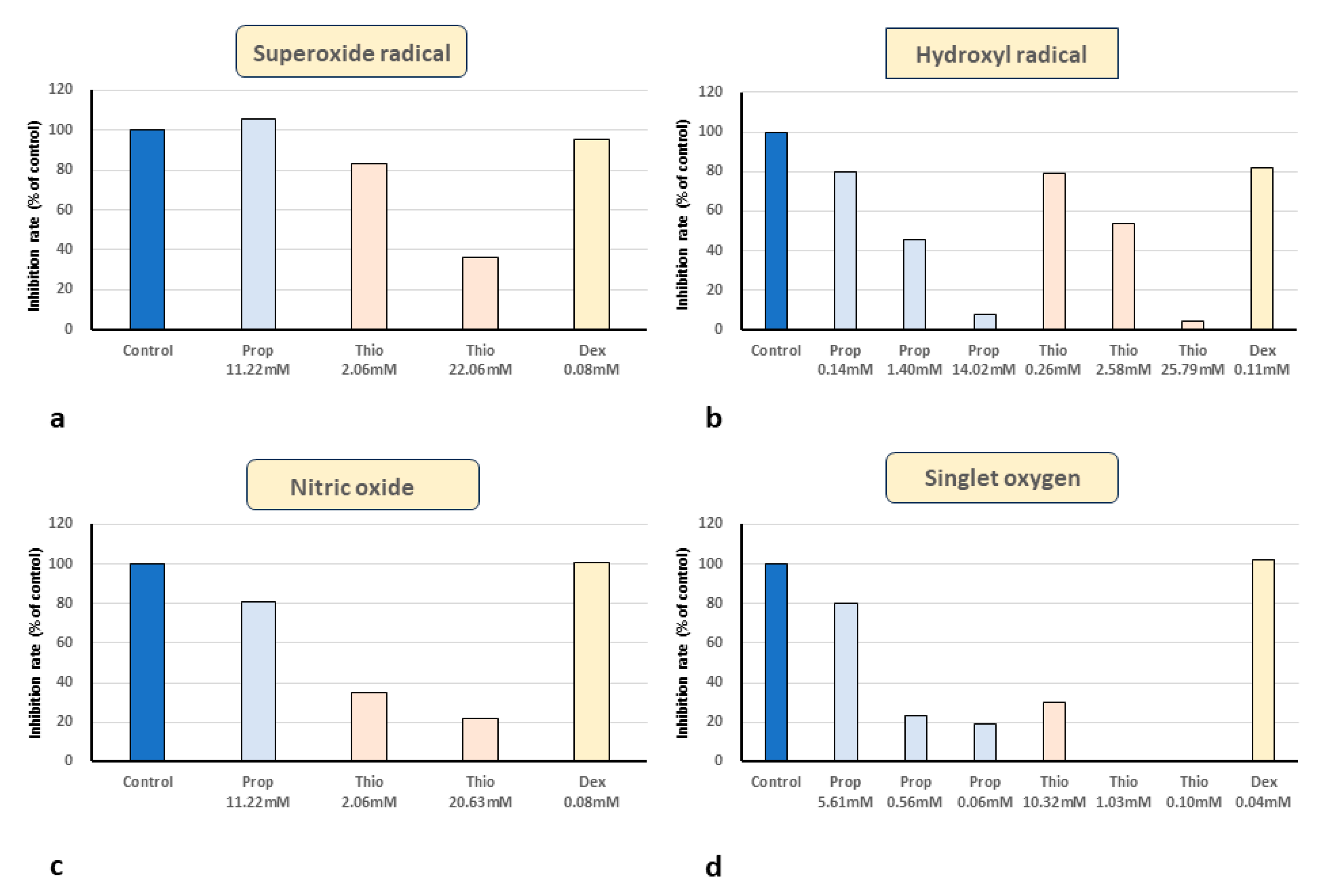
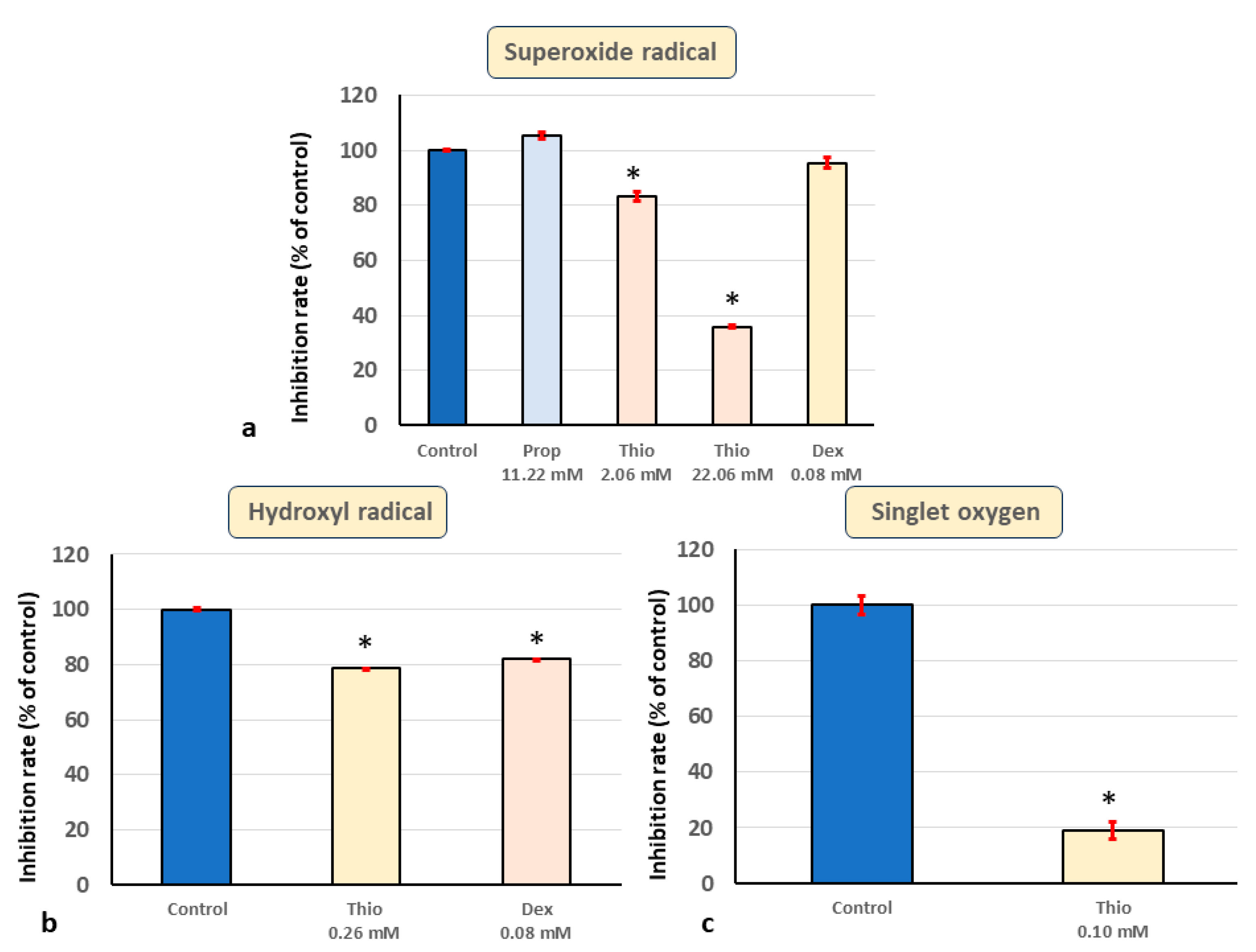
3. Results
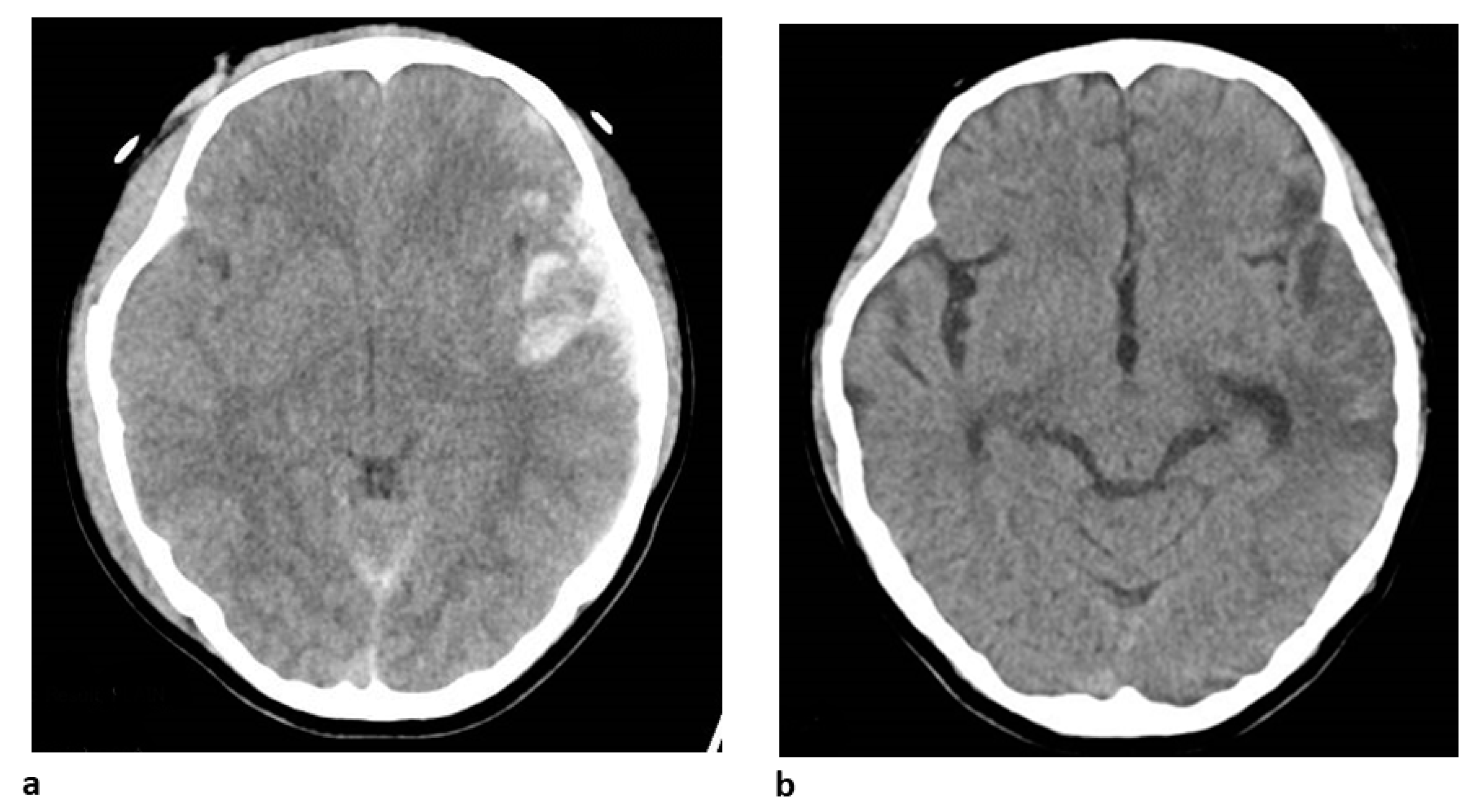
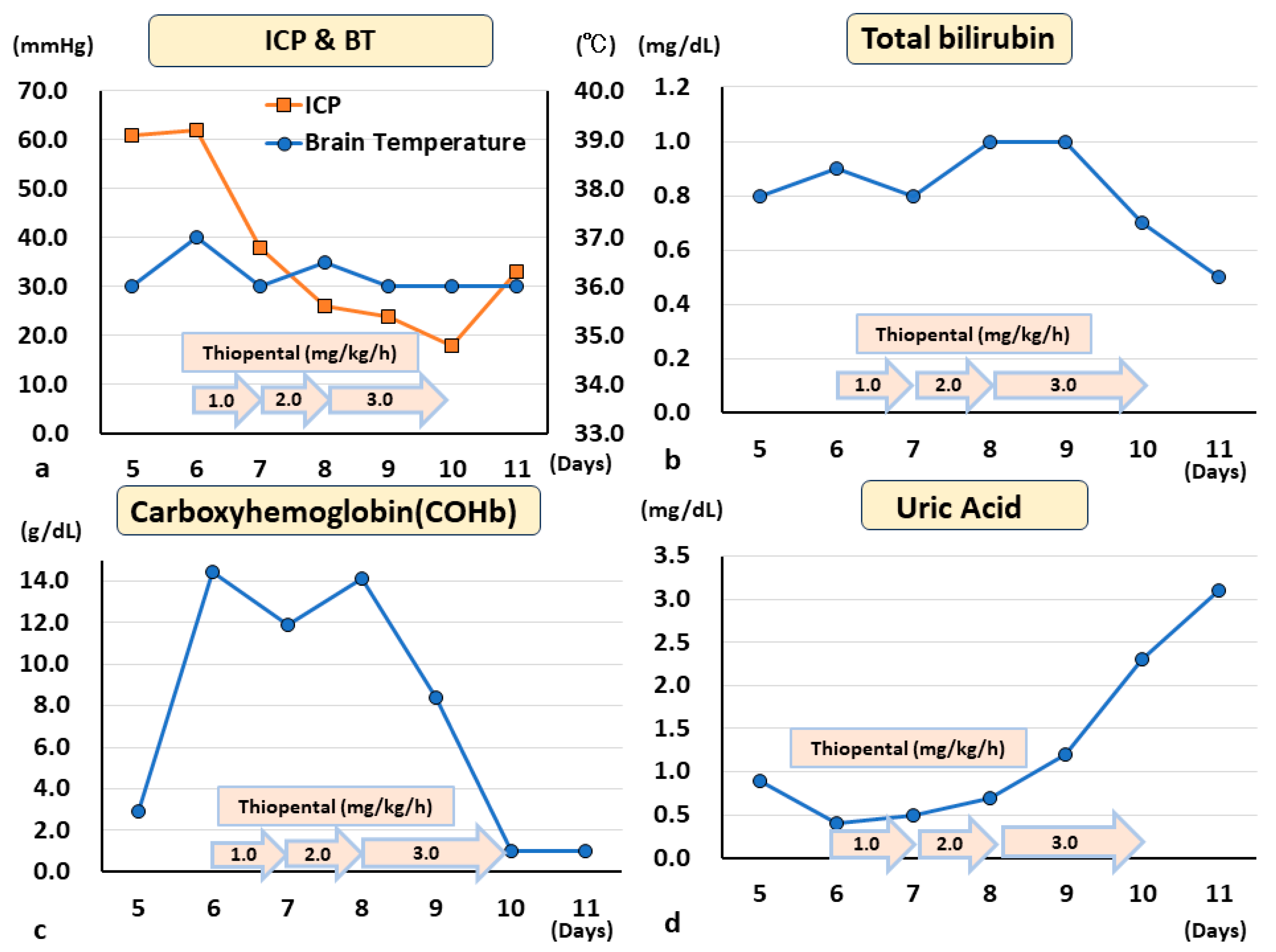
4. Clinical Case
5. Discussion
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jones, D.P. Redefining oxidative stress. Antioxid. Redox Signal. 2006, 8, 1865–1879. [Google Scholar] [CrossRef] [PubMed]
- Dohi, K.; Miyamoto, K.; Fukuda, K.; Nakamura, S.; Hayashi, M.; Ohtaki, H.; Shioda, S.; Aruga, T. Status of systemic oxidative stress during therapeutic hypothermia in patients with post-cardiac arrest syndrome. Oxidative Med. Cell. Longev. 2013, 2013, 562429. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Huynh, K.; Heshu, S.R.; Yeap, S.K.; Hazilawati, H.; Roselina, K. Water extract of brewers’ rice induces apoptosis in human colorectal cancer cells via activation of caspase-3 and caspase-8 and downregulates the Wnt/beta-catenin downstream signaling pathway in brewers’ rice-treated rats with azoxymethane-induced colon carcinogenesis. BMC Complement. Altern. Med. 2015, 15, 205. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxidative Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef] [PubMed]
- Kröncke, K.D.; Fehsel, K.; Kolb-Bachofen, V. Inducible nitric oxide synthase in human diseases. Clin. Exp. Immunol. 1998, 113, 147–156. [Google Scholar] [CrossRef]
- Geller, D.A.; Billiar, T.R. Molecular biology of nitric oxide synthases. Cancer Metastasis Rev. 1998, 17, 7–23. [Google Scholar] [CrossRef]
- Wink, D.A.; Mitchell, J.B. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998, 25, 434–456. [Google Scholar] [CrossRef]
- Dohi, K.; Ohtaki, H.; Inn, R.; Ikeda, Y.; Shioda, H.S.; Aruga, T. Peroxynitrite and caspase-3 expression after ischemia/reperfusion in mouse cardiac arrest model. Acta Neurochir. Suppl. 2003, 86, 87–91. [Google Scholar] [CrossRef]
- Biesalski, H.K.; McGregor, G.P. Antioxidant therapy in critical care--is the microcirculation the primary target? Crit. Care Med. 2007, 35, S577–S583. [Google Scholar] [CrossRef]
- Fratta Pasini, A.M.; Stranieri, C.; Cominacini, L.; Mozzini, C. Potential role of antioxidant and anti-inflammatory therapies to prevent severe SARS-Cov-2 complications. Antioxidants 2021, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Hiedra, R.; Lo, K.B.; Elbashabsheh, M.; Gul, F.; Wright, R.M.; Albano, J.; Azmaiparashvili, Z.; Patarroyo Aponte, G. The use of IV vitamin C for patients with COVID-19: A case series. Expert Rev. Anti Infect. Ther. 2020, 18, 1259–1261. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.X.; Gelb, A.W. Free radicals, antioxidants, and neurologic injury: Possible relationship to cerebral protection by anesthetics. J. Neurosurg. Anesthesiol. 2002, 14, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Ikeda, Y.; Shioda, S.; Tobe, T.; Yoshikawa, T. Edarabone scavenges nitric oxide. Redox Rep. 2002, 7, 219–222. [Google Scholar] [CrossRef]
- Dohi, K.; Satoh, K.; Mihara, Y.; Nakamura, S.; Miyake, Y.; Ohtaki, H.; Nakamachi, T.; Yoshikawa, T.; Shioda, S.; Aruga, T. Alkoxyl radical-scavenging activity of edaravone in patients with traumatic brain injury. J. Neurotrauma 2006, 23, 1591–1599. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ohtaki, H.; Dohi, K.; Tsumuraya, T.; Song, D.; Kiriyama, K.; Satoh, K.; Shimizu, A.; Aruga, T.; Shioda, S. Therapeutic time window for edaravone treatment of traumatic brain injury in mice. Biomed. Res. Int. 2013, 2013, 379206. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ohtaki, H.; Dohi, K.; Tsumuraya, T.; Nakano, H.; Kiriyama, K.; Song, D.; Aruga, T.; Shioda, S. Edaravone increases regional cerebral blood flow after traumatic brain injury in mice. Acta Neurochir. Suppl. 2013, 118, 103–109. [Google Scholar] [CrossRef]
- Dohi, K.; Satoh, K.; Nakamachi, T.; Yofu, S.; Hiratsuka, K.; Nakamura, S.; Ohtaki, H.; Yoshikawa, T.; Shioda, S.; Aruga, T. Does edaravone (MCI- 186) act as an antioxidant and a neuroprotector in experimental traumatic brain injury? Antioxid. Redox Signal. 2007, 9, 281–287. [Google Scholar] [CrossRef]
- Hughes, C.G.; McGrane, S.; Pandharipande, P.P. Sedation in the intensive care setting. Clin. Pharmacol. 2012, 4, 53–63. [Google Scholar] [CrossRef]
- Aarts, L.; van der Hee, R.; Dekker, I.; de Jong, J.; Langemeijer, H.; Bast, A. The widely used anesthetic agent propofol can replace alpha-tocopherol as an antioxidant. FEBS Lett. 1995, 357, 83–85. [Google Scholar] [CrossRef]
- Escamilla-Ocañas, C.E.; Albores-Ibarra, N. Current status and outlook for the management of intracranial hypertension after traumatic brain injury: Decompressive craniectomy, therapeutic hypothermia, and barbiturates. Neurologia 2023, 38, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’Andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free radical properties, source and targets, antioxidant consumption and health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Halliwell, B.; Whiteman, M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br. J. Pharmacol. 2004, 142, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Matsumoto, K.; Dohi, K.; Jimbo, H.; Sasaki, K.; Satoh, K. Direct superoxide scavenging activity of nonsteroidal anti-inflammatory drugs: Determination by electron spin resonance using the spin trap method. Headache 2001, 41, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Jimbo, H.; Shimazu, M.; Satoh, K. Sumatriptan scavenges superoxide, hydroxyl, and nitric oxide radicals: In vitro electron spin resonance study. Headache 2002, 42, 888–892. [Google Scholar] [CrossRef]
- Ao, Y.; Satoh, K.; Shibano, K.; Kawahito, Y.; Shioda, S. Singlet oxygen scavenging activity and cytotoxicity of essential oils from Rutaceae. J. Clin. Biochem. Nutr. 2008, 43, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Dohi, K.; Ohtaki, H.; Nakamachi, T.; Yofu, S.; Satoh, K.; Miyamoto, K.; Song, D.; Tsunawaki, S.; Shioda, S.; Aruga, T. Gp91phox (NOX2) in classically activated microglia exacerbates traumatic brain injury. J. Neuroinflamm. 2010, 7, 41. [Google Scholar] [CrossRef]
- Dohi, K.; Kraemer, B.C.; Erickson, M.A.; McMillan, P.J.; Kovac, A.; Flachbartova, Z.; Hansen, K.M.; Shah, G.N.; Sheibani, N.; Salameh, T.; et al. Molecular hydrogen in drinking water protects against neurodegenerative changes induced by traumatic brain injury. PLoS ONE 2014, 9, e108034. [Google Scholar] [CrossRef]
- Abobaker, A.; Alzwi, A.; Alraied, A.H.A. Overview of the possible role of vitamin C in management of COVID-19. Pharmacol. Rep. 2020, 72, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Mochizuki, Y.; Nakamura, Y.; Dohi, K.; Matsumoto, H.; Jimbo, H.; Hayashi, M.; Matsumoto, K.; Yoshikawa, T.; Murase, H.; et al. Protective effect of a novel vitamin E derivative on experimental traumatic brain edema in rats–preliminary study. Acta Neurochir. Suppl. 2000, 76, 343–345. [Google Scholar] [CrossRef]
- Dohi, K.; Jimbo, H.; Ikeda, Y.; Fujita, S.; Ohtaki, H.; Shioda, S.; Abe, T.; Aruga, T. Pharmacological brain cooling with indomethacin in acute hemorrhagic stroke: Antiinflammatory cytokines and antioxidative effects. Acta Neurochir. Suppl. 2006, 96, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, Y.; Matsumoto, K. Hypothermia in neurological diseases. No Shinkei 2001, 53, 513–524. [Google Scholar]
- Kurz, M.A.; Boyer, T.D.; Whalen, R.; Peterson, T.E.; Harrison, D.G. Nitroglycerin metabolism in vascular tissue: Role of glutathione S-transferases and relationship between NO. and NO2− formation. Biochem. J. 1993, 292 Pt 2, 545–550. [Google Scholar] [CrossRef]
- Harrison, D.G.; Bates, J.N. The nitrovasodilators. New ideas about old drugs. Circulation 1993, 87, 1461–1467. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, I.; Lue, T.F.; Padma-Nathan, H.; Rosen, R.C.; Steers, W.D.; Wicker, P.A. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil study group. N. Engl. J. Med. 1998, 338, 1397–1404. [Google Scholar] [CrossRef]
- Akaike, T.; Maeda, H. Quantitation of nitric oxide using 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide (PTIO). Methods Enzymol. 1996, 268, 211–221. [Google Scholar] [CrossRef]
- Marik, P.E.; Kory, P.; Varon, J.; Iglesias, J.; Meduri, G.U. MATH+ protocol for the treatment of SARS-CoV-2 infection: The scientific rationale. Expert Rev. Anti Infect. Ther. 2021, 19, 129–135. [Google Scholar] [CrossRef]
- Kondo, F.; Kondo, Y.; Gómez-Vargas, M.; Ogawa, N. Indomethacin inhibits delayed DNA fragmentation of hippocampal CA1 pyramidal neurons after transient forebrain ischemia in gerbils. Brain Res. 1998, 791, 352–356. [Google Scholar] [CrossRef]
- Han, C.; Ding, W.; Jiang, W.; Chen, Y.U.; Hang, D.; Gu, D.; Jiang, G.; Tan, Y.; Ge, Z.; Ma, T. A comparison of the effects of midazolam, propofol and dexmedetomidine on the antioxidant system: A randomized trial. Exp. Ther. Med. 2015, 9, 2293–2298. [Google Scholar] [CrossRef][Green Version]
- Akpinar, H.; Nazıroğlu, M.; Övey, İ.S.; Çiğ, B.; Akpinar, O. The neuroprotective action of dexmedetomidine on apoptosis, calcium entry and oxidative stress in cerebral ischemia-induced rats: Contribution of TRPM2 and TRPV1 channels. Sci. Rep. 2016, 6, 37196. [Google Scholar] [CrossRef] [PubMed]
- Unchiti, K.; Leurcharusmee, P.; Samerchua, A.; Pipanmekaporn, T.; Chattipakorn, N.; Chattipakorn, S.C. The potential role of dexmedetomidine on neuroprotection and its possible mechanisms: Evidence from in vitro and in vivo studies. Eur. J. Neurosci. 2021, 54, 7006–7047. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Xu, H.; Yan, J.; Zhang, L.; Lu, Y. Molecular targets and mechanism of action of dexmedetomidine in treatment of ischemia/reperfusion injury. Mol. Med. Rep. 2014, 9, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Kose, E.A.; Bakar, B.; Kasimcan, O.; Atilla, P.; Kilinc, K.; Muftuoglu, S.; Apan, A. Effects of intracisternal and intravenous dexmedetomidine on ischemia-induced brain injury in rat: A comparative study. Turk. Neurosurg. 2013, 23, 208–217. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shapiro, H.M.; Wyte, S.R.; Loeser, J. Barbiturate-augmented hypothermia for reduction of persistent intracranial hypertension. J. Neurosurg. 1974, 40, 90–100. [Google Scholar] [CrossRef]
- Bricolo, A.P.; Glick, R.P. Barbiturate effects on acute experimental intracranial hypertension. J. Neurosurg. 1981, 55, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef]
- Smith, D.S.; Rehncrona, S.; Siesjö, B.K. Inhibitory effects of different barbiturates on lipid peroxidation in brain tissue in vitro: Comparison with the effects of promethazine and chlorpromazine. Anesthesiology 1980, 53, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.G.; Davies, M.J.; Columb, M.O.; Stratford, N. Effect of propofol and thiopentone on free radical mediated oxidative stress of the erythrocyte. Br. J. Anaesth. 1996, 76, 536–543. [Google Scholar] [CrossRef]
- Lee, J.Y. Oxidative stress due to anesthesia and surgical trauma and comparison of the effects of propofol and thiopental in dogs. J. Vet. Med. Sci. 2012, 74, 663–665. [Google Scholar] [CrossRef]
- Léger, M.; Frasca, D.; Roquilly, A.; Seguin, P.; Cinotti, R.; Dahyot-Fizelier, C.; Asehnoune, K.; Le Borgne, F.; Gaillard, T.; Foucher, Y.; et al. Early use of barbiturates is associated with increased mortality in traumatic brain injury patients from a propensity score-based analysis of a prospective cohort. PLoS ONE 2022, 17, e0268013. [Google Scholar] [CrossRef]
- Kajiwara, S.; Hasegawa, Y.; Negoto, T.; Orito, K.; Kawano, T.; Yoshitomi, M.; Sakata, K.; Takeshige, N.; Yamakawa, Y.; Jono, H.; et al. Efficacy of a novel prophylactic barbiturate therapy for severe traumatic brain injuries: Step-down infusion of a barbiturate with normothermia. Neurol. Med. Chir. 2021, 61, 528–535. [Google Scholar] [CrossRef]
- Yamakawa, Y.; Morioka, M.; Negoto, T.; Orito, K.; Yoshitomi, M.; Nakamura, Y.; Takeshige, N.; Yamamoto, M.; Takeuchi, Y.; Oda, K.; et al. A novel step-down infusion method of barbiturate therapy: Its safety and effectiveness for intracranial pressure control. Pharmacol. Res. Perspect. 2021, 9, e00719. [Google Scholar] [CrossRef] [PubMed]
- Loop, T.; Humar, M.; Pischke, S.; Hoetzel, A.; Schmidt, R.; Pahl, H.L.; Geiger, K.K.; Pannen, B.H. Thiopental inhibits tumor necrosis factor alpha-induced activation of nuclear factor kappaB through suppression of kappaB kinase activity. Anesthesiology 2003, 99, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Stover, J.F.; Stocker, R. Barbiturate coma may promote reversible bone marrow suppression in patients with severe isolated traumatic brain injury. Eur. J. Clin. Pharmacol. 1998, 54, 529–534. [Google Scholar] [CrossRef]
- Barbagallo, I.; Galvano, F.; Frigiola, A.; Cappello, F.; Riccioni, G.; Murabito, P.; D’Orazio, N.; Torella, M.; Gazzolo, D.; Li Volti, G. Potential therapeutic effects of natural heme oxygenase-1 inducers in cardiovascular diseases. Antioxid. Redox Signal. 2013, 18, 507–521. [Google Scholar] [CrossRef]
- Dohi, K.; Mochizuki, Y.; Satoh, K.; Jimbo, H.; Hayashi, M.; Toyoda, I.; Ikeda, Y.; Abe, T.; Aruga, T. Transient elevation of serum bilirubin (a heme oxygenase-1 metabolite) level in hemorrhagic stroke: Bilirubin is a marker of oxidant stress. Acta Neurochir. Suppl. 2003, 86, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Yisireyili, M.; Hayashi, M.; Wu, H.; Uchida, Y.; Yamamoto, K.; Kikuchi, R.; Shoaib Hamrah, M.; Nakayama, T.; Wu Cheng, X.; Matsushita, T.; et al. Xanthine oxidase inhibition by febuxostat attenuates stress-induced hyperuricemia, glucose dysmetabolism, and prothrombotic state in mice. Sci. Rep. 2017, 7, 1266. [Google Scholar] [CrossRef] [PubMed]
- Drummond, G.S.; Baum, J.; Greenberg, M.; Lewis, D.; Abraham, N.G. HO-1 overexpression and underexpression: Clinical implications. Arch. Biochem. Biophys. 2019, 673, 108073. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Liu, H.; Liu, J.; Zhao, H.; Wang, H. Is serum total bilirubin a predictor of prognosis in arteriosclerotic cardiovascular disease? A meta-analysis. Medicine 2019, 98, e17544. [Google Scholar] [CrossRef]
- Schizodimos, T.; Soulountsi, V.; Iasonidou, C.; Kapravelos, N. An overview of management of intracranial hypertension in the intensive care unit. J. Anesth. 2020, 34, 741–757. [Google Scholar] [CrossRef]
- Vassalle, C.; Maltinti, M.; Sabatino, L. Targeting oxidative stress for disease prevention and therapy: Where do we stand, and where do we go from here. Molecules 2020, 25, 2653. [Google Scholar] [CrossRef]
- Tanaka, M.; Vecsei, L. Monitoring the redox status in multiple sclerosis. Biomedicines 2020, 8, 406. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, G.; Ohtaki, Y.; Satoh, K.; Odanaka, Y.; Katoh, A.; Suzuki, K.; Tomita, Y.; Eiraku, M.; Kikuchi, K.; Harano, K.; et al. Sedation Therapy in Intensive Care Units: Harnessing the Power of Antioxidants to Combat Oxidative Stress. Biomedicines 2023, 11, 2129. https://doi.org/10.3390/biomedicines11082129
Inoue G, Ohtaki Y, Satoh K, Odanaka Y, Katoh A, Suzuki K, Tomita Y, Eiraku M, Kikuchi K, Harano K, et al. Sedation Therapy in Intensive Care Units: Harnessing the Power of Antioxidants to Combat Oxidative Stress. Biomedicines. 2023; 11(8):2129. https://doi.org/10.3390/biomedicines11082129
Chicago/Turabian StyleInoue, Gen, Yuhei Ohtaki, Kazue Satoh, Yuki Odanaka, Akihito Katoh, Keisuke Suzuki, Yoshitake Tomita, Manabu Eiraku, Kazuki Kikuchi, Kouhei Harano, and et al. 2023. "Sedation Therapy in Intensive Care Units: Harnessing the Power of Antioxidants to Combat Oxidative Stress" Biomedicines 11, no. 8: 2129. https://doi.org/10.3390/biomedicines11082129
APA StyleInoue, G., Ohtaki, Y., Satoh, K., Odanaka, Y., Katoh, A., Suzuki, K., Tomita, Y., Eiraku, M., Kikuchi, K., Harano, K., Yagi, M., Uchida, N., & Dohi, K. (2023). Sedation Therapy in Intensive Care Units: Harnessing the Power of Antioxidants to Combat Oxidative Stress. Biomedicines, 11(8), 2129. https://doi.org/10.3390/biomedicines11082129