Metabolic Syndrome: A Strange Companion of Atrial Fibrillation; A Blessing in Disguise from the Neuropsychiatric Point of View
Abstract
1. Introduction
2. Material and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): Supplementary Data. Available online: https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/eurheartj/42/5/10.1093_eurheartj_ehaa612/3/ehaa612_supplementary_data.pdf?Expires=1629475752&Signature=kGhLjuMsKWF6KVV1fmivQpkdQ0LjMyn2epMoaoM8xkrrFh3OIIvdxl7oOxGnER-rRuN2H2ZhVTY1VK9j3HsZsYslznXMEtMnVJ~yxDg3pB3TLCzR5hr5fg6FilfLNmx82Vd5hXzF30woprzQ0zvFd5YYk8BdSzcUYI~2Fkfbh-wrISzNgoa~CrRqshNxm-fFBrBplTFItx-Q1OgrIbWxmTqP7cbI0KK5DR1qBBRBeqw-9mnaoJpz2xfGGeEiGCVPRVPpnH1HaNpSYogFMoecTpK~kYIH1yPSY4whr93TusgPy1ggm2Mxdsp846H4X9tGTgGTvBgO2W7q6mTwTLxnxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA (accessed on 30 January 2023).
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; E Dilaveris, P.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2020, 42, 4194. [Google Scholar]
- Tai, W.A. Stroke: Primary Prevention. FP Essent 2022, 512, 11–17. [Google Scholar]
- Hachinski, V.; Einhäupl, K.; Ganten, D.; Alladi, S.; Brayne, C.; Stephan, B.C.; Sweeney, M.D.; Zlokovic, B.; Iturria-Medina, Y.; Iadecola, C.; et al. Preventing dementia by preventing stroke: The Berlin Manifesto. Alzheimer’s Dement. 2019, 15, 961–984. [Google Scholar] [CrossRef]
- The IDF Consensus Worldwide Definition of the Metabolic Syndrome. 2006–2020. Available online: https://idf.org/our-activities/advocacy-awareness/resources-and-tools/60:idfconsensus-worldwide-definitionof-the-metabolic-syndrome.html (accessed on 30 January 2023).
- Tahmi, M.; Palta, P.; Luchsinger, J.A. Metabolic Syndrome and Cognitive Function. Curr. Cardiol. Rep. 2021, 23, 180. [Google Scholar] [CrossRef]
- Kirvalidze, M.; Hodkinson, A.; Storman, D.; Fairchild, T.J.; Bała, M.M.; Beridze, G.; Zuriaga, A.; Brudasca, N.I.; Brini, S. The role of glucose in cognition, risk of dementia, and related biomarkers in individuals without type 2 diabetes mellitus or the metabolic syndrome: A systematic review of observational studies. Neurosci. Biobehav. Rev. 2022, 135, 104551. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Luo, L.; Yang, M.; Hao, Q.; Yue, J.; Dong, B. Cross-Sectional Study Examining the Association between Metabolic Syndrome and Cognitive Function among the Oldest Old. J. Am. Med. Dir. Assoc. 2013, 14, 105–108. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Komulainen, P.; Lakka, T.A.; Kivipelto, M.; Hassinen, M.; Helkala, E.-L.; Haapala, I.; Nissinen, A.; Rauramaa, R. Metabolic Syndrome and Cognitive Function: A Population-Based Follow-up Study in Elderly Women. Dement. Geriatr. Cogn. Disord. 2007, 23, 29–34. [Google Scholar] [CrossRef]
- Ivanova, N.; Liu, Q.; Agca, C.; Agca, Y.; Noble, E.G.; Whitehead, S.N.; Cechetto, D.F. White matter inflammation and cognitive function in a co-morbid metabolic syndrome and prodromal Alzheimer’s disease rat model. J. Neuroinflamm. 2020, 17, 29. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S. “Cholesterol Paradox” in Atrial Fibrillation. Circ. J. 2011, 75, 2749–2750. [Google Scholar] [CrossRef]
- Alonso, A.; Yin, X.; Roetker, N.S.; Magnani, J.W.; Kronmal, R.A.; Ellinor, P.T.; Chen, L.Y.; Lubitz, S.A.; McClelland, R.L.; McManus, D.D.; et al. Blood Lipids and the Incidence of Atrial Fibrillation: The Multi-Ethnic Study of Atherosclerosis and the Framingham Heart Study. J. Am. Heart Assoc. 2014, 3, e001211. [Google Scholar] [CrossRef]
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. 2012. Available online: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf (accessed on 1 January 2016).
- Hajhosseiny, R.; Matthews, G.K.; Lip, G.Y. Metabolic syndrome, atrial fibrillation, and stroke: Tackling an emerging epidemic. Heart Rhythm. 2015, 12, 2332–2343. [Google Scholar] [CrossRef]
- Koutsonida, M.; Markozannes, G.; Bouras, E.; Aretouli, E.; Tsilidis, K.K. Metabolic syndrome and cognition: A systematic review across cognitive domains and a bibliometric analysis. Front. Psychol. 2022, 13, 981379. [Google Scholar] [CrossRef] [PubMed]
- Borshchev, Y.Y.; Uspensky, Y.P.; Galagudza, M.M. Pathogenetic pathways of cognitive dysfunction and dementia in metabolic syndrome. Life Sci. 2019, 237, 116932. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Pathak, R.K.; Mahajan, R.; Lau, D.H.; Sanders, P. The Implications of Obesity for Cardiac Arrhythmia Mechanisms and Management. Can. J. Cardiol. 2015, 31, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Lavie, C.J.; Pandey, A.; Lau, D.H.; Alpert, M.A.; Sanders, P. Obesity and Atrial Fibrillation Prevalence, Pathogenesis, and Prognosis: Effects of Weight Loss and Exercise. J. Am. Coll. Cardiol. 2017, 70, 2022–2035. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, D.; Khan, A.; Gupta, M.; Hu, Y.; Li, J.; Abedi, V.; Zand, R. Obesity and mortality after the first ischemic stroke: Is obesity paradox real? PLoS ONE 2021, 16, e0246877. [Google Scholar] [CrossRef] [PubMed]
- Feijóo-Bandín, S.; Aragón-Herrera, A.; Moraña-Fernández, S.; Anido-Varela, L.; Tarazón, E.; Roselló-Lletí, E.; Portolés, M.; Moscoso, I.; Gualillo, O.; González-Juanatey, J.R.; et al. Adipokines and Inflammation: Focus on Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 7711. [Google Scholar] [CrossRef]
- Menzaghi, C.; Trischitta, V. The Adiponectin Paradox for All-Cause and Cardiovascular Mortality. Diabetes 2018, 67, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-H. Adiponectin: A pivotal role in the protection against cerebral ischemic injury. Neuroimmunol. Neuroinflamm. 2019, 2019, 4. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, M.; Wang, M.; Wang, Z.; Wang, S.; Hu, H.; Ma, K.; Jiang, H. Association between adiponectin-to-leptin ratio and heart rate variability in new-onset paroxysmal atrial fibrillation: A retrospective cohort study. Ann. Noninvasive Electrocardiol. 2022, 27, e12896. [Google Scholar] [CrossRef]
- Hindsberger, B.; Lindegaard, B.; Andersen, L.R.; Israelsen, S.B.; Pedersen, L.; Szecsi, P.B.; Benfield, T. Circulating Adiponectin Levels Are Inversely Associated with Mortality and Respiratory Failure in Patients Hospitalized with COVID-19. Int. J. Endocrinol. 2023, 2023, 4427873. [Google Scholar] [CrossRef]
- Borghi, C.; Agabiti-Rosei, E.; Johnson, R.J.; Kielstein, J.T.; Lurbe, E.; Mancia, G.; Redon, J.; Stack, A.G.; Tsioufis, K.P. Hyperuricaemia and gout in cardiovascular, metabolic and kidney disease. Eur. J. Intern. Med. 2020, 80, 1–11. [Google Scholar] [CrossRef]
- Topiwala, A.; Mankia, K.; Bell, S.; Webb, A.; Ebmeier, K.P.; Howard, I.; Wang, C.; Alfaro-Almagro, F.; Miller, K.; Burgess, S.; et al. Association of gout with brain reserve and vulnerability to neurodegenerative disease. Nat. Commun. 2023, 14, 2844. [Google Scholar] [CrossRef]
- Takir, M.; Kostek, O.; Ozkok, A.; Elcioglu, O.C.; Bakan, A.; Erek, A.; Mutlu, H.H.; Telci, O.; Semerci, A.; Odabas, A.R.; et al. Lowering Uric Acid with Allopurinol Improves Insulin Resistance and Systemic Inflammation in Asymptomatic Hyperuricemia. J. Investig. Med. 2015, 63, 924–929. [Google Scholar] [CrossRef]
- MacIsaac, R.L.; Salatzki, J.; Higgins, P.; Walters, M.R.; Padmanabhan, S.; Dominiczak, A.F.; Touyz, R.M.; Dawson, J. Allopurinol and Cardiovascular Outcomes in Adults with Hypertension. Hypertension 2016, 67, 535–540. [Google Scholar] [CrossRef]
- Demers, J.; Ton, A.; Huynh, F.; Thibault, S.; Ducharme, A.; Paradis, P.; Nemer, M.; Fiset, C. Atrial Electrical Remodeling in Mice with Cardiac-Specific Overexpression of Angiotensin II Type 1 Receptor. J. Am. Heart Assoc. 2022, 11, e023974. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.; Zhang, Y.; Wang, Y.; Yuan, M.; Kariyawasam, S.; Tse, G.; Liu, T.; Fu, H.; Li, G. Renin–angiotensin system inhibition is associated with reduced risk of left atrial appendage thrombosis formation in patients with atrial fibrillation. Cardiol. J. 2018, 25, 611–620. [Google Scholar] [CrossRef]
- Schneider, M.P.; Hua, T.A.; Böhm, M.; Wachtell, K.; Kjeldsen, S.E.; Schmieder, R.E. Prevention of Atrial Fibrillation by Renin-Angiotensin System Inhibition: A Meta-Analysis. J. Am. Coll. Cardiol. 2010, 55, 2299–2307. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, L. Association of Atrial Fibrillation with Diabetes Mellitus, High Risk Comorbidities. Maedica 2022, 17, 143–152. [Google Scholar]

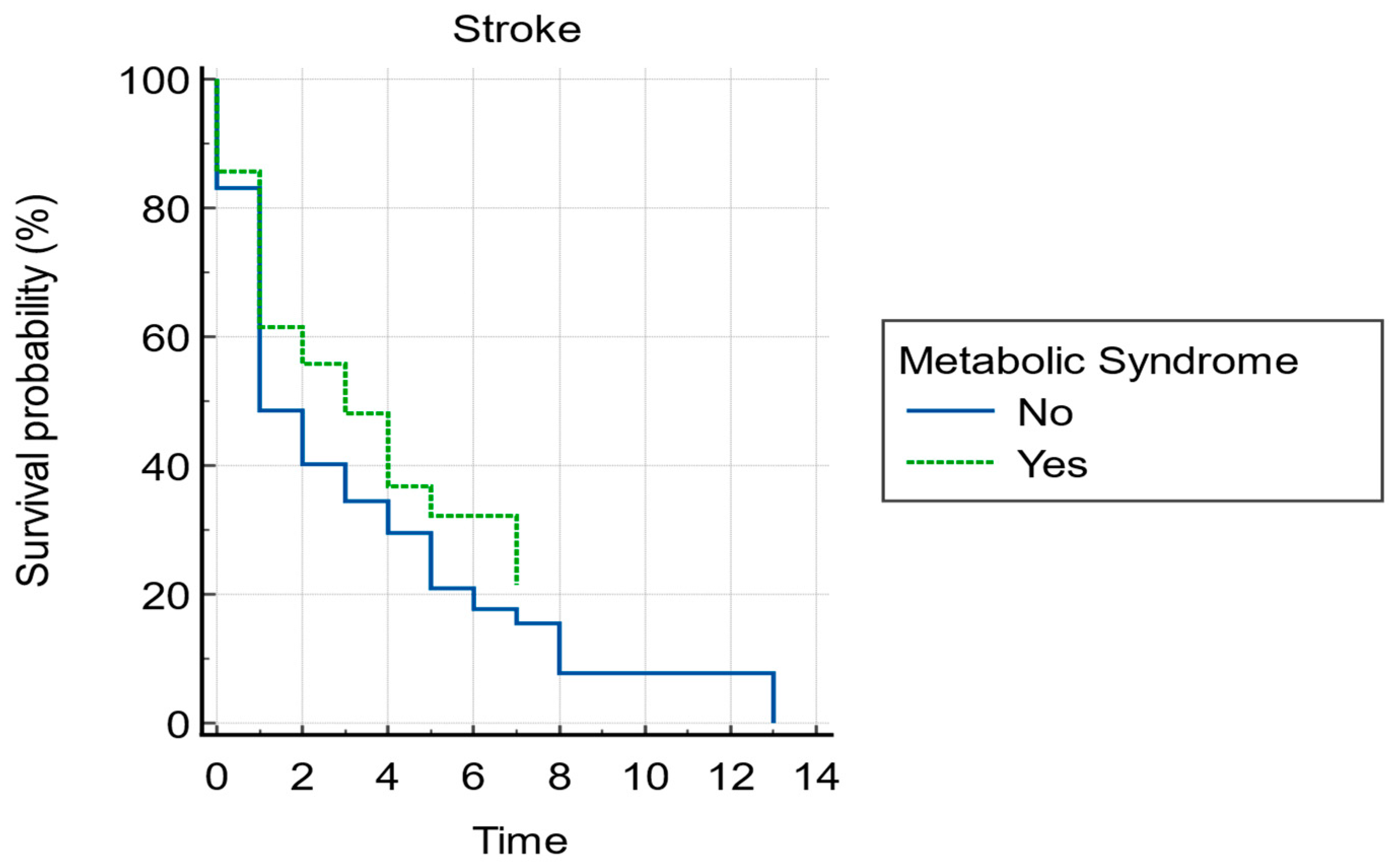
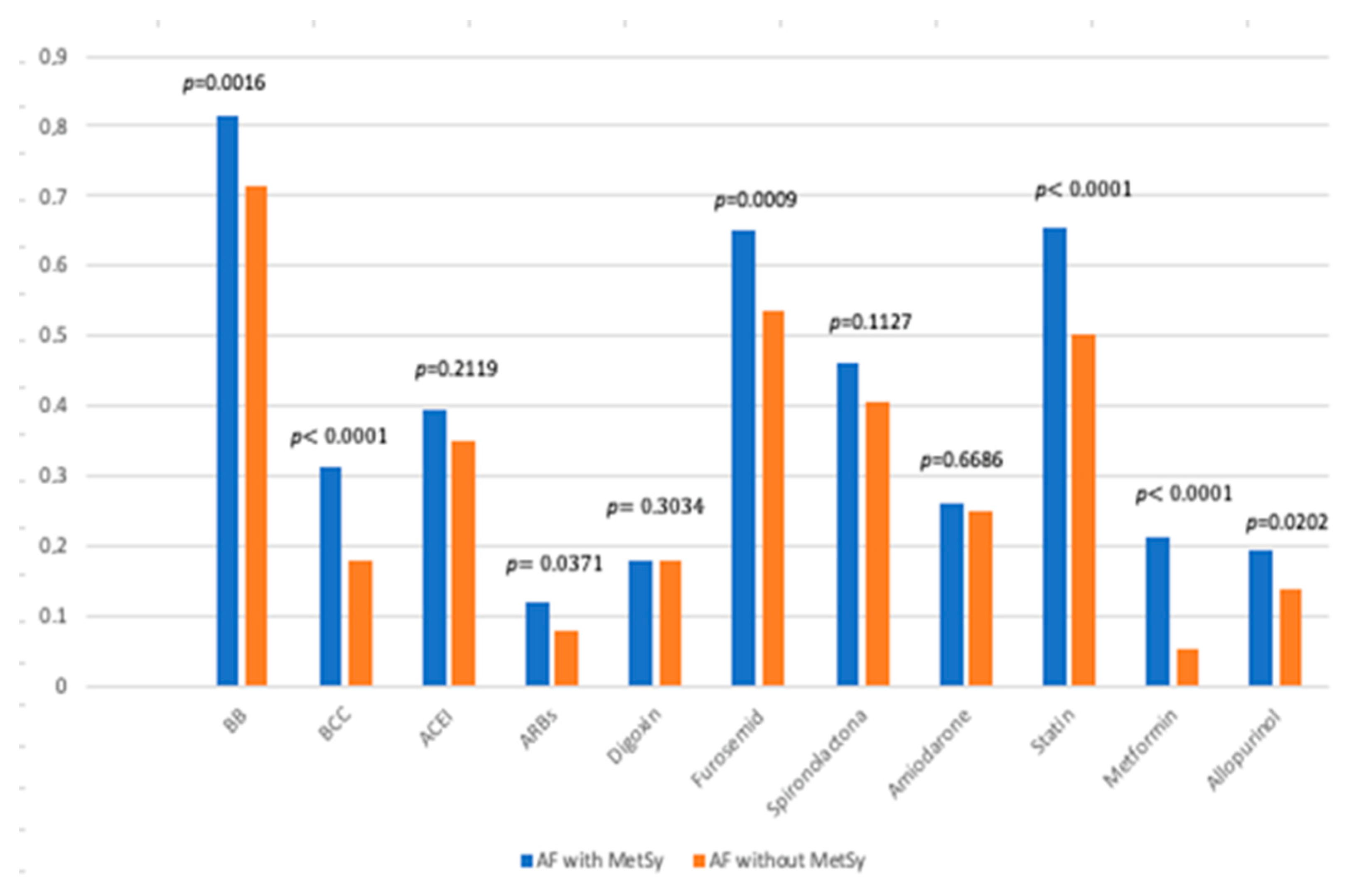
| Group | AF with MetSy 267 Patients | AF without MetSy 843 Patients | p-Value |
|---|---|---|---|
| Gender | M: 51.7% F: 48.3% | M: 47.8% F: 52.2% | 0.8847 |
| Mean age (years) | 69.50 (95% CI) for the mean 68.3611 to 70.6501 Std. Dev. 9.49 years | 73.94 95% CI for the mean 72.4875 to 73.9419 Std Dev 10.75 years | <0.0001 |
| Place of residence | U: 47.9% R: 52.1% | U: 49.9% R:50.1% | <0.5463 |
| COPD | 25.1% | 20.2% | 0.0870 |
| Asthma | 13.5% | 9.1% | <0.0406 |
| HF | 88.8% | 77.8% | <0.0001 |
| ICD | 77.2% | 63.7% | <0.0001 |
| CKD stage > 3 | 63.3% | 59.3% | 0.2410 |
| Hyperuricemia | 22.8% | 11.6% | <0.0001 |
| Group | AF and MetSy (267 Patients) | AF without MetSy (843 Patients) | p-Value |
|---|---|---|---|
| Stroke | 37.5% | 42.2% | 0.1731 |
| Dementia | 4.5% | 9.1% | <0.0001 |
| SLI | 7.1% | 10.7% | 0.0886 |
| Cognitive impairment | 10.9% | 6% | 0.0081 |
| Parkinson’s disease | 5.6% | 5.0% | 0.6818 |
| Variable | Coefficient | Std. Error | Wald | p-Value | Odds Ratio | 95% CI |
|---|---|---|---|---|---|---|
| HBP | 0.043256 | 0.32026 | 0.01824 | 0.8926 | 1.0442 | 0.5574 to 1.9561 |
| DM | 0.36730 | 0.24558 | 2.2368 | 0.1348 | 1.4438 | 0.8922 to 2.3365 |
| HyperTG | 0.34083 | 0.27572 | 1.5280 | 0.2164 | 1.4061 | 0.8191 to 2.4139 |
| HypoHDLc | 0.25701 | 0.27859 | 0.8511 | 0.3562 | 1.2931 | 0.7490 to 2.2323 |
| Obesity | −1.25852 | 0.35248 | 12.7487 | 0.0004 | 0.2841 | 0.1424 to 0.5668 |
| Constant | −2.63705 | 0.30488 | 74.8141 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosca, C.I.; Lighezan, D.F.; Nisulescu, D.-D.; Sharma, A.; Neagu, M.N.; Nistor, D.; Georgescu, D.; Kundnani, N.R. Metabolic Syndrome: A Strange Companion of Atrial Fibrillation; A Blessing in Disguise from the Neuropsychiatric Point of View. Biomedicines 2023, 11, 2012. https://doi.org/10.3390/biomedicines11072012
Rosca CI, Lighezan DF, Nisulescu D-D, Sharma A, Neagu MN, Nistor D, Georgescu D, Kundnani NR. Metabolic Syndrome: A Strange Companion of Atrial Fibrillation; A Blessing in Disguise from the Neuropsychiatric Point of View. Biomedicines. 2023; 11(7):2012. https://doi.org/10.3390/biomedicines11072012
Chicago/Turabian StyleRosca, Ciprian Ilie, Daniel Florin Lighezan, Daniel-Dumitru Nisulescu, Abhinav Sharma, Marioara Nicula Neagu, Daciana Nistor, Doina Georgescu, and Nilima Rajpal Kundnani. 2023. "Metabolic Syndrome: A Strange Companion of Atrial Fibrillation; A Blessing in Disguise from the Neuropsychiatric Point of View" Biomedicines 11, no. 7: 2012. https://doi.org/10.3390/biomedicines11072012
APA StyleRosca, C. I., Lighezan, D. F., Nisulescu, D.-D., Sharma, A., Neagu, M. N., Nistor, D., Georgescu, D., & Kundnani, N. R. (2023). Metabolic Syndrome: A Strange Companion of Atrial Fibrillation; A Blessing in Disguise from the Neuropsychiatric Point of View. Biomedicines, 11(7), 2012. https://doi.org/10.3390/biomedicines11072012











