Expression of Amine Oxidase Proteins in Adrenal Cortical Neoplasm and Pheochromocytoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Tissue Microarray
2.3. Immunohistochemistry
2.4. Interpretation of Immunohistochemical Staining
2.5. Statistical Analysis
3. Results
3.1. Clinicopathologic Characteristics
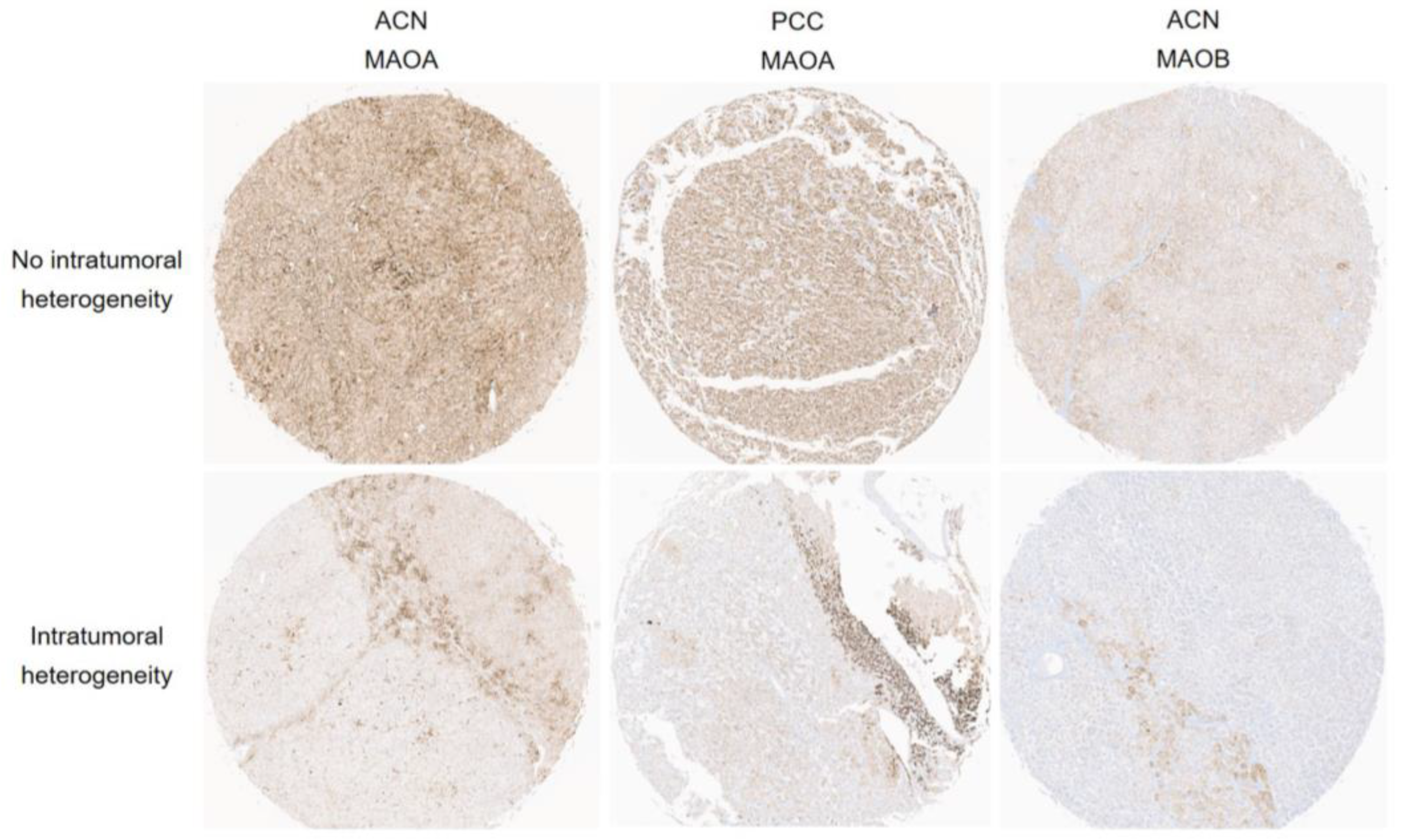
3.2. Expression of Amine Oxidase Family in Adrenal Cortical Neoplasm and Pheochromocytoma
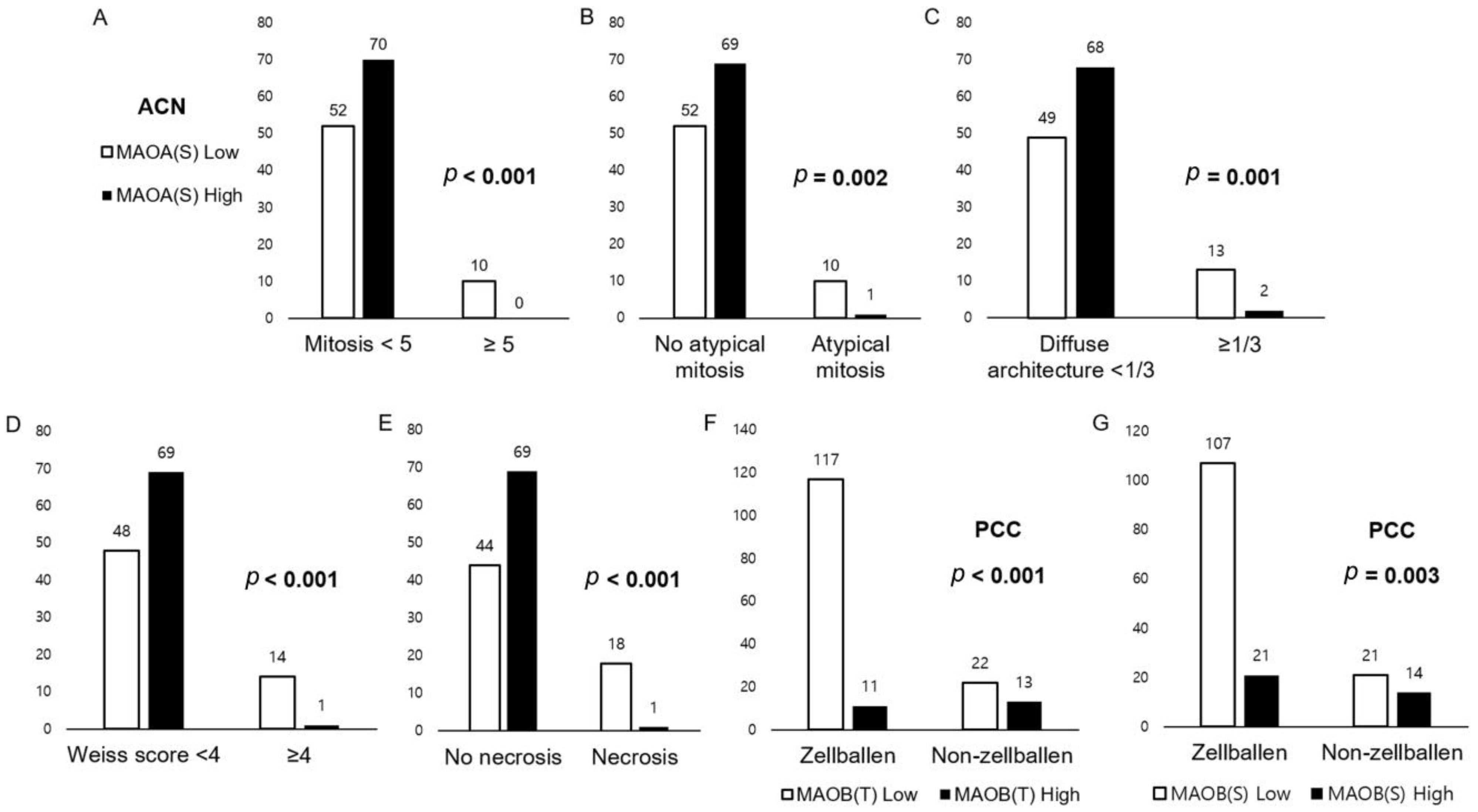
3.3. Relationship between Expression of Amine Oxidase Family and Pathologic Factors of Adrenal Neoplasm
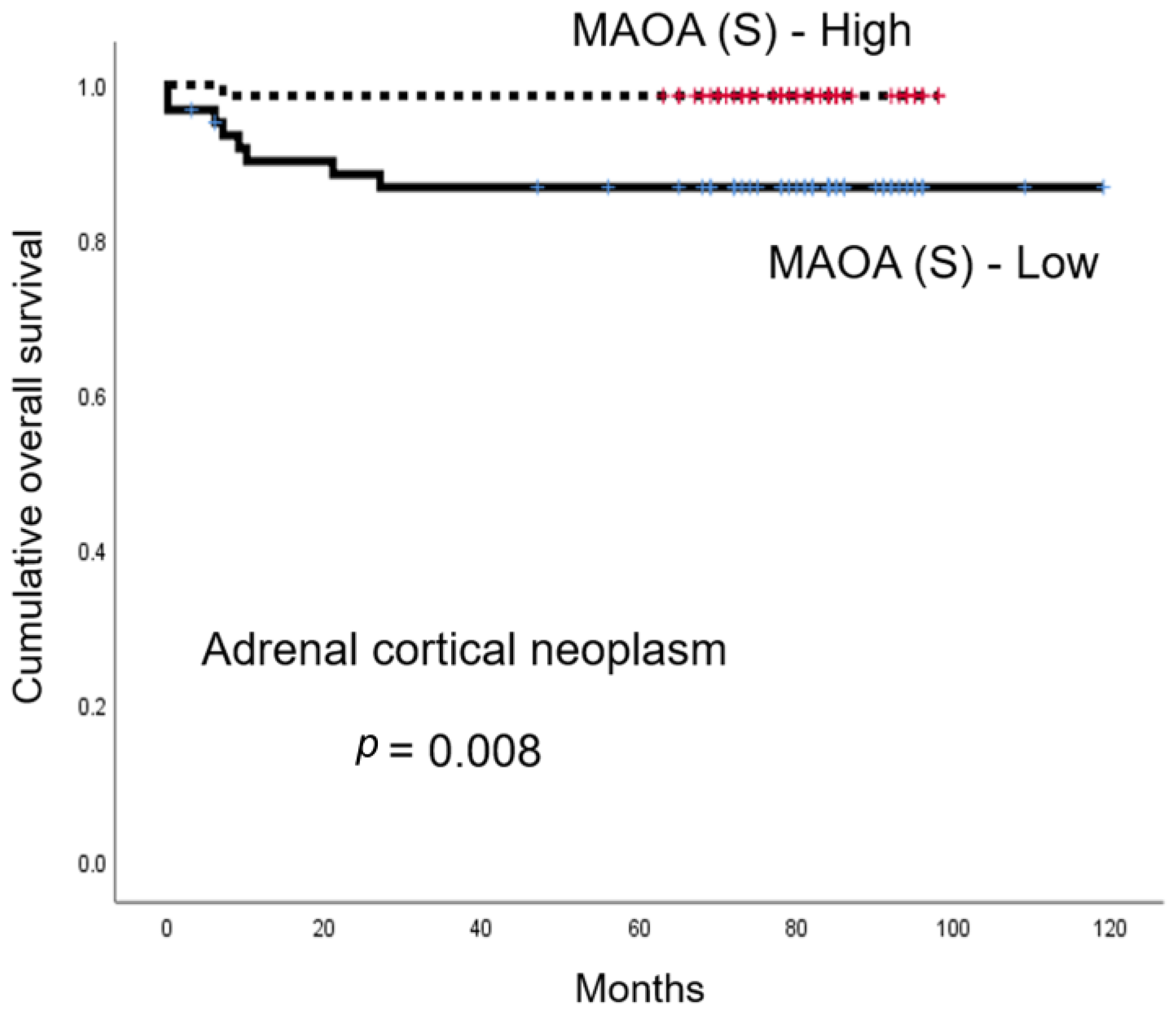
3.4. The Influence of Amine Oxidase Family Expression on the Prognosis of Adrenal Cortical Neoplasm and Pheochromocytoma
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Endocrine and Neuroendocrine Tumours [Internet], 5th ed.; International Agency for Research on Cancer: Lyon, France, 2022; Volume 10. [Google Scholar]
- Weiss, L.M. Comparative histologic study of 43 metastasizing and nonmetastasizing adrenocortical tumors. Am. J. Surg. Pathol. 1984, 8, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.M.; Medeiros, L.J.; Vickery, A.L., Jr. Pathologic features of prognostic significance in adrenocortical carcinoma. Am. J. Surg. Pathol. 1989, 13, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Aubert, S.; Wacrenier, A.; Leroy, X.; Devos, P.; Carnaille, B.; Proye, C.; Wemeau, J.L.; Lecomte-Houcke, M.; Leteurtre, E. Weiss system revisited: A clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am. J. Surg. Pathol. 2002, 26, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Pennanen, M.; Heiskanen, I.; Sane, T.; Remes, S.; Mustonen, H.; Haglund, C.; Arola, J. Helsinki score-a novel model for prediction of metastases in adrenocortical carcinomas. Hum. Pathol. 2015, 46, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Duregon, E.; Fassina, A.; Volante, M.; Nesi, G.; Santi, R.; Gatti, G.; Cappellesso, R.; Dalino Ciaramella, P.; Ventura, L.; Gambacorta, M.; et al. The reticulin algorithm for adrenocortical tumor diagnosis: A multicentric validation study on 245 unpublished cases. Am. J. Surg. Pathol. 2013, 37, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Bisceglia, M.; Ludovico, O.; Di Mattia, A.; Ben-Dor, D.; Sandbank, J.; Pasquinelli, G.; Lau, S.K.; Weiss, L.M. Adrenocortical oncocytic tumors: Report of 10 cases and review of the literature. Int. J. Surg. Pathol. 2004, 12, 231–243. [Google Scholar] [CrossRef]
- Wieneke, J.A.; Thompson, L.D.; Heffess, C.S. Adrenal cortical neoplasms in the pediatric population: A clinicopathologic and immunophenotypic analysis of 83 patients. Am. J. Surg. Pathol. 2003, 27, 867–881. [Google Scholar] [CrossRef]
- Juhlin, C.C.; Bertherat, J.; Giordano, T.J.; Hammer, G.D.; Sasano, H.; Mete, O. What did we learn from the molecular biology of adrenal cortical neoplasia? From histopathology to translational genomics. Endocr. Pathol. 2021, 32, 102–133. [Google Scholar] [CrossRef]
- Thompson, L.D. Pheochromocytoma of the adrenal gland scaled score (PASS) to separate benign from malignant neoplasms: A clinicopathologic and immunophenotypic study of 100 cases. Am. J. Surg. Pathol. 2002, 26, 551–566. [Google Scholar] [CrossRef]
- Kimura, N.; Takayanagi, R.; Takizawa, N.; Itagaki, E.; Katabami, T.; Kakoi, N.; Rakugi, H.; Ikeda, Y.; Tanabe, A.; Nigawara, T.; et al. Pathological grading for predicting metastasis in phaeochromocytoma and paraganglioma. Endocr. Relat. Cancer 2014, 21, 405–414. [Google Scholar] [CrossRef]
- Koh, J.M.; Ahn, S.H.; Kim, H.; Kim, B.J.; Sung, T.Y.; Kim, Y.H.; Hong, S.J.; Song, D.E.; Lee, S.H. Validation of pathological grading systems for predicting metastatic potential in pheochromocytoma and paraganglioma. PLoS ONE 2017, 12, e0187398. [Google Scholar] [CrossRef] [PubMed]
- Pierre, C.; Agopiantz, M.; Brunaud, L.; Battaglia-Hsu, S.F.; Max, A.; Pouget, C.; Nomine, C.; Lomazzi, S.; Vignaud, J.M.; Weryha, G.; et al. COPPS, a composite score integrating pathological features, PS100 and SDHB losses, predicts the risk of metastasis and progression-free survival in pheochromocytomas/paragangliomas. Virchows Arch. 2019, 474, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Mondovì, B.; Finazzi Agrò, A. Structure and function of amine oxidase. Adv. Exp. Med. Biol. 1982, 148, 141–153. [Google Scholar] [PubMed]
- Kumar, V.; Dooley, D.M.; Freeman, H.C.; Guss, J.M.; Harvey, I.; McGuirl, M.A.; Wilce, M.C.; Zubak, V.M. Crystal structure of a eukaryotic (pea seedling) copper-containing amine oxidase at 2.2 a resolution. Structure 1996, 4, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.L.; Kearns, G.L. Histamine: New thoughts about a familiar mediator. Clin. Pharmacol. Ther. 2011, 89, 189–197. [Google Scholar] [CrossRef]
- McGrath, A.P.; Hilmer, K.M.; Collyer, C.A.; Shepard, E.M.; Elmore, B.O.; Brown, D.E.; Dooley, D.M.; Guss, J.M. Structure and inhibition of human diamine oxidase. Biochemistry 2009, 48, 9810–9822. [Google Scholar] [CrossRef]
- Kaitaniemi, S.; Elovaara, H.; Grön, K.; Kidron, H.; Liukkonen, J.; Salminen, T.; Salmi, M.; Jalkanen, S.; Elima, K. The unique substrate specificity of human AOC2, a semicarbazide-sensitive amine oxidase. Cell Mol. Life Sci. 2009, 66, 2743–2757. [Google Scholar] [CrossRef]
- Smith, D.J.; Salmi, M.; Bono, P.; Hellman, J.; Leu, T.; Jalkanen, S. Cloning of vascular adhesion protein 1 reveals a novel multifunctional adhesion molecule. J. Exp. Med. 1998, 188, 17–27. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. Vascular adhesion protein-1: A cell surface amine oxidase in translation. Antioxid. Redox Signal. 2019, 30, 314–332. [Google Scholar] [CrossRef]
- Csiszar, K. Lysyl oxidases: A novel multifunctional amine oxidase family. Prog. Nucleic Acid. Res. Mol. Biol. 2001, 70, 1–32. [Google Scholar]
- Edmondson, D.E.; Newton-Vinson, P. The covalent fad of monoamine oxidase: Structural and functional role and mechanism of the flavinylation reaction. Antioxid. Redox Signal. 2001, 3, 789–806. [Google Scholar] [CrossRef]
- Yamada, M.; Yasuhara, H. Clinical pharmacology of MAO inhibitors: Safety and future. Neurotoxicology 2004, 25, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Reynaud, C.; Ferreras, L.; Di Mauro, P.; Kan, C.; Croset, M.; Bonnelye, E.; Pez, F.; Thomas, C.; Aimond, G.; Karnoub, A.E.; et al. Lysyl oxidase is a strong determinant of tumor cell colonization in bone. Cancer Res. 2017, 77, 268–278. [Google Scholar] [CrossRef]
- Ye, M.; Song, Y.; Pan, S.; Chu, M.; Wang, Z.W.; Zhu, X. Evolving roles of lysyl oxidase family in tumorigenesis and cancer therapy. Pharmacol. Ther. 2020, 215, 107633. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ou, W.; Tang, W.; Huang, Z.; Zhu, Z.; Ding, W.; Fu, J.; Zhu, Y.; Liu, C.; Xu, W.; et al. Increased AOC1 expression promotes cancer progression in colorectal cancer. Front. Oncol. 2021, 11, 657210. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Xu, Y.; Xiong, J.H.; Zhang, J.H.; Wu, J.; Luo, J.; Xiong, J.P. AOC1 contributes to tumor progression by promoting the AKT and EMT pathways in gastric cancer. Cancer Manag. Res. 2020, 12, 1789–1798. [Google Scholar] [CrossRef]
- Ding, Q.; Lin, D.; Zhou, Y.; Li, F.; Lai, J.; Duan, J.; Chen, J.; Jiang, C. Downregulation of amine oxidase copper containing 1 inhibits tumor progression by suppressing IL-6/JAK/STAT3 pathway activation in hepatocellular carcinoma. Oncol. Lett. 2021, 22, 857. [Google Scholar] [CrossRef]
- Sun, W.Y.; Choi, J.; Cha, Y.J.; Koo, J.S. Evaluation of the expression of amine oxidase proteins in breast cancer. Int. J. Mol. Sci. 2017, 18, 2775. [Google Scholar] [CrossRef]
- Lai, Y.C.; Chang, S.J.; Kostoro, J.; Kwan, A.L.; Chai, C.Y. Vascular adhesion protein-1 as indicator of breast cancer tumor aggressiveness and invasiveness. Apmis 2018, 126, 755–761. [Google Scholar] [CrossRef]
- Chang, C.Y.; Wu, K.L.; Chang, Y.Y.; Tsai, P.H.; Hung, J.Y.; Chang, W.A.; Jian, S.F.; Huang, Y.C.; Chong, I.W.; Tsai, Y.M.; et al. Amine oxidase, copper containing 3 exerts anti-mesenchymal transformation and enhances CD4(+) t-cell recruitment to prolong survival in lung cancer. Oncol. Rep. 2021, 46, 203. [Google Scholar] [CrossRef]
- Liu, F.; Hu, L.; Ma, Y.; Huang, B.; Xiu, Z.; Zhang, P.; Zhou, K.; Tang, X. Increased expression of monoamine oxidase a is associated with epithelial to mesenchymal transition and clinicopathological features in non-small cell lung cancer. Oncol. Lett. 2018, 15, 3245–3251. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guo, L.; Sun, Z.; Yang, G.; Guo, J.; Chen, K.; Xiao, R.; Yang, X.; Sheng, L. Monoamine oxidase a is a major mediator of mitochondrial homeostasis and glycolysis in gastric cancer progression. Cancer Manag. Res. 2020, 12, 8023–8035. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.B.; Shao, C.; Li, X.; Li, Q.; Hu, P.; Shi, C.; Li, Y.; Chen, Y.T.; Yin, F.; Liao, C.P.; et al. Monoamine oxidase a mediates prostate tumorigenesis and cancer metastasis. J. Clin. Investig. 2014, 124, 2891–2908. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, M.A.; Baskin, D.S. Monoamine oxidase B levels are highly expressed in human gliomas and are correlated with the expression of HiF-1α and with transcription factors Sp1 and Sp3. Oncotarget 2016, 7, 3379–3393. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Chien, M.H.; Lai, T.C.; Su, C.Y.; Jan, Y.H.; Hsiao, M.; Chen, C.L. Monoamine oxidase B expression correlates with a poor prognosis in colorectal cancer patients and is significantly associated with epithelial-to-mesenchymal transition-related gene signatures. Int. J. Mol. Sci. 2020, 21, 2813. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Park, S.Y.; Kim, H.; Sun, P.L.; Jin, Y.; Cho, S.K.; Kim, K.; Lee, C.T.; Chung, J.H. Membranous insulin-like growth factor-1 receptor (IGF1R) expression is predictive of poor prognosis in patients with epidermal growth factor receptor (EGFR)-mutant lung adenocarcinoma. J. Pathol. Transl. Med. 2015, 49, 382–388. [Google Scholar] [CrossRef]
- Grouzmann, E.; Matter, M.; Bilz, S.; Herren, A.; Triponez, F.; Henzen, C.; Kim, K.S.; Zulewski, H.; Buclin, T.; Brakch, N.; et al. Monoamine oxidase a down-regulation contributes to high metanephrine concentration in pheochromocytoma. J. Clin. Endocrinol. Metab. 2012, 97, 2773–2781. [Google Scholar] [CrossRef]
- Peters, T.M.A.; Lammerts van Bueren, I.; Geurtz, B.; Coene, K.L.M.; de Leeuw, N.; Brunner, H.G.; Jónsson, J.J.; Willemsen, M.; Wevers, R.A.; Verbeek, M.M. Monoamine oxidase a activity in fibroblasts as a functional confirmation of MAOA variants. JIMD Rep. 2021, 58, 114–121. [Google Scholar] [CrossRef]
- Groshong, R.; Gibson, D.A.; Baldessarini, R.J. Monoamine oxidase activity in cultured human skin fibroblasts. Clin. Chim. Acta 1977, 80, 113–120. [Google Scholar] [CrossRef]
- Denney, R.M. Relationship between monoamine oxidase (MAO) a specific activity and proportion of human skin fibroblasts which express the enzyme in culture. J. Neural Transm. Suppl. 1998, 52, 17–27. [Google Scholar]
- Sturza, A.; Leisegang, M.S.; Babelova, A.; Schröder, K.; Benkhoff, S.; Loot, A.E.; Fleming, I.; Schulz, R.; Muntean, D.M.; Brandes, R.P. Monoamine oxidases are mediators of endothelial dysfunction in the mouse aorta. Hypertension 2013, 62, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Lalor, P.F.; Edwards, S.; McNab, G.; Salmi, M.; Jalkanen, S.; Adams, D.H. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J. Immunol. 2002, 169, 983–992. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. Different forms of human vascular adhesion protein-1 (VAP-1) in blood vessels in vivo and in cultured endothelial cells: Implications for lymphocyte-endothelial cell adhesion models. Eur. J. Immunol. 1995, 25, 2803–2812. [Google Scholar] [CrossRef]
- Hashimoto, B.E.; Filly, R.A.; Callen, P.W. Fetal pseudoascites: Further anatomic observations. J. Ultrasound Med. 1986, 5, 151–152. [Google Scholar] [CrossRef]
- Hsia, L.T.; Ashley, N.; Ouaret, D.; Wang, L.M.; Wilding, J.; Bodmer, W.F. Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers. Proc. Natl. Acad. Sci. USA 2016, 113, E2162–E2171. [Google Scholar] [CrossRef] [PubMed]
- de Fraipont, F.; El Atifi, M.; Gicquel, C.; Bertagna, X.; Chambaz, E.M.; Feige, J.J. Expression of the angiogenesis markers vascular endothelial growth factor-a, thrombospondin-1, and platelet-derived endothelial cell growth factor in human sporadic adrenocortical tumors: Correlation with genotypic alterations. J. Clin. Endocrinol. Metab. 2000, 85, 4734–4741. [Google Scholar] [PubMed]
- Pereira, S.S.; Costa, M.M.; Guerreiro, S.G.; Monteiro, M.P.; Pignatelli, D. Angiogenesis and lymphangiogenesis in the adrenocortical tumors. Pathol. Oncol. Res. 2018, 24, 689–693. [Google Scholar] [CrossRef]
- Sasano, H.; Ohashi, Y.; Suzuki, T.; Nagura, H. Vascularity in human adrenal cortex. Mod. Pathol. 1998, 11, 329–333. [Google Scholar]
- Rooijens, P.P.; de Krijger, R.R.; Bonjer, H.J.; van der Ham, F.; Nigg, A.L.; Bruining, H.A.; Lamberts, S.W.; van der Harst, E. The significance of angiogenesis in malignant pheochromocytomas. Endocr. Pathol. 2004, 15, 39–45. [Google Scholar] [CrossRef]
- Zielke, A.; Middeke, M.; Hoffmann, S.; Colombo-Benkmann, M.; Barth, P.; Hassan, I.; Wunderlich, A.; Hofbauer, L.C.; Duh, Q.Y. VEGF-mediated angiogenesis of human pheochromocytomas is associated to malignancy and inhibited by anti-VEGF antibodies in experimental tumors. Surgery 2002, 132, 1056–1063, discussion 1063. [Google Scholar] [CrossRef]
- Li, J.; Pu, T.; Yin, L.; Li, Q.; Liao, C.P.; Wu, B.J. MAOA-mediated reprogramming of stromal fibroblasts promotes prostate tumorigenesis and cancer stemness. Oncogene 2020, 39, 3305–3321. [Google Scholar] [CrossRef] [PubMed]
- Rybinski, B.; Yun, K. Addressing intra-tumoral heterogeneity and therapy resistance. Oncotarget 2016, 7, 72322–72342. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Shema, E.; Loi, S.; Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat. Med. 2021, 27, 212–224. [Google Scholar] [CrossRef]
- McGranahan, N.; Swanton, C. Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef]
- Gulbahce, H.E.; Gamez, R.; Dvorak, L.; Forster, C.; Varghese, L. Concordance between tissue microarray and whole-section estrogen receptor expression and intratumoral heterogeneity. Appl. Immunohistochem. Mol. Morphol. 2012, 20, 340–343. [Google Scholar] [CrossRef] [PubMed]
- Kyndi, M.; Sørensen, F.B.; Knudsen, H.; Overgaard, M.; Nielsen, H.M.; Andersen, J.; Overgaard, J. Tissue microarrays compared with whole sections and biochemical analyses. A subgroup analysis of dbcg 82 b&c. Acta Oncol. 2008, 47, 591–599. [Google Scholar]
- Lin, Y.; Hatem, J.; Wang, J.; Quinn, A.; Hicks, D.; Tang, P. Tissue microarray-based immunohistochemical study can significantly underestimate the expression of HER2 and progesterone receptor in ductal carcinoma in situ of the breast. Biotech. Histochem. 2011, 86, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Flamand, V.; Zhao, H.; Peehl, D.M. Targeting monoamine oxidase a in advanced prostate cancer. J. Cancer Res. Clin. Oncol. 2010, 136, 1761–1771. [Google Scholar] [CrossRef]
- Zhao, H.; Flamand, V.; Peehl, D.M. Anti-oncogenic and pro-differentiation effects of clorgyline, a monoamine oxidase a inhibitor, on high grade prostate cancer cells. BMC Med. Genomics 2009, 2, 55. [Google Scholar] [CrossRef]
- Kushal, S.; Wang, W.; Vaikari, V.P.; Kota, R.; Chen, K.; Yeh, T.S.; Jhaveri, N.; Groshen, S.L.; Olenyuk, B.Z.; Chen, T.C.; et al. Monoamine oxidase A (MAO A) inhibitors decrease glioma progression. Oncotarget 2016, 7, 13842–13853. [Google Scholar] [CrossRef]
- Li, P.C.; Siddiqi, I.N.; Mottok, A.; Loo, E.Y.; Wu, C.H.; Cozen, W.; Steidl, C.; Shih, J.C. Monoamine oxidase a is highly expressed in classical hodgkin lymphoma. J. Pathol. 2017, 243, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, M.A.; Livingston, A.D.; Gist, T.L.; Ghosh, P.; Han, J.; Baskin, D.S. Successful treatment of intracranial glioblastoma xenografts with a monoamine oxidase B-activated pro-drug. EBioMedicine 2015, 2, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, W.; Gong, N.; Liu, P. Sodium danshensu inhibits the progression of lung cancer by regulating PI3K/Akt signaling pathway. Drug Dev. Res. 2022, 83, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.E.; Agus, D.B.; Dorff, T.B.; Pinski, J.K.; Quinn, D.I.; Castellanos, O.; Gilmore, P.; Shih, J.C. Phase 2 trial of monoamine oxidase inhibitor phenelzine in biochemical recurrent prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bondareva, A.; Downey, C.M.; Ayres, F.; Liu, W.; Boyd, S.K.; Hallgrimsson, B.; Jirik, F.R. The lysyl oxidase inhibitor, beta-aminopropionitrile, diminishes the metastatic colonization potential of circulating breast cancer cells. PLoS ONE 2009, 4, e5620. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Li, W.; Chen, J.; Xiao, X.; Wang, Y.; Yan, G.; Chen, L. Inactivation of lysyl oxidase by β-aminopropionitrile inhibits hypoxia-induced invasion and migration of cervical cancer cells. Oncol. Rep. 2013, 29, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Boufraqech, M.; Nilubol, N.; Zhang, L.; Gara, S.K.; Sadowski, S.M.; Mehta, A.; He, M.; Davis, S.; Dreiling, J.; Copland, J.A.; et al. miR30a inhibits LOX expression and anaplastic thyroid cancer progression. Cancer Res. 2015, 75, 367–377. [Google Scholar] [CrossRef]
- Natarajan, S.; Foreman, K.M.; Soriano, M.I.; Rossen, N.S.; Shehade, H.; Fregoso, D.R.; Eggold, J.T.; Krishnan, V.; Dorigo, O.; Krieg, A.J.; et al. Collagen remodeling in the hypoxic tumor-mesothelial niche promotes ovarian cancer metastasis. Cancer Res. 2019, 79, 2271–2284. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Huang, Y.; Ma, Y.; Jin, X.; Wang, H.; Wang, J. Inhibition of lysyl oxidase expression by dextran sulfate affects invasion and migration of gastric cancer cells. Int. J. Mol. Med. 2018, 42, 2737–2749. [Google Scholar] [PubMed]


| H-Score (Mean ± SD) | Total N = 295 (%) | Adrenal Cortical Neoplasm n = 132 (%) | Pheochromocytoma n = 163 (%) | p-Value |
|---|---|---|---|---|
| MAOA (T) | 164.4 ± 90.9 | 180.6 ± 90.5 | 150.8 ± 89.3 | 0.005 |
| MAOA (S) | 129.7 ± 79.4 | 126.7 ± 80.8 | 132.2 ± 78.5 | 0.555 |
| MAOB (T) | 17.4 ± 39.3 | 24.4 ± 40.7 | 11.8 ± 37.4 | 0.006 |
| MAOB (S) | 4.0 ± 19.6 | 3.1 ± 7.1 | 4.8 ± 25.6 | 0.469 |
| LOX (T) | 179.4 ± 67.3 | 163.3 ± 66.1 | 192.3 ± 65.6 | <0.001 |
| LOX (S) | 158.5 ± 68.7 | 142.1 ± 62.8 | 171.8 ± 70.6 | <0.001 |
| AOC3 (T) | 56.7 ± 44.5 | 46.1 ± 47.9 | 65.4 ± 39.7 | <0.001 |
| AOC3 (S) | 27.2 ± 29.0 | 26.7 ± 32.4 | 27.5 ± 26.0 | 0.798 |
| Parameters | Total N = 132 (%) | Adrenal Cortical Adenoma, n = 115 (%) | Adrenal Cortical Carcinoma, n = 17 (%) | p-Value |
|---|---|---|---|---|
| MAOA (T) | 0.606 | |||
| Low | 54 (40.9) | 46 (40.0) | 8 (47.1) | |
| High | 78 (59.1) | 69 (60.0) | 9 (52.9) | |
| MAOA (S) | <0.001 | |||
| Low | 62 (47.0) | 46 (40.0) | 16 (94.1) | |
| High | 70 (53.0) | 69 (60.0) | 1 (5.9) | |
| MAOB (T) | 0.341 | |||
| Low | 105 (79.5) | 93 (80.9) | 12 (70.6) | |
| High | 27 (20.5) | 22 (19.1) | 5 (29.4) | |
| MAOB (S) | 0.003 | |||
| Low | 93 (70.5) | 76 (66.1) | 17 (100.0) | |
| High | 39 (29.5) | 39 (33.9) | 0 (0.0) | |
| LOX (T) | 0.788 | |||
| Low | 48 (36.4) | 41 (35.7) | 7 (41.2) | |
| High | 84 (63.6) | 74 (64.3) | 10 (58.8) | |
| LOX (S) | 0.794 | |||
| Low | 56 (42.4) | 48 (41.7) | 8 (47.1) | |
| High | 76 (57.6) | 67 (58.3) | 9 (52.9) | |
| AOC3 (T) | 0.196 | |||
| Low | 72 (54.5) | 60 (52.2) | 12 (70.6) | |
| High | 60 (45.5) | 55 (47.8) | 5 (29.4) | |
| AOC3 (S) | 0.022 | |||
| Low | 93 (70.5) | 77 (67.0) | 16 (94.1) | |
| High | 39 (29.5) | 38 (33.0) | 1 (5.9) |
| Parameters | Total N = 163 (%) | GAPP < 3 n = 113 (%) | GAPP ≥ 3 n = 50 (%) | p-Value |
|---|---|---|---|---|
| MAOA (T) | 0.610 | |||
| Low | 67 (41.1) | 48 (42.5) | 19 (38.0) | |
| High | 96 (58.9) | 65 (57.5) | 31 (62.0) | |
| MAOA (S) | 0.235 | |||
| Low | 81 (49.7) | 60 (53.1) | 21 (42.0) | |
| High | 82 (50.3) | 53 (46.9) | 29 (58.0) | |
| MAOB (T) | 0.033 | |||
| Low | 139 (85.3) | 101 (89.4) | 38 (76.0) | |
| High | 24 (14.7) | 12 (10.6) | 12 (24.0) | |
| MAOB (S) | 0.215 | |||
| Low | 128 (78.5) | 92 (81.4) | 36 (72.0) | |
| High | 35 (21.5) | 21 (18.6) | 14 (28.0) | |
| LOX (T) | 0.728 | |||
| Low | 63 (38.7) | 45 (39.8) | 18 (36.0) | |
| High | 100 (61.3) | 68 (60.2) | 32 (64.0) | |
| LOX (S) | 0.732 | |||
| Low | 70 (42.9) | 50 (44.2) | 20 (40.0) | |
| High | 93 (57.1) | 63 (55.8) | 30 (60.0) | |
| AOC3 (T) | 0.864 | |||
| Low | 68 (41.7) | 48 (42.5) | 20 (40.0) | |
| High | 95 (58.3) | 65 (57.5) | 65 (57.5) | |
| AOC3 (S) | 0.731 | |||
| Low | 97 (59.5) | 66 (58.4) | 31 (62.0) | |
| High | 66 (40.5) | 47 (41.6) | 19 (38.0) |
| Parameter | No. of Patients /Recurrence /Death | Disease-Free Survival | Overall Survival | ||
|---|---|---|---|---|---|
| Mean Survival Months (95% CI) | p-Value | Mean Survival Months (95% CI) | p-Value | ||
| MAOA (T) | 0.839 | 0.096 | |||
| Low | 54/1/6 | 107 (103–110) | 97 (89–106) | ||
| High | 78/2/3 | 116 (112–120) | 114 (110–119) | ||
| MAOA (S) | - | 0.008 | |||
| Low | 62/3/8 | - | 104 (95–113) | ||
| High | 70/0/1 | - | 96 (94–99) | ||
| MAOB (T) | - | 0.850 | |||
| Low | 105/3/7 | - | 111 (106–116) | ||
| High | 27/0/2 | - | 101 (90–111) | ||
| MAOB (S) | - | - | |||
| Low | 93/3/9 | - | - | ||
| High | 39/0/0 | - | - | ||
| LOX (T) | 0.306 | 0.345 | |||
| Low | 48/3/2 | 105 (99–110) | 105 (99–110) | ||
| High | 84/1/7 | 117 (115–120) | 109 (102–116) | ||
| LOX (S) | 0.423 | 0.535 | |||
| Low | 56/2/3 | 105 (100–110) | 103 (98–109) | ||
| High | 76/1/6 | 117 (114–120) | 110 (103–116) | ||
| AOC3 (T) | - | 0.988 | |||
| Low | 72/3/5 | - | 111 (105–117) | ||
| High | 60/0/4 | - | 91 (85–97) | ||
| AOC3 (S) | - | - | |||
| Low | 93/3/9 | - | - | ||
| High | 39/0/0 | - | - | ||
| Parameter | No. of Patients /Recurrence /Death | Disease-Free Survival | Overall Survival | ||
|---|---|---|---|---|---|
| Mean Survival Months (95% CI) | p-Value | Mean Survival Months (95% CI) | p-Value | ||
| MAOA (T) | - | 0.094 | |||
| Low | 67/0/7 | - | 113 (102–124) | ||
| High | 96/3/3 | - | 160 (153–167) | ||
| MAOA (S) | 0.730 | 0.829 | |||
| Low | 81/1/5 | 138 (134–143) | 128 (118–138) | ||
| High | 82/2/5 | 154 (145–163) | 152 (140–165) | ||
| MAOB (T) | - | - | |||
| Low | 139/3/10 | - | - | ||
| High | 24/0/0 | - | - | ||
| MAOB (S) | - | - | |||
| Low | 128/3/10 | - | - | ||
| High | 35/0/0 | - | - | ||
| LOX (T) | 0.313 | 0.157 | |||
| Low | 63/2/6 | 154 (146–163) | 148 (134–162) | ||
| High | 100/1/4 | 151 (144–159) | 154 (142–165) | ||
| LOX (S) | 0.349 | 0.217 | |||
| Low | 70/2/6 | 154 (146–163) | 148 (134–162) | ||
| High | 93/1/4 | 151 (143–159) | 153 (142–165) | ||
| AOC3 (T) | 0.770 | 0.908 | |||
| Low | 68/1/4 | 157 (151–163) | 149 (138–160) | ||
| High | 95/2/6 | 151 (141–160) | 149 (136–163) | ||
| AOC3 (S) | 0.195 | 0.099 | |||
| Low | 97/1/9 | 159 (155–162) | 142 (130–153) | ||
| High | 66/2/1 | 143 (121–164) | 163 (156–170) | ||
| Parameter | Hazard Ratio | 95% CI | p-Value |
|---|---|---|---|
| Fuhrman grade | 0.797 | ||
| 1 or 2 versus 3 or 4 | 0.654 | 0.026–16.63 | |
| Mitosis (/50 HPFs) | 0.393 | ||
| ≤5 versus >5 | 3.315 | 0.212–51.76 | |
| Atypical mitosis | 0.362 | ||
| Absent versus present | 2.589 | 0.335–19.99 | |
| Clear cell proportion | 0.240 | ||
| ≥25% versus <25% | 5.684 | 0.313–103 | |
| Diffuse architecture (proportion) | 0.042 | ||
| <1/3 versus ≥1/3 | 12.796 | 1.094–149 | |
| Venous invasion | 0.002 | ||
| Absent versus present | 60.934 | 4.605–806 | |
| Capsular invasion | 0.965 | ||
| Absent versus present | 0.954 | 0.115–7.885 | |
| Weiss score | 0.839 | ||
| <4 versus ≥4 | 0.680 | 0.017–27.86 | |
| MAOA (S) expression | 0.423 | ||
| Low versus high | 0.215 | 0.005–9.273 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.K.; Koo, J.S. Expression of Amine Oxidase Proteins in Adrenal Cortical Neoplasm and Pheochromocytoma. Biomedicines 2023, 11, 1896. https://doi.org/10.3390/biomedicines11071896
Kim EK, Koo JS. Expression of Amine Oxidase Proteins in Adrenal Cortical Neoplasm and Pheochromocytoma. Biomedicines. 2023; 11(7):1896. https://doi.org/10.3390/biomedicines11071896
Chicago/Turabian StyleKim, Eun Kyung, and Ja Seung Koo. 2023. "Expression of Amine Oxidase Proteins in Adrenal Cortical Neoplasm and Pheochromocytoma" Biomedicines 11, no. 7: 1896. https://doi.org/10.3390/biomedicines11071896
APA StyleKim, E. K., & Koo, J. S. (2023). Expression of Amine Oxidase Proteins in Adrenal Cortical Neoplasm and Pheochromocytoma. Biomedicines, 11(7), 1896. https://doi.org/10.3390/biomedicines11071896






