The Value of Prolactin, a Panel of Cytokines, and the Soluble Human Epidermal Growth Factor Receptor 2 in the Prediction of Rapid Progression and Shorter Survival during Palliative Chemotherapy of Colorectal Cancer Patients
Abstract
1. Introduction
2. Materials and Patients
2.1. Patients
2.2. Assays
2.3. Data Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Predictive Value of Prolactin
4.2. Predictive Value of IP-10 (IP-10, CXCL10)
4.3. Predictive Value of sHER2
4.4. Predictive Value of IL-8 and IL-6
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Cancer. Available online: https://www.who.int/news-room/fact-=sheets/detail/cancer (accessed on 14 July 2021).
- Kow, A.W.C. Hepatic metastasis from colorectal cancer. J. Gastrointest. Oncol. 2019, 10, 1274–1298. [Google Scholar] [CrossRef]
- Long, T.; Raufman, J. The diagnostic and prognostic role of cytokines in colon cancer. Gastrointest. Cancer Targets Ther. 2011, 1, 27–39. [Google Scholar]
- Hegde, P.S.; Karanikas, V.; Evers, S. The Where, the When, and the How of Immune Monitoring for Cancer Immunotherapies in the Era of Checkpoint Inhibition. Clin. Cancer Res. 2016, 22, 1865–1874. [Google Scholar] [CrossRef] [PubMed]
- Czajka-Francuz, P.; Francuz, T.; Cisoń-Jurek, S.; Czajka, A.; Fajkis, M.; Szymczak, B.; Kozaczka, M.; Malinowski, K.P.; Zasada, W.; Wojnar, J.; et al. Serum cytokine profile as a potential prognostic tool in colorectal cancer patients—One center study. Rep. Pract. Oncol. Radiother. 2020, 25, 867–875. [Google Scholar] [CrossRef]
- Patel, D.D.; Bhatavdekar, J.M.; Ghosh, N.; Vora, H.H.; Karelia, N.H.; Shah, N.G.; Suthar, T.P.; Balar, D.B.; Trivedi, C.R. Plasma prolactin in patients with colorectal cancer. Value in follow-up and as a prognosticator. Cancer 1994, 73, 570–574. [Google Scholar] [CrossRef]
- Soroush, A.R.; Zadeh, H.M.; Moemeni, M.; Shakiba, B.; Elmi, S. Plasma prolactin in patients with colorectal cancer. BMC Cancer 2004, 4, 97. [Google Scholar] [CrossRef]
- Bhatavdekar, J.M.; Patel, D.D.; Giri, D.D.; Karelia, N.H.; Vora, H.H.; Ghosh, N.; Shah, N.G.; Trivedi, S.N.; Balar, D.B. Comparison of plasma prolactin and CEA in monitoring patients with adenocarcinoma of colon and rectum. Br. J. Cancer 1992, 66, 977–980. [Google Scholar] [CrossRef]
- Mahboob, S.; Ahn, S.B.; Cheruku, H.R.; Cantor, D.; Rennel, E.; Fredriksson, S.; Edfeldt, G.; Breen, E.J.; Khan, A.; Mohamedali, A.; et al. A novel multiplexed immunoassay identifies CEA, IL-8 and prolactin as prospective markers for Dukes’ stages A-D colorectal cancers. Clin. Proteom. 2015, 12, 10. [Google Scholar] [CrossRef]
- Bhatavdekar, J.M.; Shah, N.G.; Balar, D.B.; Patel, D.D.; Bhaduri, A.; Trivedi, S.N.; Karelia, N.H.; Ghosh, N.; Shukla, M.K.; Giri, D.D. Plasma Prolactin as an Indicator of Disease Progression in Advanced Breast Cancer. Cancer 1990, 65, 2028–2032. [Google Scholar] [CrossRef]
- Becker, D.J.; Vinik, A.I.; Pimstone, B.L.; Paul, M. Prolactin responses to Thyrotropin Releasing Hormone in Protein Calorie Malnutrition. J. Clin. Endocrinol. Metab. 1975, 41, 782. [Google Scholar] [CrossRef]
- Navarro, I.; Batista, K.; Schraner, M.; Riediger, T. Brainstem prolactin-releasing peptide contributes to cancer anorexia-cachexia syndrome in rats. Neuropharmacology 2020, 180, 108289. [Google Scholar] [CrossRef]
- Faupel-Badger, J.M.; Ginsburg, E.; Fleming, J.M.; Susser, L.; Doucet, T.; Vonderhaar, B.K. 16-kDa Prolactin Reduces Angiogenesis, but Not Growth of Human Breast Cancer Tumors In Vivo. Horm. Cancer 2010, 1, 71–79. [Google Scholar] [CrossRef]
- Bernard, V.; Young, J.; Chanson, P.; Binart, N. New insights in prolactin: Pathological implications. Nat. Rev. Endocrinol. 2015, 11, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Karin, N.; Razon, H. Chemokines beyond chemo-attraction: CXCL10 and its significant role in cancer and autoimmunity. Cytokine 2018, 109, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Zumwalt, T.J.; Arnold, M.; Goel, A.; Boland, C.R. Active secretion of CXCL10 and CCL5 from colorectal cancer microenvironments associates with GranzymeB+ CD8+ T-cell infiltration. Oncotarget 2015, 6, 2981–2991. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Wang, S.; Ni, H.; Zhao, P.; Chen, G.; Xu, B.; Yuan, L. The role of CXCR3 and its ligands in cancer. Front. Oncol. 2022, 12, 1022688. [Google Scholar] [CrossRef]
- Song, W.; Yin, H.; Han, C.; Mao, Q.; Tang, J.; Ji, Z.; Yan, X.; Wang, L.; Liu, S.; Ai, C. The role of CXCL10 in prognosis of patients with colon cancer and tumor microenvironment remodeling. Medicine 2021, 100, e27224. [Google Scholar] [CrossRef]
- Wightman, S.; Uppal, A.; Pitroda, S.; Ganai, S.; Burnette, B.; Stack, M.; Oshima, G.; Khan, S.; Huang, X.; Posner, M.C.; et al. Oncogenic CXCL10 signalling drives metastasis development and poor clinical outcome. Br. J. Cancer 2015, 113, 327–335. [Google Scholar] [CrossRef]
- Dufour, J.H.; Dziejman, M.; Liu, M.T.; Leung, J.H.; Lane, T.E.; Luster, A.D. IFN-γ-Inducible Protein 10 (IP-10; CXCL10)-Deficient Mice Reveal a Role for IP-10 in Effector T Cell Generation and Trafficking1. J. Immunol. 2002, 168, 3195–3204. [Google Scholar] [CrossRef]
- Mohammadpoor, A.H.; Ghaffarzadegan, K.; Hasanpoor, M.; Izanloo, A.; Mehri, S.; Jannati, M.; Elyasi, S. Evaluation of Serum CXCL10 Level as a Prognostic Marker in Colorectal Cancer Patients: A Retrospective Cohort Study. Rep. Radiother. Oncol. 2021, 8, e122874. [Google Scholar] [CrossRef]
- Afrasânie, V.A.; Marinca, M.V.; Alexa-Stratulat, T.; Gafton, B.; Paduraru, M.; Adavidoaiei, A.M.; Miron, L.; Rusu, C. KRAS, NRAS, BRAF, HER2 and microsatellite instability in metastatic colorectal cancer—Practical implications for the clinician. Radiol. Oncol. 2019, 53, 265–274. [Google Scholar] [CrossRef]
- Ivanova, M.; Venetis, K.; Guerini-Rocco, E.; Bottiglieri, L.; Mastropasqua, M.G.; Garrone, O.; Fusco, N.; Ghidini, M. HER2 in Metastatic Colorectal Cancer: Pathology, Somatic Alterations, and Perspectives for Novel Therapeutic Schemes. Life 2022, 12, 1403. [Google Scholar] [CrossRef]
- Moreno-Aspitia, A.; Hillman, D.W.; Dyar, S.H.; Tenner, K.S.; Gralow, J.; Kaufman, P.A.; Davidson, N.E.; Lafky, J.M.; Reinholz, M.M.; Lingle, W.L.; et al. Soluble human epidermal growth factor receptor 2 (HER2) levels in patients with HER2-positive breast cancer receiving chemotherapy with or without trastuzumab: Results from North Central Cancer Treatment Group adjuvant trial N9831. Cancer 2013, 119, 2675–2682. [Google Scholar] [CrossRef]
- Xia, W.; Chen, W.; Zhang, Z.; Wu, D.; Wu, P.; Chen, Z.; Li, C.; Huang, J. Prognostic Value, Clinicopathologic Features and Diagnostic Accuracy of Interleukin-8 in Colorectal Cancer: A Meta-Analysis. PLoS ONE 2015, 10, e0123484. [Google Scholar] [CrossRef]
- Bie, Y.; Ge, W.; Yang, Z.; Cheng, Z.; Zhao, Z.; Li, S.; Wang, W.; Wang, Y.; Zhao, X.; Yin, Z.; et al. The Crucial Role of CXCL8 and Its Receptors in Colorectal Liver Metastasis. Dis. Markers 2019, 2019, 8023460. [Google Scholar] [CrossRef]
- Conciatori, F.; Bazzichetto, C.; Falcone, I.; Ferretti, G.; Cognetti, F.; Milella, M.; Ciuffreda, L. Colorectal cancer stem cells properties and features: Evidence of interleukin-8 involvement. Cancer Drug Resist. 2019, 2, 968–979. [Google Scholar] [CrossRef]
- Long, X.; Ye, Y.; Zhang, L.; Liu, P.; Yu, W.; Wei, F.; Ren, X.; Yu, J. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways. Int. J. Oncol. 2016, 48, 5–12. [Google Scholar] [CrossRef]
- Bazzichetto, C.; Milella, M.; Zampiva, I.; Simionato, F.; Amoreo, C.A.; Buglioni, S.; Pacelli, C.; Le Pera, L.; Colombo, T.; Bria, E.; et al. Interleukin-8 in Colorectal Cancer: A Systematic Review and Meta- Analysis of Its Potential Role as a Prognostic Biomarker. Biomedicines 2022, 10, 2631. [Google Scholar] [CrossRef]
- Pączek, S.; Łukaszewicz-Zając, M.; Gryko, M.; Mroczko, P.; Kulczyńska-Przybik, A.; Mroczko, B. CXCL-8 in Preoperative Colorectal Cancer Patients: Significance for Diagnosis and Cancer Progression. Int. J. Mol. Sci. 2020, 21, 2040. [Google Scholar] [CrossRef]
- Burz, C.; Bojan, A.; Balacescu, L.; Pop, V.V.; Silaghi, C.; Lupan, I.; Aldea, C.; Sur, D.; Samasca, G.; Cainap, C.; et al. Interleukin 8 as predictive factor for response to chemotherapy in colorectal cancer patients. Acta Clin. Belg. 2021, 76, 113–118. [Google Scholar] [CrossRef]
- Xu, J.; Ye, Y.; Zhang, H.; Szmitkowski, M.; Mäkinen, M.J.; Li, P.; Xia, D.; Yang, J.; Wu, Y.; Wu, H. Diagnostic and Prognostic Value of Serum Interleukin-6 in Colorectal Cancer. Medicine 2016, 95, e2502. [Google Scholar] [CrossRef]
- Cui, G.; Yuan, A.; Sun, Z.; Zheng, W.; Pang, Z. IL-1_/IL-6 network in the tumor microenvironment of human colorectal cancer. Pathol. Res. Pract. 2018, 214, 986–992. [Google Scholar] [CrossRef]
- Chonov, D.C.; Ignatova, M.M.K.; Ananiev, J.R.; Gulubova, M.V. IL-6 Activities in the Tumour Microenvironment. Part 1. Open Access Maced. J. Med. Sci. 2019, 7, 2391–2398. [Google Scholar] [CrossRef]
- Waniczek, D.; Lorenc, Z.; Snietura, M.; Wesecki, M.; Kopec, A.; Muc-Wierzgo, M. Tumor-Associated Macrophages and Regulatory T Cells Infiltration and the Clinical Outcome in Colorectal Cancer. Arch. Immunol. Ther. Exp. 2017, 65, 445–454. [Google Scholar] [CrossRef]
- Kasprzak, A. The Role of Tumor Microenvironment Cells in Colorectal Cancer (CRC) Cachexia. Int. J. Mol. Sci. 2021, 22, 1565. [Google Scholar] [CrossRef]
- Xu, W.; He, Y.; Wang, Y.; Li, X.; Young, J.; Ioannidis, J.P.A.; Dunlop, M.G.; Theodoratou, E. Risk factors and risk prediction models for colorectal cancer metastasis and recurrence: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 172. [Google Scholar] [CrossRef]
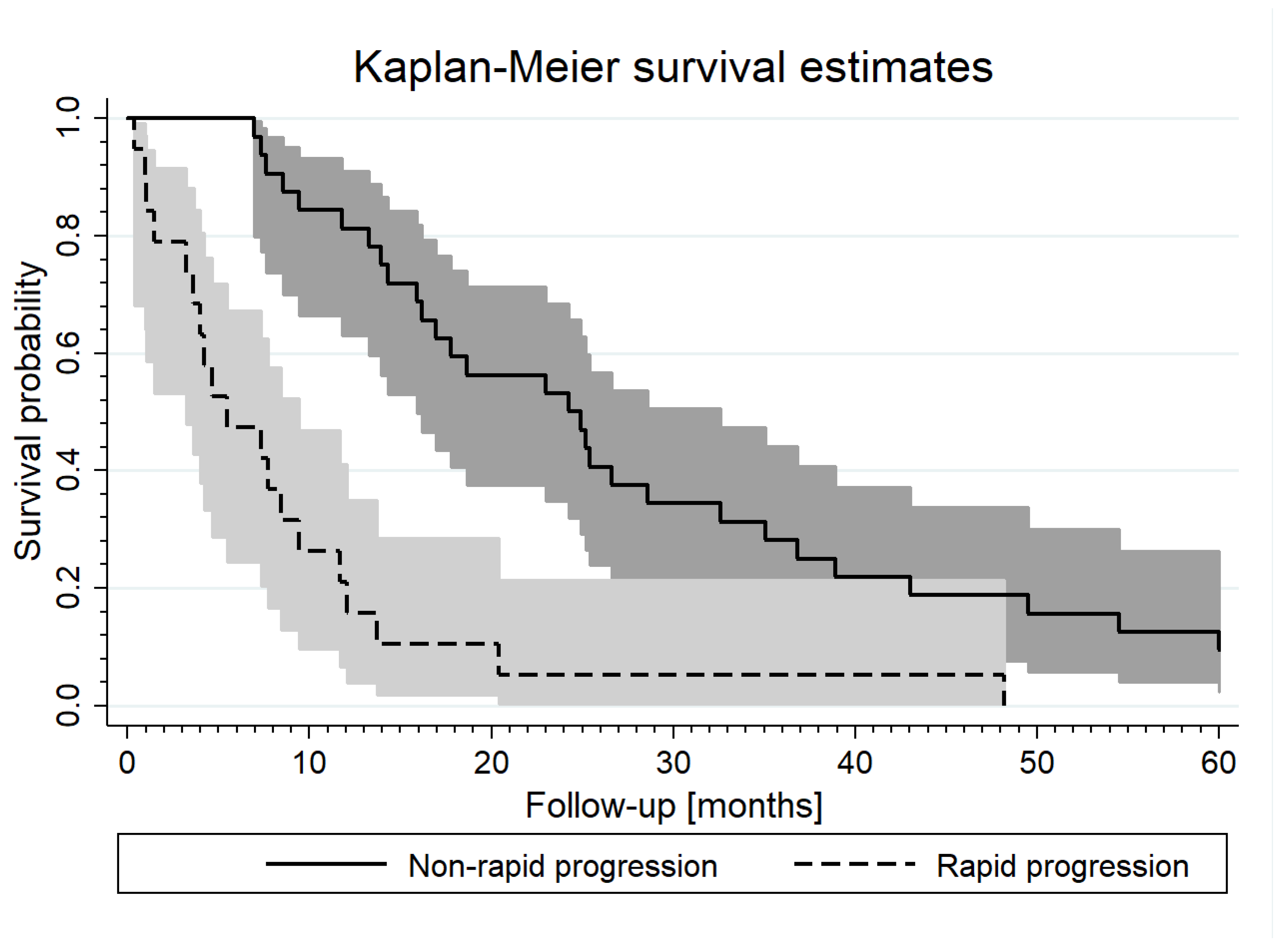
| All | Non-Rapid Progression | Rapid Progression | p | |
|---|---|---|---|---|
| N (%) | 51 (100%) | 32 (62.7) | 19 (37.3) | |
| Female, N (%) | 28 (54.9%) | 20 (62.5%) | 8 (42.1) | 0.16 |
| Age [years] | 66 ± 9 | 64 ± 7 | 68 ± 10 | 0.11 |
| BMI [kg/m2] | 26.1 ± 4.5 | 26.4 ± 4.6 | 25.8 ± 4.5 | 0.66 |
| WHO, N (%) | ||||
| 0 | 37 (72.6) | 25 (78.1) | 12 (63.2) | 0.51 |
| 1 | 12 (23.5) | 6 (18.8) | 6 (31.6) | |
| 2 | 2 (3.9) | 1 (3.1) | 1 (5.2) | |
| Cancer location, N (%) | 0.28 | |||
| Right colon | 13 (25.5) | 7 (21.9) | 6 (31.6) | |
| Left colon | 12 (23.5) | 9 (28.1) | 3 (15.8) | |
| Sigmoid | 13 (25.5) | 10 (31.2) | 3 (15.8) | |
| Rectum | 13 (25.5) | 6 (18.8) | 7 (36.8) | |
| Clinical stage, N (%) | ||||
| II/III | 16 (31.40 | 10 (31.2) | 6 (31.6) | 0.98 |
| IV | 35 (68.6) | 22 (68.8) | 13 (68.4) | |
| Grade, N (%) | ||||
| 1 + 2 | 39 (76.5) | 29 (90.6) | 10 (52.6) | <0.01 |
| 3 | 12 (23.5) | 3 (9.4) | 9 (47.4) | |
| RAS mutation, N (%) | 18 (75.0) | 14 (73.7) | 4 (80.0) | 1.00 |
| Surgery type, N (%) | ||||
| Hemicolectomy | 17 (33.3) | 9 (28.1) | 8 (42.1) | 0.06 |
| Segmental resection | 14 (27.4) | 13 (40.6) | 1 (5.3) | |
| Lower anterior resection | 12 (23.5) | 6 (18.8) | 6 (31.6) | |
| Colostomy | 8 (15.7) | 4 (12.5) | 4 (21.0) | |
| Radiation therapy, N (%) | 12 (23.53) | 7 (21.9) | 5 (26.3) | 0.72 |
| Chemotherapy, N (%) | ||||
| Initially palliative | 23 (45.1) | 15 (46.9) | 8 (42.1) | 0.74 |
| Palliative after radical therapy | 28 (54.9) | 17 (53.1) | 11 (57.9) | |
| 5-FU monotherapy | 11 (21.6) | 5 (15.6) | 6 (31.6) | 0.55 |
| FOLFIRI | 31 (60.8) | 21 (65.6) | 10 (52.6) | |
| FOLFOX-4 | 7 (13.7) | 5 (15.6) | 2 (10.5) | |
| FOLFOX4+bevacizumab | 2 (3.9) | 1 (3.1) | 1 (5.3) |
| All Patients [N = 51] | Non-Rapid Progressors [N = 32] | Rapid Progressors [N = 19] | p | |
|---|---|---|---|---|
| Hemoglobin [g/dL] | 12.2 ± 1.6 | 12.4 ± 1.6 | 11.8 ± 1.6 | 0.26 |
| Red blood cells [106/μL] | 4.34 ± 0.45 | 4.38 ± 0.49 | 4.28 ± 0.38 | 0.46 |
| White blood cells [103/μL] | 7.7 ± 3.2 | 7.8 ± 3.2 | 7.6 ± 3.2 | 0.86 |
| Platelets [103/μL] | 291 ± 133 | 300 ± 125 | 276 ± 147 | 0.53 |
| CEA [ng/mL] | 22.8 (4.8–100.8) | 29.5 (4.8–92.1) | 20.4 (5.3–335.5) | 0.99 |
| Ca19-9 [U/mL] | 15.2 (7.2–92.2) | 31.9 (7.2–122.0) | 11.1 (5.8–40.3) | 0.26 |
| AST [IU/L] | 23 (16–39) | 21 (14–34) | 24 (17–44) | 0.16 |
| ALT [IU/L] | 21 (16–37) | 20 (15–38) | 21 (16–27) | 0.84 |
| Parameter | Non-Rapid Progressors [N = 32] | Rapid Progressors [N = 19] | p |
|---|---|---|---|
| IL-8 [pg/mL] | 19.8 (15.6–29.3) | 21.7 (16.6–44.4) | 0.08 |
| IP-10 [pg/dL] | 6.6 (4.4–81.3) | 7.4 (6.2–103.4) | 0.08 |
| PRL [ng/mL] | 19.5 (13.6–50.7) | 17.9 (10.0–29.4) | <0.05 |
| Progression | ||
|---|---|---|
| HR | ±95% CI | |
| Male vs. Female | 1.04 | 0.59–1.85 |
| Age [per decade] | 1.49 $ | 1.00–2.22 |
| BMI [per kg/m2] | 0.99 | 0.93–1.05 |
| WHO 1/2 vs. 0 | 1.54 | 0.82–2.89 |
| Cancer location | ||
| Right colon | 1.65 | 0.72–3.80 |
| Left colon | 0.86 | 0.37–2.00 |
| Sigmoid | Ref. | |
| Rectum | 2.88 * | 1.23–6.76 |
| Clinical Stage VI vs. II/III | 1.14 | 0.61–2.12 |
| Grade 3 vs. 1/2 | 3.28 # | 1.64–6.59 |
| RAS mutation | 1.22 | 0.45–3.33 |
| Surgery type | ||
| Hemicolectomy | Ref. | |
| Segmental resection | 0.64 | 0.30–1.37 |
| Lower anterior resection | 1.60 | 0.75–3.42 |
| Abdominoperineal resection | 1.62 | 0.68–3.86 |
| Radiation therapy | 1.15 | 0.58–2.26 |
| Chemotherapy | ||
| Initially palliative | Ref. | |
| Palliative after radical therapy | 0.84 | 0.47–1.51 |
| Rapid progression | – | – |
| Initial values of: | ||
| CEA [per ng/dL] | 1.02 | 0.98–1.07 |
| Ca19-9 [per U/dL] | 1.03 | 0.83–1.28 |
| Baseline values | ||
| IL-6 [per pg/mL] | 1.27 * | 1.03–1.56 |
| IL-8 [per 10 pg/mL] | 1.10 ** | 1.03–1.17 |
| IP-10 [per pg/dL] | 1.08 * | 1.01–1.16 |
| sHER2 [per ng/mL] | 1.05 * | 1.01–1.09 |
| PRL [per 10 ng/mL] | 0.95 $ | 0.90–1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cisoń-Jurek, S.; Czajka-Francuz, P.; Francuz, T.; Owczarek, A.J.; Szymczak, B.; Wojnar, J.; Chudek, J. The Value of Prolactin, a Panel of Cytokines, and the Soluble Human Epidermal Growth Factor Receptor 2 in the Prediction of Rapid Progression and Shorter Survival during Palliative Chemotherapy of Colorectal Cancer Patients. Biomedicines 2023, 11, 2014. https://doi.org/10.3390/biomedicines11072014
Cisoń-Jurek S, Czajka-Francuz P, Francuz T, Owczarek AJ, Szymczak B, Wojnar J, Chudek J. The Value of Prolactin, a Panel of Cytokines, and the Soluble Human Epidermal Growth Factor Receptor 2 in the Prediction of Rapid Progression and Shorter Survival during Palliative Chemotherapy of Colorectal Cancer Patients. Biomedicines. 2023; 11(7):2014. https://doi.org/10.3390/biomedicines11072014
Chicago/Turabian StyleCisoń-Jurek, Sylwia, Paulina Czajka-Francuz, Tomasz Francuz, Aleksander J. Owczarek, Bożena Szymczak, Jerzy Wojnar, and Jerzy Chudek. 2023. "The Value of Prolactin, a Panel of Cytokines, and the Soluble Human Epidermal Growth Factor Receptor 2 in the Prediction of Rapid Progression and Shorter Survival during Palliative Chemotherapy of Colorectal Cancer Patients" Biomedicines 11, no. 7: 2014. https://doi.org/10.3390/biomedicines11072014
APA StyleCisoń-Jurek, S., Czajka-Francuz, P., Francuz, T., Owczarek, A. J., Szymczak, B., Wojnar, J., & Chudek, J. (2023). The Value of Prolactin, a Panel of Cytokines, and the Soluble Human Epidermal Growth Factor Receptor 2 in the Prediction of Rapid Progression and Shorter Survival during Palliative Chemotherapy of Colorectal Cancer Patients. Biomedicines, 11(7), 2014. https://doi.org/10.3390/biomedicines11072014







