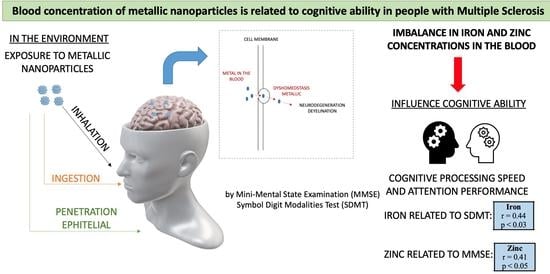
The Blood Concentration of Metallic Nanoparticles Is Related to Cognitive Performance in People with Multiple Sclerosis: An Exploratory Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Protocol and Data Analysis
2.3. Statistical Analysis
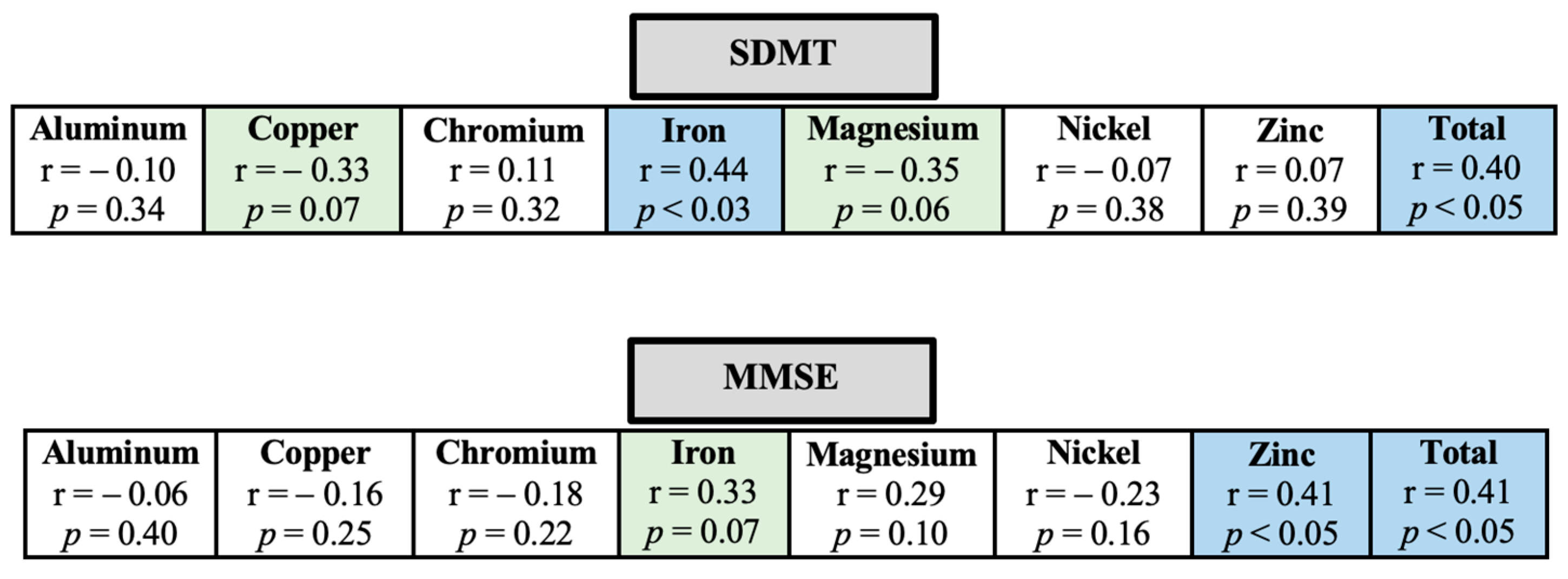
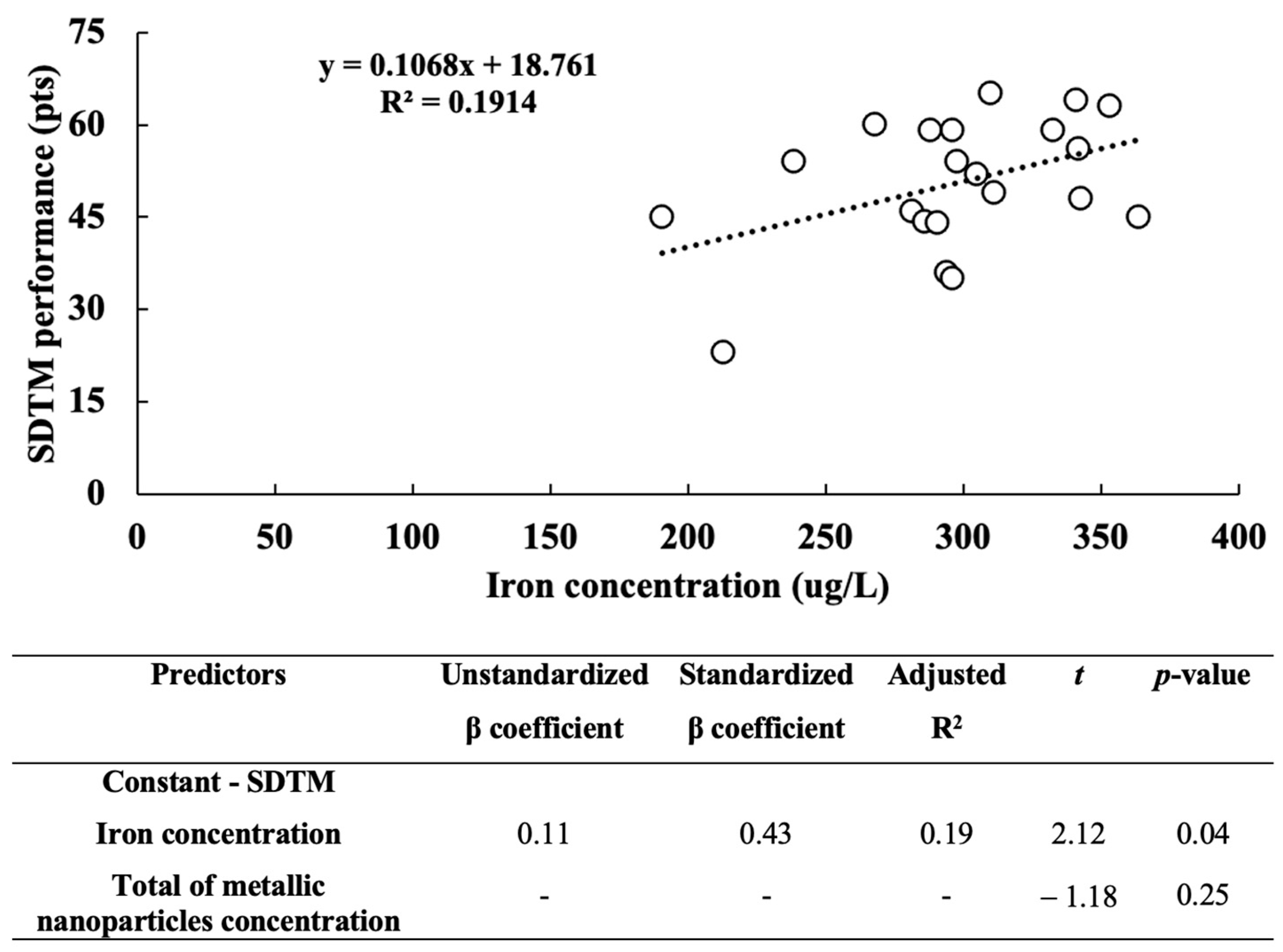
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dutta, R.; Trapp, B.D. Mechanisms of Neuronal Dysfunction and Degeneration in Multiple Sclerosis. Prog. Neurobiol. 2011, 93, 1–12. [Google Scholar] [CrossRef]
- Brummer, T.; Muthuraman, M.; Steffen, F.; Uphaus, T.; Minch, L.; Person, M.; Zipp, F.; Groppa, S.; Bittner, S.; Fleischer, V. Improved Prediction of Early Cognitive Impairment in Multiple Sclerosis Combining Blood and Imaging Biomarkers. Brain Commun. 2022, 4, fcac153. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive Impairment in Multiple Sclerosis: Clinical Management, MRI, and Therapeutic Avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef] [PubMed]
- Frndak, S.E.; Kordovski, V.M.; Cookfair, D.; Rodgers, J.D.; Weinstock-Guttman, B.; Benedict, R.H.B. Disclosure of Disease Status among Employed Multiple Sclerosis Patients: Association with Negative Work Events and Accommodations. Mult. Scler. 2015, 21, 225–234. [Google Scholar] [CrossRef]
- Ruano, L.; Portaccio, E.; Goretti, B.; Niccolai, C.; Severo, M.; Patti, F.; Cilia, S.; Gallo, P.; Grossi, P.; Ghezzi, A.; et al. Age and Disability Drive Cognitive Impairment in Multiple Sclerosis across Disease Subtypes. Mult. Scler. J. 2017, 23, 1258–1267. [Google Scholar] [CrossRef]
- Johnen, A.; Landmeyer, N.C.; Bürkner, P.-C.; Wiendl, H.; Meuth, S.G.; Holling, H. Distinct Cognitive Impairments in Different Disease Courses of Multiple Sclerosis—A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2017, 83, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.; DeLuca, J.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R. Validity of the Symbol Digit Modalities Test as a Cognition Performance Outcome Measure for Multiple Sclerosis. Mult. Scler. J. 2017, 23, 721–733. [Google Scholar] [CrossRef]
- Rademacher, T.-D.; Meuth, S.G.; Wiendl, H.; Johnen, A.; Landmeyer, N.C. Molecular Biomarkers and Cognitive Impairment in Multiple Sclerosis: State of the Field, Limitations, and Future Direction—A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2023, 146, 105035. [Google Scholar] [CrossRef]
- Modica, C.M.; Zivadinov, R.; Dwyer, M.G.; Bergsland, N.; Weeks, A.R.; Benedict, R.H.B. Iron and Volume in the Deep Gray Matter: Association with Cognitive Impairment in Multiple Sclerosis. Am. J. Neuroradiol. 2015, 36, 57–62. [Google Scholar] [CrossRef]
- Oset, M.; Stasiolek, M.; Matysiak, M. Cognitive Dysfunction in the Early Stages of Multiple Sclerosis—How Much and How Important? Curr. Neurol. Neurosci. Rep. 2020, 20, 22. [Google Scholar] [CrossRef]
- Migliore, S.; Ghazaryan, A.; Simonelli, I.; Pasqualetti, P.; Squitieri, F.; Curcio, G.; Landi, D.; Palmieri, M.G.; Moffa, F.; Filippi, M.M.; et al. Cognitive Impairment in Relapsing-Remitting Multiple Sclerosis Patients with Very Mild Clinical Disability. Behav. Neurol. 2017, 2017, 7404289. [Google Scholar] [CrossRef]
- de Oliveira, M.; Gianeti, T.M.R.; da Rocha, F.C.G.; Lisboa-Filho, P.N.; Piacenti-Silva, M. A Preliminary Study of the Concentration of Metallic Elements in the Blood of Patients with Multiple Sclerosis as Measured by ICP-MS. Sci. Rep. 2020, 10, 13112. [Google Scholar] [CrossRef] [PubMed]
- Crichton, R.R.; Dexter, D.T.; Ward, R.J. Metal Based Neurodegenerative Diseases—From Molecular Mechanisms to Therapeutic Strategies. Coord. Chem. Rev. 2008, 252, 1189–1199. [Google Scholar] [CrossRef]
- Cabral Pinto, M.M.S.; Marinho-Reis, A.P.; Almeida, A.; Ordens, C.M.; Silva, M.M.V.G.; Freitas, S.; Simões, M.R.; Moreira, P.I.; Dinis, P.A.; Diniz, M.L.; et al. Human Predisposition to Cognitive Impairment and Its Relation with Environmental Exposure to Potentially Toxic Elements. Environ. Geochem. Health 2018, 40, 1767–1784. [Google Scholar] [CrossRef] [PubMed]
- Nirooei, E.; Kashani, S.M.A.; Owrangi, S.; Malekpour, F.; Niknam, M.; Moazzen, F.; Nowrouzi-Sohrabi, P.; Farzinmehr, S.; Akbari, H. Blood Trace Element Status in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2021, 200, 13–26. [Google Scholar] [CrossRef]
- Chen, P.; Miah, M.R.; Aschner, M. Metals and Neurodegeneration. F1000Research 2016, 5, 366. [Google Scholar] [CrossRef]
- Singh, A.V.; Khare, M.; Gade, W.N.; Zamboni, P. Theranostic Implications of Nanotechnology in Multiple Sclerosis: A Future Perspective. Autoimmune Dis. 2012, 2012, 160830. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A Flexible Statistical Power Analysis Program for the Social, Behavioral, and Biomedical Sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating Neurologic Impairment in Multiple Sclerosis: An Expanded Disability Status Scale (EDSS). Neurology 1983, 33, 1444. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of Multiple Sclerosis: 2017 Revisions of the McDonald Criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, M.H.; Souza, G.B.; Oliveira, R.V.; Forato, L.A.; Nóbrega, J.A.; Nogueira, A.R.A. Microwave-Assisted Digestion Procedures for Biological Samples with Diluted Nitric Acid: Identification of Reaction Products. Talanta 2009, 79, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Bocca, B.; Forte, G.; Petrucci, F.; Senofonte, O.; Violante, N.; Alimonti, A. Development of Methods for the Quantification of Essential and Toxic Elements in Human Biomonitoring. Ann. Ist. Super. Sanita 2005, 41, 165–170. [Google Scholar] [PubMed]
- Boringa, J.B.; Lazeron, R.H.; Reuling, I.E.; Adèr, H.J.; Pfennings, L.; Lindeboom, J.; de Sonneville, L.M.; Kalkers, N.F.; Polman, C.H. The Brief Repeatable Battery of Neuropsychological Tests: Normative Values Allow Application in Multiple Sclerosis Clinical Practice. Mult. Scler. 2001, 7, 263–267. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Arevalo-Rodriguez, I.; Smailagic, N.; Roqué I Figuls, M.; Ciapponi, A.; Sanchez-Perez, E.; Giannakou, A.; Pedraza, O.L.; Bonfill Cosp, X.; Cullum, S. Mini-Mental State Examination (MMSE) for the Detection of Alzheimer’s Disease and Other Dementias in People with Mild Cognitive Impairment (MCI). Cochrane Database Syst. Rev. 2015, 2015, CD010783. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988. [Google Scholar]
- LoPresti, P. Serum-Based Biomarkers in Neurodegeneration and Multiple Sclerosis. Biomedicines 2022, 10, 1077. [Google Scholar] [CrossRef]
- Dales, J.-P.; Desplat-Jégo, S. Metal Imbalance in Neurodegenerative Diseases with a Specific Concern to the Brain of Multiple Sclerosis Patients. Int. J. Mol. Sci. 2020, 21, 9105. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Biological Metals and Metal-Targeting Compounds in Major Neurodegenerative Diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef]
- Ferrero, M.E. Neuron Protection by EDTA May Explain the Successful Outcomes of Toxic Metal Chelation Therapy in Neurodegenerative Diseases. Biomedicines 2022, 10, 2476. [Google Scholar] [CrossRef]
- Bittner, S.; Oh, J.; Havrdová, E.K.; Tintoré, M.; Zipp, F. The Potential of Serum Neurofilament as Biomarker for Multiple Sclerosis. Brain 2021, 144, 2954–2963. [Google Scholar] [CrossRef]
- Hametner, S.; Dal Bianco, A.; Trattnig, S.; Lassmann, H. Iron Related Changes in MS Lesions and Their Validity to Characterize MS Lesion Types and Dynamics with Ultra-High Field Magnetic Resonance Imaging. Brain Pathol. 2018, 28, 743–749. [Google Scholar] [CrossRef]
- Skjørringe, T.; Møller, L.B.; Moos, T. Impairment of Interrelated Iron- and Copper Homeostatic Mechanisms in Brain Contributes to the Pathogenesis of Neurodegenerative Disorders. Front. Pharmacol. 2012, 3, 169. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, K.J. The Interaction of Zinc and the Blood-Brain Barrier under Physiological and Ischemic Conditions. Toxicol. Appl. Pharmacol. 2019, 364, 114–119. [Google Scholar] [CrossRef]
- Johnson, S. The Possible Role of Gradual Accumulation of Copper, Cadmium, Lead and Iron and Gradual Depletion of Zinc, Magnesium, Selenium, Vitamins B2, B6, D, and E Essential Fatty Acids in Multiple Sclerosis. Med. Hypotheses 2000, 55, 239–241. [Google Scholar] [CrossRef]
- Ontaneda, D.; Thompson, A.J.; Fox, R.J.; Cohen, J.A. Progressive Multiple Sclerosis: Prospects for Disease Therapy, Repair, and Restoration of Function. Lancet 2017, 389, 1357–1366. [Google Scholar] [CrossRef]
- Fujiwara, E.; Kmech, J.A.; Cobzas, D.; Sun, H.; Seres, P.; Blevins, G.; Wilman, A.H. Cognitive Implications of Deep Gray Matter Iron in Multiple Sclerosis. AJNR. Am. J. Neuroradiol. 2017, 38, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.; Buchheit, C.L.; Berman, N.E.J.; LeVine, S.M. Pathogenic Implications of Iron Accumulation in Multiple Sclerosis. J. Neurochem. 2012, 120, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Molina-Holgado, F.; Hider, R.C.; Gaeta, A.; Williams, R.; Francis, P. Metals Ions and Neurodegeneration. BioMetals 2007, 20, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Bartzokis, G. Age-Related Myelin Breakdown: A Developmental Model of Cognitive Decline and Alzheimer’s Disease. Neurobiol. Aging 2004, 25, 5–62. [Google Scholar] [CrossRef]
- da Silva Castro, Á.; da Silva Albuquerque, L.; de Melo, M.L.P.; D’Almeida, J.A.C.; Braga, R.A.M.; de Assis, R.C.; do Nascimento Marreiro, D.; Matos, W.O.; Maia, C.S.C. Relationship between Zinc-Related Nutritional Status and the Progression of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022, 66, 104063. [Google Scholar] [CrossRef] [PubMed]
- McAllum, E.J.; Finkelstein, D.I. Metals in Alzheimer’s and Parkinson’s Disease: Relevance to Dementia with Lewy Bodies. J. Mol. Neurosci. 2016, 60, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Jung, J.; Suh, S. The Emerging Role of Zinc in the Pathogenesis of Multiple Sclerosis. Int. J. Mol. Sci. 2017, 18, 2070. [Google Scholar] [CrossRef] [PubMed]
| Individual Characteristics | |
|---|---|
| Sex (female/male) | 12/9 |
| Age (years) | 32 ± 7 (45–18) |
| Height (m) | 1.68 ± 0.02 (1.89–1.55) |
| Body mass (kg) | 74.4 ± 3.1(94.6–51.1) |
| Clinical characteristics | |
| EDSS (points) | 2.5 ± 1.1 (4.5–1.0) |
| Relapse time (months) | 38 ± 30 (94–3) |
| Multiple sclerosis onset (months) | 92 ± 77 (276–5) |
| Cognitive characteristics | |
| SDMT (points) | 50.49 ± 10.70 (65–23) |
| MMSE (points) | 29.05 ± 0.80 (30–28) |
| Blood concentration of metallic nanoparticles | |
| Aluminum (ug/L) | 7.56 ± 2.52 (16.18–4.39) |
| Copper (ug/L) | 0.98 ± 0.39 (1.78–0.44) |
| Chromium (ug/L) | 0.39 ± 0.11 (0.65–0.29) |
| Iron (ug/L) | 297.16 ± 43.84 (363.58–190.74) |
| Magnesium (ug/L) | 28.69 ± 5.59 (41.77–20.03) |
| Nickel (ug/L) | 0.31 ± 0.48 (1.69–0.04) |
| Zinc (ug/L) | 3.20 ± 0.85 (4.58–1.63) |
| Total (ug/L) | 338.30 ± 42.23 (404.92–235.56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, M.; Santinelli, F.B.; Lisboa-Filho, P.N.; Barbieri, F.A. The Blood Concentration of Metallic Nanoparticles Is Related to Cognitive Performance in People with Multiple Sclerosis: An Exploratory Analysis. Biomedicines 2023, 11, 1819. https://doi.org/10.3390/biomedicines11071819
de Oliveira M, Santinelli FB, Lisboa-Filho PN, Barbieri FA. The Blood Concentration of Metallic Nanoparticles Is Related to Cognitive Performance in People with Multiple Sclerosis: An Exploratory Analysis. Biomedicines. 2023; 11(7):1819. https://doi.org/10.3390/biomedicines11071819
Chicago/Turabian Stylede Oliveira, Marcela, Felipe Balistieri Santinelli, Paulo Noronha Lisboa-Filho, and Fabio Augusto Barbieri. 2023. "The Blood Concentration of Metallic Nanoparticles Is Related to Cognitive Performance in People with Multiple Sclerosis: An Exploratory Analysis" Biomedicines 11, no. 7: 1819. https://doi.org/10.3390/biomedicines11071819
APA Stylede Oliveira, M., Santinelli, F. B., Lisboa-Filho, P. N., & Barbieri, F. A. (2023). The Blood Concentration of Metallic Nanoparticles Is Related to Cognitive Performance in People with Multiple Sclerosis: An Exploratory Analysis. Biomedicines, 11(7), 1819. https://doi.org/10.3390/biomedicines11071819