The Role of the IGF2 Methylation Score in Diagnosing Adrenocortical Tumors with Unclear Malignant Potential—Feasibility of Formalin-Fixed Paraffin-Embedded Tissue
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Case Series of ACT with Unclear Malignant Potential
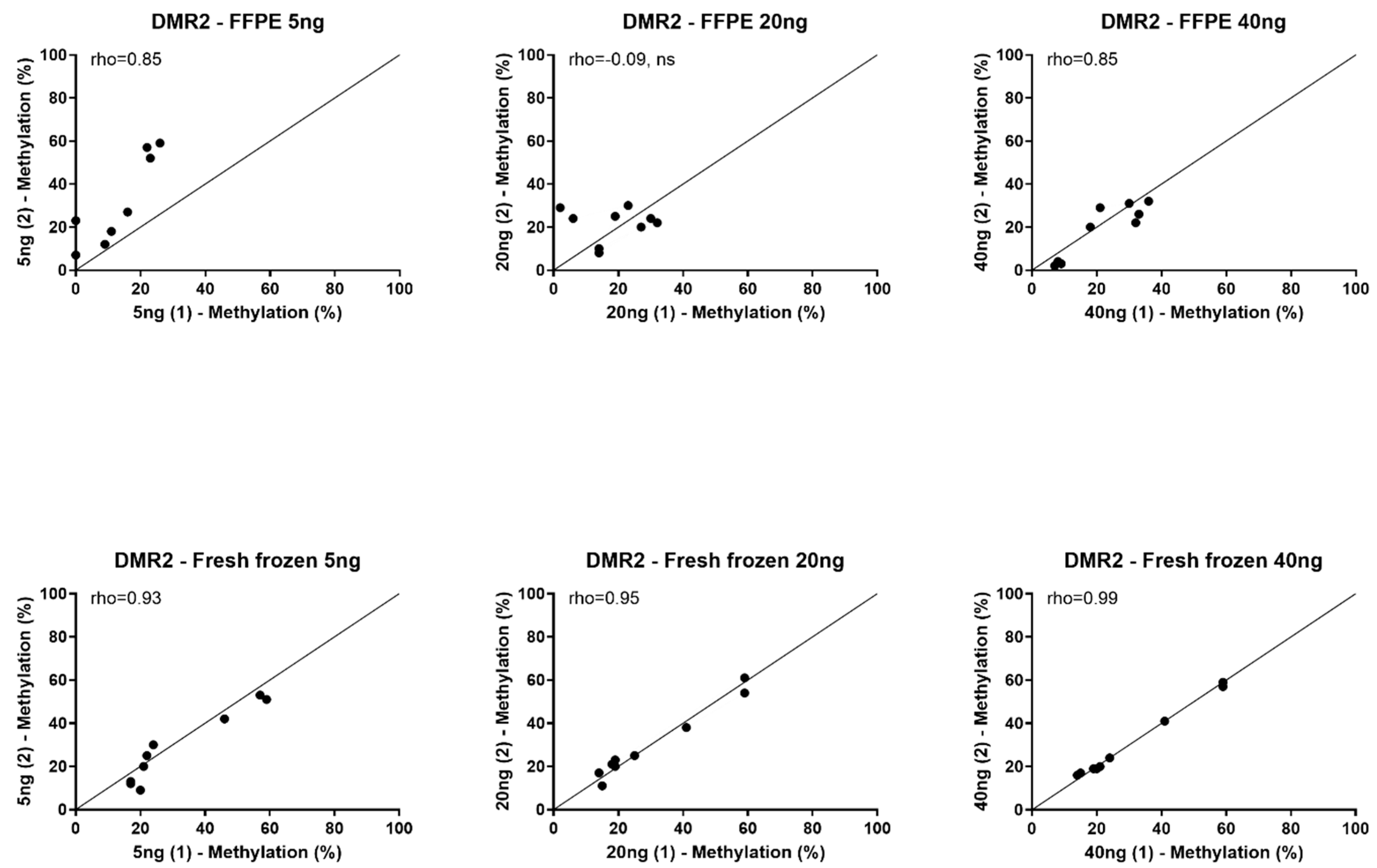
3.2. Feasibility of Using FFPE Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fassnacht, M.; Dekkers, O.; Else, T.; Baudin, E.; Berruti, A.; de Krijger, R.R.; Haak, H.R.; Mihai, R.; Assie, G.; Terzolo, M. European Society of Endocrinology Clinical Practice Guidelines on the Management of Adrenocortical Carcinoma in Adults, in Collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018, 179, G1–G46. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; de la Fouchardiere, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A.; et al. Adrenocortical Carcinomas and Malignant Phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of Adrenal Incidentalomas: European Society of Endocrinology Clinical Practice Guideline in Collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Bancos, I.; Ferrante di Ruffano, L.; Chortis, V.; Davenport, C.; Bayliss, S.; Sahdev, A.; Guest, P.; Fassnacht, M.; Deeks, J.J.; et al. MANAGEMENT OF ENDOCRINE DISEASE: Imaging for the Diagnosis of Malignancy in Incidentally Discovered Adrenal Masses: A Systematic Review and Meta-Analysis. Eur. J. Endocrinol. 2016, 175, 51. [Google Scholar] [CrossRef] [PubMed]
- Petersenn, S.; Richter, P.A.; Broemel, T.; Ritter, C.O.; Deutschbein, T.; Beil, F.U.; Allolio, B.; Fassnacht, M.; German ACC Study Group. Computed Tomography Criteria for Discrimination of Adrenal Adenomas and Adrenocortical Carcinomas: Analysis of the German ACC Registry. Eur. J. Endocrinol. 2015, 172, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Bancos, I.; Taylor, A.E.; Chortis, V.; Sitch, A.J.; Jenkinson, C.; Davidge-Pitts, C.J.; Lang, K.; Tsagarakis, S.; Macech, M.; Riester, A.; et al. Urine Steroid Metabolomics for the Differential Diagnosis of Adrenal Incidentalomas in the EURINE-ACT Study: A Prospective Test Validation Study. Lancet Diabetes Endocrinol. 2020, 8, 773–781. [Google Scholar] [CrossRef]
- Tissier, F.; Aubert, S.; Leteurtre, E.; Al Ghuzlan, A.; Patey, M.; Decaussin, M.; Doucet, L.; Gobet, F.; Hoang, C.; Mazerolles, C.; et al. Adrenocortical Tumors: Improving the Practice of the Weiss System through Virtual Microscopy: A National Program of the French Network INCa-COMETE. Am. J. Surg. Pathol. 2012, 36, 1194–1201. [Google Scholar] [CrossRef]
- Jouinot, A.; Bertherat, J. MANAGEMENT OF ENDOCRINE DISEASE: Adrenocortical Carcinoma: Differentiating the Good from the Poor Prognosis Tumors. Eur. J. Endocrinol. 2018, 178, R215–R230. [Google Scholar] [CrossRef]
- Lippert, J.; Fassnacht, M.; Ronchi, C.L. The Role of Molecular Profiling in Adrenocortical Carcinoma. Clin. Endocrinol. 2022, 97, 460–472. [Google Scholar] [CrossRef]
- Tabano, S.; Colapietro, P.; Cetin, I.; Grati, F.R.; Zanutto, S.; Mando, C.; Antonazzo, P.; Pileri, P.; Rossella, F.; Larizza, L.; et al. Epigenetic Modulation of the IGF2/H19 Imprinted Domain in Human Embryonic and Extra-Embryonic Compartments and its Possible Role in Fetal Growth Restriction. Epigenetics 2010, 5, 313–324. [Google Scholar] [CrossRef]
- Creemers, S.G.; van Koetsveld, P.M.; van Kemenade, F.J.; Papathomas, T.G.; Franssen, G.J.; Dogan, F.; Eekhoff, E.M.; van der Valk, P.; de Herder, W.W.; Janssen, J.A.; et al. Methylation of IGF2 Regulatory Regions to Diagnose Adrenocortical Carcinomas. Endocr. Relat. Cancer 2016, 23, 727–737. [Google Scholar] [CrossRef]
- Creemers, S.G.; Feelders, R.A.; Valdes, N.; Ronchi, C.L.; Volante, M.; van Hemel, B.M.; Luconi, M.; Ettaieb, M.H.T.; Mannelli, M.; Chiara, M.D.; et al. The IGF2 Methylation Score for Adrenocortical Cancer: An ENSAT Validation Study. Endocr. Relat. Cancer 2020, 27, 541–550. [Google Scholar] [CrossRef]
- Ettaieb, M.H.; Duker, J.C.; Feelders, R.A.; Corssmit, E.P.; Menke-van der Houven van Oordt, C.W.; Timmers, H.J.; Kerstens, M.N.; Wilmink, J.W.; Zelissen, P.M.; Havekes, B.; et al. Synchronous Vs. Metachronous Metastases in Adrenocortical Carcinoma: An Analysis of the Dutch Adrenal Network. Horm. Cancer 2016, 7, 336–344. [Google Scholar] [CrossRef]
- Glenn, J.A.; Else, T.; Hughes, D.T.; Cohen, M.S.; Jolly, S.; Giordano, T.J.; Worden, F.P.; Gauger, P.G.; Hammer, G.D.; Miller, B.S. Longitudinal Patterns of Recurrence in Patients with Adrenocortical Carcinoma. Surgery 2019, 165, 186–195. [Google Scholar] [CrossRef]
- Gicquel, C.; Bertagna, X.; Gaston, V.; Coste, J.; Louvel, A.; Baudin, E.; Bertherat, J.; Chapuis, Y.; Duclos, J.M.; Schlumberger, M.; et al. Molecular Markers and Long-Term Recurrences in a Large Cohort of Patients with Sporadic Adrenocortical Tumors. Cancer Res. 2001, 61, 6762–6767. [Google Scholar]
- Muth, A.; Taft, C.; Hammarstedt, L.; Bjorneld, L.; Hellstrom, M.; Wangberg, B. Patient-Reported Impacts of a Conservative Management Programme for the Clinically Inapparent Adrenal Mass. Endocrine 2013, 44, 228–236. [Google Scholar] [CrossRef]
- Wu, J.; Salva, K.A.; Stutz, N.; Longley, B.J.; Spiegelman, V.S.; Wood, G.S. Quantitative Gene Analysis of Methylation and Expression (Q-GAME) in Fresh Or Fixed Cells and Tissues. Exp. Dermatol. 2014, 23, 304–309. [Google Scholar] [CrossRef]
- Leong, K.J.; James, J.; Wen, K.; Taniere, P.; Morton, D.G.; Bach, S.P.; Matthews, G.M. Impact of Tissue Processing, Archiving and Enrichment Techniques on DNA Methylation Yield in Rectal Carcinoma. Exp. Mol. Pathol. 2013, 95, 343–349. [Google Scholar] [CrossRef]
- Jeschke, J.; Bizet, M.; Desmedt, C.; Calonne, E.; Dedeurwaerder, S.; Garaud, S.; Koch, A.; Larsimont, D.; Salgado, R.; Van den Eynden, G.; et al. DNA Methylation-Based Immune Response Signature Improves Patient Diagnosis in Multiple Cancers. J. Clin. Investig. 2017, 127, 3090–3102. [Google Scholar] [CrossRef]
- Song, Y.S.; Kim, Y.; Cho, N.Y.; Yang, H.K.; Kim, W.H.; Kang, G.H. Methylation Status of Long Interspersed Element-1 in Advanced Gastric Cancer and its Prognostic Implication. Gastric Cancer 2016, 19, 98–106. [Google Scholar] [CrossRef]
- Min, J.; Choi, B.; Han, T.S.; Lee, H.J.; Kong, S.H.; Suh, Y.S.; Kim, T.H.; Choe, H.N.; Kim, W.H.; Hur, K.; et al. Methylation Levels of LINE-1 as a Useful Marker for Venous Invasion in both FFPE and Frozen Tumor Tissues of Gastric Cancer. Mol. Cells 2017, 40, 346–354. [Google Scholar] [PubMed]
- Wen, X.; Jeong, S.; Kim, Y.; Bae, J.M.; Cho, N.Y.; Kim, J.H.; Kang, G.H. Improved Results of LINE-1 Methylation Analysis in Formalin-Fixed, Paraffin-Embedded Tissues with the Application of a Heating Step during the DNA Extraction Process. Clin. Epigenetics 2017, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Quillien, V.; Lavenu, A.; Ducray, F.; Joly, M.O.; Chinot, O.; Fina, F.; Sanson, M.; Carpentier, C.; Karayan-Tapon, L.; Rivet, P.; et al. Validation of the High-Performance of Pyrosequencing for Clinical MGMT Testing on a Cohort of Glioblastoma Patients from a Prospective Dedicated Multicentric Trial. Oncotarget 2016, 7, 61916–61929. [Google Scholar] [CrossRef] [PubMed]
- Mikeska, T.; Bock, C.; El-Maarri, O.; Hubner, A.; Ehrentraut, D.; Schramm, J.; Felsberg, J.; Kahl, P.; Buttner, R.; Pietsch, T.; et al. Optimization of Quantitative MGMT Promoter Methylation Analysis using Pyrosequencing and Combined Bisulfite Restriction Analysis. J. Mol. Diagn. 2007, 9, 368–381. [Google Scholar] [CrossRef]
- Tournier, B.; Chapusot, C.; Courcet, E.; Martin, L.; Lepage, C.; Faivre, J.; Piard, F. Why do Results Conflict regarding the Prognostic Value of the Methylation Status in Colon Cancers? the Role of the Preservation Method. BMC Cancer 2012, 12, 12. [Google Scholar] [CrossRef]
- Lattanzio, L.; Borgognone, M.; Mocellini, C.; Giordano, F.; Favata, E.; Fasano, G.; Vivenza, D.; Monteverde, M.; Tonissi, F.; Ghiglia, A.; et al. MGMT Promoter Methylation and Glioblastoma: A Comparison of Analytical Methods and of Tumor Specimens. Int. J. Biol. Markers 2015, 30, 208. [Google Scholar] [CrossRef]
- Bak, S.T.; Staunstrup, N.H.; Starnawska, A.; Daugaard, T.F.; Nyengaard, J.R.; Nyegaard, M.; Borglum, A.; Mors, O.; Dorph-Petersen, K.A.; Nielsen, A.L. Evaluating the Feasibility of DNA Methylation Analyses using Long-Term Archived Brain Formalin-Fixed Paraffin-Embedded Samples. Mol. Neurobiol. 2018, 55, 668–681. [Google Scholar] [CrossRef]
- Reuter, C.; Preece, M.; Banwait, R.; Boer, S.; Cuzick, J.; Lorincz, A.; Nedjai, B. Consistency of the S5 DNA Methylation Classifier in Formalin-Fixed Biopsies Versus Corresponding Exfoliated Cells for the Detection of Pre-Cancerous Cervical Lesions. Cancer Med. 2021, 10, 2668–2679. [Google Scholar] [CrossRef]
- Yu, C.; Dugue, P.; Dowty, J.G.; Hammet, F.; Joo, J.E.; Wong, E.M.; Hosseinpour, M.; Giles, G.G.; Hopper, J.L.; Nguyen-Dumont, T.; et al. Repeatability of Methylation Measures using a QIAseq Targeted Methyl Panel and Comparison with the Illumina HumanMethylation450 Assay. BMC Res. Notes 2021, 14, 394. [Google Scholar] [CrossRef]
- de Ruijter, T.C.; de Hoon, J.P.; Slaats, J.; de Vries, B.; Janssen, M.J.; van Wezel, T.; Aarts, M.J.; van Engeland, M.; Tjan-Heijnen, V.C.; Van Neste, L.; et al. Formalin-Fixed, Paraffin-Embedded (FFPE) Tissue Epigenomics using Infinium HumanMethylation450 BeadChip Assays. Lab. Investig. 2015, 95, 833–842. [Google Scholar] [CrossRef]
- Ohara, K.; Arai, E.; Takahashi, Y.; Fukamachi, Y.; Ito, N.; Maeshima, A.M.; Fujimoto, H.; Yoshida, T.; Kanai, Y. Feasibility of Methylome Analysis using Small Amounts of Genomic DNA from Formalin-Fixed Paraffin-Embedded Tissue. Pathol. Int. 2018, 68, 633–635. [Google Scholar] [CrossRef]
- Kerachian, M.A.; Javadmanesh, A.; Azghandi, M.; Mojtabanezhad Shariatpanahi, A.; Yassi, M.; Shams Davodly, E.; Talebi, A.; Khadangi, F.; Soltani, G.; Hayatbakhsh, A.; et al. Crosstalk between DNA Methylation and Gene Expression in Colorectal Cancer, a Potential Plasma Biomarker for Tracing this Tumor. Sci. Rep. 2020, 10, 2813. [Google Scholar] [CrossRef]
- Yokoyama, S.; Iwaya, H.; Akahane, T.; Hamada, T.; Higashi, M.; Hashimoto, S.; Tanoue, S.; Ohtsuka, T.; Ido, A.; Tanimoto, A. Sequential Evaluation of MUC Promoter Methylation using Next-Generation Sequencing-Based Custom-made Panels in Liquid-Based Cytology Specimens of Pancreatic Cancer. Diagn. Cytopathol. 2022, 50, 499–507. [Google Scholar] [CrossRef]
- Espinal, A.C.; Wang, D.; Yan, L.; Liu, S.; Tang, L.; Hu, Q.; Morrison, C.D.; Ambrosone, C.B.; Higgins, M.J.; Sucheston-Campbell, L.E. A Methodological Study of Genome-Wide DNA Methylation Analyses using Matched Archival Formalin-Fixed Paraffin Embedded and Fresh Frozen Breast Tumors. Oncotarget 2017, 8, 14821–14829. [Google Scholar] [CrossRef]
- Moran, B.; Das, S.; Smeets, D.; Peutman, G.; Klinger, R.; Fender, B.; Connor, K.; Ebert, M.; Gaiser, T.; Prehn, J.H.; et al. Assessment of Concordance between Fresh-Frozen and Formalin-Fixed Paraffin Embedded Tumor DNA Methylation using a Targeted Sequencing Approach. Oncotarget 2017, 8, 48126–48137. [Google Scholar] [CrossRef]
- Jasmine, F.; Rahaman, R.; Roy, S.; Raza, M.; Paul, R.; Rakibuz-Zaman, M.; Paul-Brutus, R.; Dodsworth, C.; Kamal, M.; Ahsan, H.; et al. Interpretation of Genome-Wide Infinium Methylation Data from Ligated DNA in Formalin-Fixed, Paraffin-Embedded Paired Tumor and Normal Tissue. BMC Res. Notes 2012, 5, 117. [Google Scholar] [CrossRef]
- Hamilton, M.G.; Roldan, G.; Magliocco, A.; McIntyre, J.B.; Parney, I.; Easaw, J.C. Determination of the Methylation Status of MGMT in Different Regions within Glioblastoma Multiforme. J. Neurooncol. 2011, 102, 255–260. [Google Scholar] [CrossRef]
- Garcia-Gimenez, J.L.; Mena-Molla, S.; Beltran-Garcia, J.; Sanchis-Gomar, F. Challenges in the Analysis of Epigenetic Biomarkers in Clinical Samples. Clin. Chem. Lab. Med. 2017, 55, 1474–1477. [Google Scholar] [CrossRef]
- Srinivasan, M.; Sedmak, D.; Jewell, S. Effect of Fixatives and Tissue Processing on the Content and Integrity of Nucleic Acids. Am. J. Pathol. 2002, 161, 1961–1971. [Google Scholar] [CrossRef]
- Senguven, B.; Baris, E.; Oygur, T.; Berktas, M. Comparison of Methods for the Extraction of DNA from Formalin-Fixed, Paraffin-Embedded Archival Tissues. Int. J. Med. Sci. 2014, 11, 494–499. [Google Scholar] [CrossRef]
- Potluri, K.; Mahas, A.; Kent, M.N.; Naik, S.; Markey, M. Genomic DNA Extraction Methods using Formalin-Fixed Paraffin-Embedded Tissue. Anal. Biochem. 2015, 486, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.; Davidsson, S.; Fridfeldt, J.; Giunchi, F.; Fiano, V.; Grasso, C.; Zelic, R.; Richiardi, L.; Andren, O.; Pettersson, A.; et al. Quantity and Quality of Nucleic Acids Extracted from Archival Formalin Fixed Paraffin Embedded Prostate Biopsies. BMC Med. Res. Methodol. 2018, 18, 161. [Google Scholar] [CrossRef] [PubMed]
- Duregon, E.; Volante, M.; Rapa, I.; Vatrano, S.; Papotti, M. Dissecting Morphological and Molecular Heterogeneity in Adrenocortical Carcinoma. Turk. Patoloji Derg. 2015, 31 (Suppl. S1), 98–104. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Q.; Li, J.; Tan, A.Y.; Vedururu, R.; Pang, J.M.; Do, H.; Ellul, J.; Doig, K.; Bell, A.; MacArthur, G.A.; et al. Sequence Artefacts in a Prospective Series of Formalin-Fixed Tumours Tested for Mutations in Hotspot Regions by Massively Parallel Sequencing. BMC Med. Genom. 2014, 7, 23. [Google Scholar] [CrossRef]
- Do, H.; Dobrovic, A. Sequence Artifacts in DNA from Formalin-Fixed Tissues: Causes and Strategies for Minimization. Clin. Chem. 2015, 61, 64–71. [Google Scholar] [CrossRef]
- Kerkhofs, T.M.; Verhoeven, R.H.; Bonjer, H.J.; van Dijkum, E.J.; Vriens, M.R.; De Vries, J.; Van Eijck, C.H.; Bonsing, B.A.; Van de Poll-Franse, L.V.; Haak, H.R.; et al. Surgery for Adrenocortical Carcinoma in the Netherlands: Analysis of the National Cancer Registry Data. Eur. J. Endocrinol. 2013, 169, 83–89. [Google Scholar] [CrossRef]

| Case | Diagnosis Pathologist | Diagnosis IGF2 Methylation Score | Treatment | History |
|---|---|---|---|---|
| 1 | Weiss 3, unclear malignancy, 3.8 cm, PET positive | 3.51, ACC | Adjuvant mitotane | 4 years disease-free |
| 2 | Weiss 3, adenoma, 10 cm, PET positive | 3.34, ACC | Watchful waiting | 4 years disease-free |
| 3 | Weiss 3, oncocytairy adenoma, 5.5 cm, PET positive high uptake | 3.08, unclear | Watchful waiting | 4 years disease-free |
| 4 | Weiss 1, 1 minor LWB criteria oncocytairy adenoma, 9.5 cm | 4.29, ACC | Watchful waiting | 2 years disease-free, discharged from follow-up |
| 5 | Weiss 1, partially invasive adenoma, 6 cm | 4.20, ACC | Watchful waiting | 8 years disease-free |
| 6 | Weiss 3 | 4.68, ACC | Watchful waiting | 2-year death from positive lymph nodes and bone metastases |
| 7 | Weiss 3 | No fresh frozen material | Adjuvant mitotane | 1-year abdominal wall/omental metastases (resection); 2-year bone metastasis (RT/resection); 3-year lung metastasis (mitotane); 4-year death from disease |
| 8 | Weiss 3 | No fresh frozen material | Adjuvant mitotane | 10-years death from other cause, no disease recurrence |
| 9 | Weiss 3 | No fresh frozen material | Watchful waiting | 4-year death from peritonitis carcinomatosis |
| 10 | Weiss 2, cortisol and androgen production | No fresh frozen material | Adjuvant mitotane | 12-years disease-free, discharged from follow-up |
| 11 | Weiss 3 | No fresh frozen material | Adjuvant mitotane | 9 years disease-free |
| 12 | Weiss 3 | No fresh frozen material | Watchful waiting | 3-year liver metastases (RFA/mitotane); 7-year recurrent liver metastases (resection/mitotane); 9 years disease-free |
| 13 | Adenoma with necrosis, 10 cm | No fresh frozen material | Watchful waiting | 17-year recurrence 30 cm (resection); 20 years disease-free |
| 14 | Adenoma (upon revision after recurrence Weiss 4) | No fresh frozen material | Watchful waiting | 7-year lung metastases (mitotane discontinued for liver toxicity, resection, RT); 10-year abdominal metastases (EDP); 12-year death from lung and abdominal disease |
| 15 | Weiss 3, 7.8 cm | No fresh frozen material | Watchful waiting | 4 years disease-free |
| 16 | Weiss 2, 2.9 cm, cortisol production | No fresh frozen material | Watchful waiting | 4 years disease-free, discharged from follow-up |
| 17 | Weiss 3, cortisol production | No fresh frozen material | Watchful waiting | 4 years disease-free |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steenaard, R.V.; Feelders, R.A.; Dogan, F.; van Koetsveld, P.M.; Creemers, S.G.; Ettaieb, M.H.T.; van Kemenade, F.J.; Haak, H.R.; Hofland, L.J. The Role of the IGF2 Methylation Score in Diagnosing Adrenocortical Tumors with Unclear Malignant Potential—Feasibility of Formalin-Fixed Paraffin-Embedded Tissue. Biomedicines 2023, 11, 2013. https://doi.org/10.3390/biomedicines11072013
Steenaard RV, Feelders RA, Dogan F, van Koetsveld PM, Creemers SG, Ettaieb MHT, van Kemenade FJ, Haak HR, Hofland LJ. The Role of the IGF2 Methylation Score in Diagnosing Adrenocortical Tumors with Unclear Malignant Potential—Feasibility of Formalin-Fixed Paraffin-Embedded Tissue. Biomedicines. 2023; 11(7):2013. https://doi.org/10.3390/biomedicines11072013
Chicago/Turabian StyleSteenaard, Rebecca V., Richard A. Feelders, Fadime Dogan, Peter M. van Koetsveld, Sara G. Creemers, Madeleine H. T. Ettaieb, Folkert J. van Kemenade, Harm R. Haak, and Leo J. Hofland. 2023. "The Role of the IGF2 Methylation Score in Diagnosing Adrenocortical Tumors with Unclear Malignant Potential—Feasibility of Formalin-Fixed Paraffin-Embedded Tissue" Biomedicines 11, no. 7: 2013. https://doi.org/10.3390/biomedicines11072013
APA StyleSteenaard, R. V., Feelders, R. A., Dogan, F., van Koetsveld, P. M., Creemers, S. G., Ettaieb, M. H. T., van Kemenade, F. J., Haak, H. R., & Hofland, L. J. (2023). The Role of the IGF2 Methylation Score in Diagnosing Adrenocortical Tumors with Unclear Malignant Potential—Feasibility of Formalin-Fixed Paraffin-Embedded Tissue. Biomedicines, 11(7), 2013. https://doi.org/10.3390/biomedicines11072013





