Lipoprotein(a) As a Potential Predictive Factor for Earlier Aortic Valve Replacement in Patients with Bicuspid Aortic Valve
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Echocardiography
2.3. Laboratory Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoffman, J.I.E.; Kaplan, S. The Incidence of Congenital Heart Disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, F.; Guzzardi, D.G.; Bancone, C.; Fedak, P.W.M.; Della Corte, A. The Science of BAV Aortopathy. Prog. Cardiovasc. Dis. 2020, 63, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Rahatianpur, M.; Bakhtiary, F.; Vázquez-Jiménez, J.; Dähnert, I.; Kostelka, M. The Association of Bicuspid Aortic Valve on Long-Term Outcome Following One-Stage Repair of Aortic Arch Obstruction Associated with Ventricular Septal Defect. Cardiol. Young 2022, 33, 227–234. [Google Scholar] [CrossRef]
- Hecht, S.; Butcher, S.C.; Pio, S.M.; Kong, W.K.F.; Singh, G.K.; Ng, A.C.T.; Perry, R.; Poh, K.K.; Almeida, A.G.; González, A.; et al. Impact of Left Ventricular Ejection Fraction on Clinical Outcomes in Bicuspid Aortic Valve Disease. J. Am. Coll. Cardiol. 2022, 80, 1071–1084. [Google Scholar] [CrossRef]
- Butcher, S.C.; Pio, S.M.; Kong, W.K.F.; Singh, G.K.; Ng, A.C.T.; Perry, R.; Sia, C.H.; Poh, K.K.; Almeida, A.G.; González, A.; et al. Left Ventricular Remodelling in Bicuspid Aortic Valve Disease. Eur. Heart J. Cardiovasc. Imaging 2022, 23, 1669–1679. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Tastet, L.; Capoulade, R.; Larose, É.; Bédard, É.; Arsenault, M.; Chetaille, P.; Dumesnil, J.G.; Mathieu, P.; Clavel, M.A.; et al. Effect of Age and Aortic Valve Anatomy on Calcification and Haemodynamic Severity of Aortic Stenosis. Heart 2017, 103, 32–39. [Google Scholar] [CrossRef]
- Siu, S.C.; Silversides, C.K. Bicuspid Aortic Valve Disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Elkoumy, A.; Jose, J.; Terkelsen, C.J.; Nissen, H.; Gunasekaran, S.; Abdelshafy, M.; Seth, A.; Elzomor, H.; Kumar, S.; Bedogni, F.; et al. Safety and Efficacy of Myval Implantation in Patients with Severe Bicuspid Aortic Valve Stenosis—A Multicenter Real-World Experience. J. Clin. Med. 2022, 11, 443. [Google Scholar] [CrossRef]
- Mundal, L.J.; Hovland, A.; Igland, J.; Veierød, M.B.; Holven, K.B.; Bogsrud, M.P.; Tell, G.S.; Leren, T.P.; Retterstøl, K. Association of Low-Density Lipoprotein Cholesterol with Risk of Aortic Valve Stenosis in Familial Hypercholesterolemia. JAMA Cardiol. 2019, 4, 1156–1159. [Google Scholar] [CrossRef]
- Phua, K.; Chew, N.W.; Kong, W.K.; Tan, R.-S.; Ye, L.; Poh, K.-K. The Mechanistic Pathways of Oxidative Stress in Aortic Stenosis and Clinical Implications. Theranostics 2022, 12, 5189–5203. [Google Scholar] [CrossRef]
- Hofmanis, J.; Hofmane, D.; Svirskis, S.; Mackevics, V.; Tretjakovs, P.; Lejnieks, A.; Signorelli, S.S. HDL-C Role in Acquired Aortic Valve Stenosis Patients and Its Relationship with Oxidative Stress. Med. 2019, 55, 416. [Google Scholar] [CrossRef] [PubMed]
- Perera, S.; Wijesinghe, N.; Ly, E.; Devlin, G.; Pasupati, S. Outcomes of Patients with Untreated Severe Aortic Stenosis in Real-World Practice. N. Z. Med. J. 2011, 124, 40–48. [Google Scholar] [PubMed]
- Otto, C.M.; Prendergast, B. Aortic-Valve Stenosis—From Patients at Risk to Severe Valve Obstruction. N. Engl. J. Med. 2014, 371, 744–756. [Google Scholar] [CrossRef] [PubMed]
- Schnitzler, J.G.; Ali, L.; Groenen, A.G.; Kaiser, Y.; Kroon, J. Lipoprotein(a) as Orchestrator of Calcific Aortic Valve Stenosis. Biomolecules 2019, 9, 760. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Faggiano, A.; Bernardi, N.; Carugo, S.; Giammanco, A.; Faggiano, P. Lipoprotein(a) and Aortic Valve Stenosis: A Casual or Causal Association? Nutr. Metab. Cardiovasc. Dis. 2022, 32, 309–317. [Google Scholar] [CrossRef]
- Cegla, J.; France, M.; Marcovina, S.M.; Neely, R.D.G. Lp(a): When and How to Measure It. Ann. Clin. Biochem. 2021, 58, 16–21. [Google Scholar] [CrossRef]
- Langsted, A.; Nordestgaard, B.G. Antisense Oligonucleotides Targeting Lipoprotein(a). Curr. Atheroscler. Rep. 2019, 21, 30. [Google Scholar] [CrossRef]
- Schmidt, K.; Noureen, A.; Kronenberg, F.; Utermann, G. Structure, Function, and Genetics of Lipoprotein (a). J. Lipid Res. 2016, 57, 1339–1359. [Google Scholar] [CrossRef]
- Boffa, M.B.; Koschinsky, M.L. Oxidized Phospholipids as a Unifying Theory for Lipoprotein(a) and Cardiovascular Disease. Nat. Rev. Cardiol. 2019, 16, 305–318. [Google Scholar] [CrossRef]
- Yeang, C.; Wilkinson, M.J.; Tsimikas, S. Lipoprotein(a) and Oxidized Phospholipids in Calcific Aortic Valve Stenosis. Curr. Opin. Cardiol. 2016, 31, 440–450. [Google Scholar] [CrossRef]
- Nurmohamed, N.S.; Collard, D.; Reeskamp, L.F.; Kaiser, Y.; Kroon, J.; Tromp, T.R.; van den Born, B.J.H.; Coppens, M.; Vlaar, A.P.J.; Beudel, M.; et al. Lipoprotein(a), Venous Thromboembolism and COVID-19: A Pilot Study. Atherosclerosis 2022, 341, 43–49. [Google Scholar] [CrossRef]
- Ugovšek, S.; Šebeštjen, M. Lipoprotein(a)—The Crossroads of Atherosclerosis, Atherothrombosis and Inflammation. Biomolecules 2022, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, R.; Devillers, R.; Perrot, N.; Després, A.A.; Boulanger, M.C.; Mitchell, P.L.; Guertin, J.; Couture, P.; Boffa, M.B.; Scipione, C.A.; et al. Interaction of Autotaxin With Lipoprotein(a) in Patients With Calcific Aortic Valve Stenosis. JACC Basic to Transl. Sci. 2020, 5, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, P.; Boulanger, M.C. Autotaxin and Lipoprotein Metabolism in Calcific Aortic Valve Disease. Front. Cardiovasc. Med. 2019, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.; Clark, J.R.; Koschinsky, M.L.; Boffa, M.B. Lipoprotein(a): Expanding Our Knowledge of Aortic Valve Narrowing. Trends Cardiovasc. Med. 2021, 31, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Srivastava, S.; Velmurugan, M. Newer Perspectives of Coronary Artery Disease in Young. World J. Cardiol. 2016, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Pineda, J.; Marín, F.; Marco, P.; Roldán, V.; Valencia, J.; Ruiz-Nodar, J.M.; Sogorb, F.; Lip, G.Y.H. Premature Coronary Artery Disease in Young (Age<45) Subjects: Interactions of Lipid Profile, Thrombophilic and Haemostatic Markers. Int. J. Cardiol. 2009, 136, 222–225. [Google Scholar] [CrossRef]
- Nakamura, K.; Ohkawa, R.; Okubo, S.; Tozuka, M.; Okada, M.; Aoki, S.; Aoki, J.; Arai, H.; Ikeda, H.; Yatomi, Y. Measurement of Lysophospholipase D/Autotaxin Activity in Human Serum Samples. Clin. Biochem. 2007, 40, 274–277. [Google Scholar] [CrossRef]
- Capoulade, R.; Chan, K.L.; Yeang, C.; Mathieu, P.; Bossé, Y.; Dumesnil, J.G.; Tam, J.W.; Teo, K.K.; Mahmut, A.; Yang, X.; et al. Oxidized Phospholipids, Lipoprotein(a), and Progression of Calcific Aortic Valve Stenosis. J. Am. Coll. Cardiol. 2015, 66, 1236–1246. [Google Scholar] [CrossRef]
- Kronenberg, F. Lipoprotein(a) Measurement Issues: Are We Making a Mountain out of a Molehill? Atherosclerosis 2022, 349, 123–135. [Google Scholar] [CrossRef]
- Gotoh, T.; Kuroda, T.; Yamasawa, M.; Nishinaga, M.; Mitsuhashi, T.; Seino, Y.; Nagoh, N.; Kayaba, K.; Yamada, S.; Matsuo, H.; et al. Correlation between Lipoprotein(a) and Aortic Valve Sclerosis Assessed by Echocardiography (the JMS Cardiac Echo and Cohort Study). Am. J. Cardiol. 1995, 76, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Steffen, B.T.; Budoff, M.; Post, W.S.; Thanassoulis, G.; Kestenbaum, B.; Mcconnell, J.P.; Warnick, R.; Guan, W.; Tsai, M.Y. Lipoprotein(a) Levels Are Associated with Subclinical Calcific Aortic Valve Disease in White and Black Individuals: The Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1003–1009. [Google Scholar] [CrossRef]
- Stewart, B.F.; Siscovick, D.; Lind, B.K.; Gardin, J.M.; Gottdiener, J.S.; Smith, V.E.; Kitzman, D.W.; Otto, C.M. Clinical Factors Associated with Calcific Aortic Valve Disease. J. Am. Coll. Cardiol. 1997, 29, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, B.J.; Boekholdt, S.M.; Dubé, M.P.; Rhéaume, É.; Wareham, N.J.; Khaw, K.T.; Sandhu, M.S.; Tardif, J.C. Lipoprotein(a) Levels, Genotype, and Incident Aortic Valve Stenosis a Prospective Mendelian Randomization Study and Replication in a Case-Control Cohort. Circ. Cardiovasc. Genet. 2014, 7, 304–310. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Elevated Lipoprotein(a) and Risk of Aortic Valve Stenosis in the General Population. J. Am. Coll. Cardiol. 2014, 63, 470–477. [Google Scholar] [CrossRef]
- Langsted, A.; Varbo, A.; Kamstrup, P.R.; Nordestgaard, B.G. Elevated Lipoprotein(a) Does Not Cause Low-Grade Inflammation despite Causal Association with Aortic Valve Stenosis and Myocardial Infarction: A Study of 100 578 Individuals from the General Population. J. Clin. Endocrinol. Metab. 2015, 100, 2690–2699. [Google Scholar] [CrossRef] [PubMed]
- Sticchi, E.; Giusti, B.; Cordisco, A.; Gori, A.M.; Sereni, A.; Sofi, F.; Mori, F.; Colonna, S.; Fugazzaro, M.P.; Pepe, G.; et al. Role of Lipoprotein (a) and LPA KIV2 Repeat Polymorphism in Bicuspid Aortic Valve Stenosis and Calcification: A Proof of Concept Study. Intern. Emerg. Med. 2019, 14, 45–50. [Google Scholar] [CrossRef]
- Ker, J. Bicuspid Aortic Valve Disease and Lipoprotein(a)—A Concept Worth Exploring? Int. J. Cardiol. 2014, 174, 197–203. [Google Scholar] [CrossRef]
- Torzewski, M.; Ravandi, A.; Yeang, C.; Edel, A.; Bhindi, R.; Kath, S.; Twardowski, L.; Schmid, J.; Yang, X.; Franke, U.F.W.; et al. Lipoprotein(a)-Associated Molecules Are Prominent Components in Plasma and Valve Leaflets in Calcific Aortic Valve Stenosis. JACC Basic to Transl. Sci. 2017, 2, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Nsaibia, M.J.; Mahmut, A.; Boulanger, M.C.; Arsenault, B.J.; Bouchareb, R.; Simard, S.; Witztum, J.L.; Clavel, M.A.; Pibarot, P.; Bossé, Y.; et al. Autotaxin Interacts with Lipoprotein(a) and Oxidized Phospholipids in Predicting the Risk of Calcific Aortic Valve Stenosis in Patients with Coronary Artery Disease. J. Intern. Med. 2016, 280, 509–517. [Google Scholar] [CrossRef]
- Junco-Vicente, A.; Solache-Berrocal, G.; del Río-García, Á.; Rolle-Sóñora, V.; Areces, S.; Morís, C.; Martín, M.; Rodríguez, I. IL6 Gene Polymorphism Association with Calcific Aortic Valve Stenosis and Influence on Serum Levels of Interleukin-6. Front. Cardiovasc. Med. 2022, 9, 989539. [Google Scholar] [CrossRef] [PubMed]
- Wypasek, E.; Potaczek, D.P.; Lamplmayr, M.; Sadowski, J.; Undas, A. Interleukin-6 Receptor ASP358ALA Gene Polymorphism Is Associated with Plasma C-Reactive Protein Levels and Severity of Aortic Valve Stenosis. Clin. Chem. Lab. Med. 2014, 52, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Wade, D.P.; Clarke, J.G.; Lindahl, G.E.; Liu, A.C.; Zysow, B.R.; Meer, K.; Schwartz, K.; Lawn, R.M. 5’ Control Regions of the Apolipoprotein(a) Gene and Members of the Related Plasminogen Gene Family. Proc. Natl. Acad. Sci. USA 1993, 90, 1369–1373. [Google Scholar] [CrossRef] [PubMed]
- Novaro, G.M.; Katz, R.; Aviles, R.J.; Gottdiener, J.S.; Cushman, M.; Psaty, B.M.; Otto, C.M.; Griffin, B.P. Clinical Factors, But Not C-Reactive Protein, Predict Progression of Calcific Aortic-Valve Disease. The Cardiovascular Health Study. J. Am. Coll. Cardiol. 2007, 50, 1992–1998. [Google Scholar] [CrossRef]
- Hsu, S.Y.; Hung, K.C.; Chang, S.H.; Wen, M.S.; Hsieh, I.C. C-Reactive Protein in Predicting Coronary Artery Disease in Subjects with Aortic Valve Sclerosis before Diagnostic Coronary Angiography. Am. J. Med. Sci. 2006, 331, 264–269. [Google Scholar] [CrossRef]
- Wypasek, E.; Wypasek, E.; Potaczek, D.P.; Undas, A. Association of the C-Reactive Protein Gene (CRP) Rs1205 C>T Polymorphism with Aortic Valve Calcification in Patients with Aortic Stenosis. Int. J. Mol. Sci. 2015, 16, 23745–23759. [Google Scholar] [CrossRef]
- Topçiu-Shufta, V.; Haxhibeqiri, V.; Begolli, L.; Baruti-Gafurri, Z.; Veseli, S.; Haxhibeqiri, S.; Miftari, R.; Kurti, L.; Avdiu, D. Correlation of Inflammation and Lipoprotein (a) with Hypercoagulability in Hemodialysis Patients. Med. Arch. 2015, 69, 232–235. [Google Scholar] [CrossRef]
- Gracia Baena, J.M.; Calaf Vall, I.; Zielonka, M.; Marsal Mora, J.R.; Godoy, P.; Worner Diz, F. Risk Factors and Comorbidities Associated with Severe Aortic Stenosis: A Case-Control Study. Rev. Clin. Esp. 2021, 221, 249–257. [Google Scholar] [CrossRef]
- Sequeira Gross, T.M.; Kuntze, T.; Bernhardt, A.; Reichenspurner, H.; von Kodolitsch, Y.; Girdauskas, E. Markers of Lipid Metabolism Do Not Correlate with the Expression of Aortopathy in Patients with Bicuspid Aortic Valve Disease. J. Heart Valve Dis. 2016, 25, 534–542. [Google Scholar]
- Alegret, J.M.; Masana, L.; Martinez-micaelo, N.; Heras, M.; Beltrán-debón, R. LDL Cholesterol and Apolipoprotein B Are Associated with Ascending Aorta Dilatation in Bicuspid Aortic Valve Patients. QJM 2015, 108, 795–801. [Google Scholar] [CrossRef]
- Behbodikhah, J.; Ahmed, S.; Elyasi, A.; Kasselman, L.J.; De Leon, J.; Glass, A.D.; Reiss, A.B. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites 2021, 11, 690. [Google Scholar] [CrossRef] [PubMed]

| All Patients | Age < 45 | p | Age > 45 | p | |||
|---|---|---|---|---|---|---|---|
| AVS– | AVS+ | AVS– | AVS+ | ||||
| n | 75 | 13 | 9 | 18 | 35 | ||
| Age, years | 54 (44–65) | 39 (31–41) | 40 (34–43) | 0.367 | 55 (49–61) | 65 (59–69) | 0.002 |
| BMI, kg/m2 | 27 (24–30) | 27 (24–32) | 24 (23–27) | 0.190 | 27 (25–30) | 28 (26–31) | 0.247 |
| Sex (male), N (%) | 21 (28%) | 2 (11%) | 4 (44%) | 0.132 | 3 (16%) | 12 (35%) | 0.147 |
| Smoking habits, N (%) | 24 (42%) | 3 (23%) | 2 (22%) | 0.919 | 7 (39%) | 12 (35%) | 0.962 |
| Hypertension, N (%) | 39 (52%) | 2 (15%) | 1 (11%) | 0.854 | 13 (72%) | 23 (65%) | 0.795 |
| Diabetes, N (%) | 9 (15%) | 1 (1%) | 0 | 0.421 | 2 (11%) | 6 (17%) | 0.434 |
| Statins therapy, N (%) | 31 (41%) | 3 (23%) | 1 (11%) | 0.435 | 5 (27%) | 22 (62) | 0.010 |
| CAD, N (%) | 21 (28%) | 0 | 1 (12%) | 0.191 | 6 (33%) | 14 (40%) | 0.518 |
| Family history of CVD, N (%) | 37 (49%) | 6 (46%) | 6 (67%) | 0.342 | 10 (55%) | 15 (43%) | 0.345 |
| Age < 45 | Age > 45 | |||||
|---|---|---|---|---|---|---|
| AVS– | AVS+ | p | AVS– | AVS+ | p | |
| LVEF (%) | 60 (40–64) | 53 (32–70) | 0.965 | 60 (55–64) | 60 (51–64) | 0.965 |
| AVR, N (%) | 10 (76%) | 7 (77%) | 0.7 | 15 (83%) | 14 (40%) | 0.18 |
| Aortic aneurysm, N (%) | 8 (61%) | 5 (55%) | 0.8 | 17 (94%) | 20 (57%) | 0.35 |
| AVA (cm2) | 2.4 (2.1–2.5) | 1.3 (0.9–1.4) | <0.001 | 2.5 (2.2–2.6) | 0.9 (0.8–1.2) | <0.001 |
| Vmax (m/s) | 1.7 (1.6–2) | 3.6 (2.4–4.0) | <0.001 | 1.7 (1.5–1.8) | 3.9 (3.4–4.4) | <0.001 |
| PGmean (mmHg) | 7 (6–14) | 32 (15–36) | <0.001 | 7 (6.5–8) | 40.5 (25–46.5) | <0.001 |
| All Patients | Age < 45 | Age > 45 | |||||
|---|---|---|---|---|---|---|---|
| AVS– | AVS+ | p | AVS– | AVS+ | p | ||
| TC [mg/dL] | 140 (120–159) | 151 (139–166) | 150 (126–164) | 0.441 | 144 (124–167) | 128 (113–147) | 0.065 |
| HDL-C [mg/dL] | 51 (46–58) | 52 (47–55) | 48 (45–52) | 0.705 | 51 (46–60) | 50 (42–58) | 0.428 |
| LDL-C [mg/dL] | 99 (83–108) | 101 (997–114) | 107 (94–110) | 0.449 | 99 (91–119) | 86 (79–105) | 0.060 |
| TG [mg/dL] | 90 (61–123) | 83 (47–113) | 63 (52–108) | 0.659 | 105 (69–138) | 87 (68–113) | 0.272 |
| Apo B [mg/dL] | 98 (81–110) | 95 (77–100) | 110 (102–132) | 0.035 | 100 (93–114) | 83 (77–105) | 0.087 |
| Apo AI [mg/dL] | 152 (139–173) | 150 (126–168) | 143 (131–151) | 0.395 | 167 (139–183) | 155 (146–173) | 0.655 |
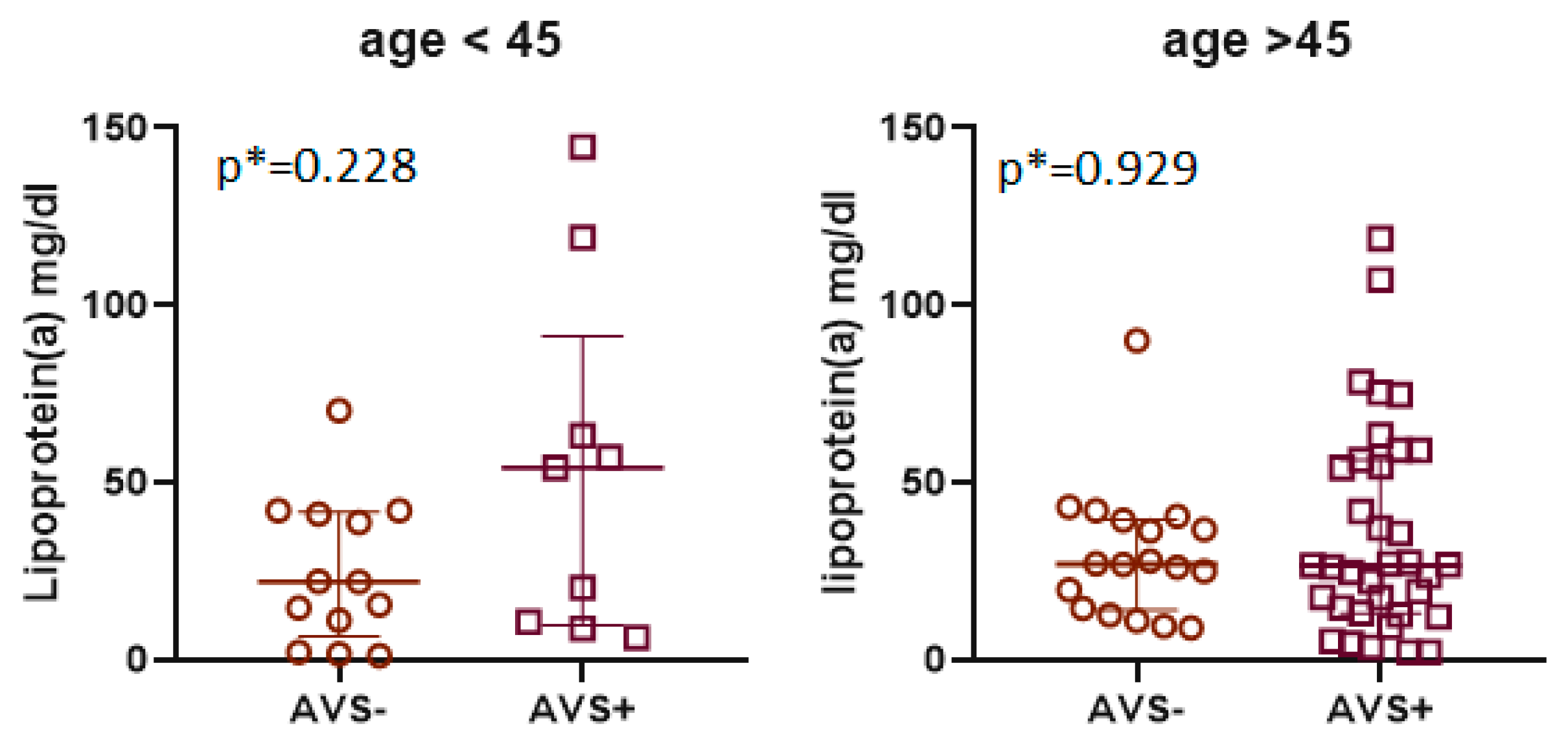
| Lp(a) [mg/dL] | 26 (12–47) | 22 (6.5–41) | 54 (10–91) | 0.228 | 27 (14–40) | 26 (13–56) | 0.929 |
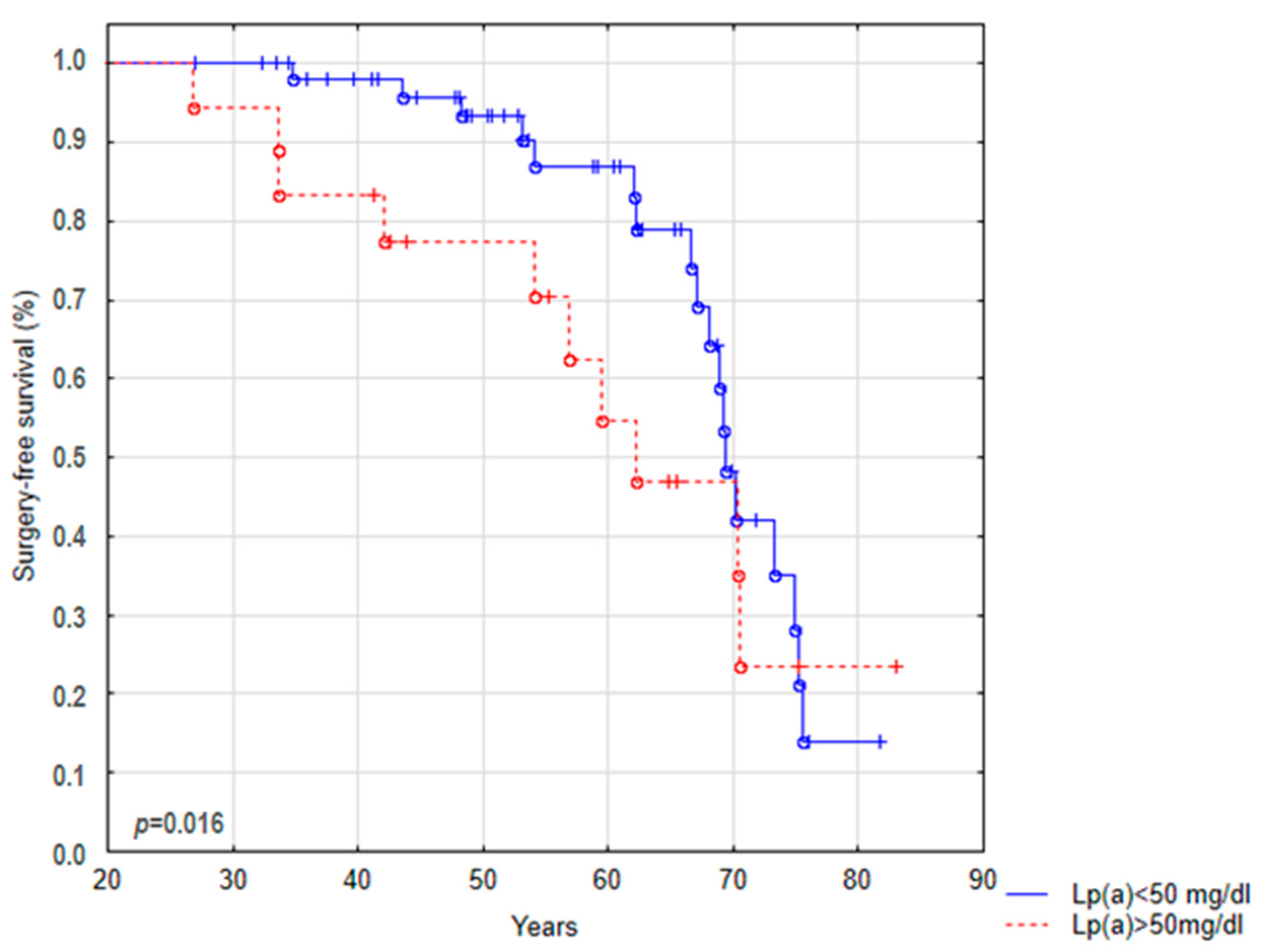
| Lp(a) < 50 mg/dL [n(%)] | 58 (76%) | 12 (93%) | 4 (44%) | 0.036 | 17 (94%) | 24 (69%) | 0.033 |
| Lp(a) > 50 mg/dL [n(%)] | 18 (24%) | 1 (7%) | 5 (56%) | 1 (6%) | 11 (31%) | ||
| CRP [mg/L] | 1.17 (0.5–2.6) | 1.2 (0.9–2.9) | 1.2 (0.4–2.2) | 0.656 | 1.0 (0.9–6.7) | 1.2 (0.4–2.2) | 0.373 |
| IL-6 [pg/mL] | 3 (1.5–8) | 1.5 (1.5–2.7) | 5.9 (1.5–9.7) | 0.043 | 3.4 (1.5–8.6) | 3.5 (1.5–8.7) | 0.710 |
| ATX [U] | 14 (11–17) | 15 (13–18) | 16 (14–19) | 0.455 | 13 (11–17) | 13 (12–16) | 0.875 |
| ATX [ng/mL] | 4.2 (1.9–6.5) | 1.6 (1.1–5.6) | 4.3 (2.3–6.5) | 0.141 | 3.4 (1.5–8.6) | 4.4 (2.4–6.7) | 0.962 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzesińska, A.; Nowak, M.; Mickiewicz, A.; Chyła-Danił, G.; Ćwiklińska, A.; Koper-Lenkiewicz, O.M.; Kamińska, J.; Matowicka-Karna, J.; Gruchała, M.; Jankowski, M.; et al. Lipoprotein(a) As a Potential Predictive Factor for Earlier Aortic Valve Replacement in Patients with Bicuspid Aortic Valve. Biomedicines 2023, 11, 1823. https://doi.org/10.3390/biomedicines11071823
Krzesińska A, Nowak M, Mickiewicz A, Chyła-Danił G, Ćwiklińska A, Koper-Lenkiewicz OM, Kamińska J, Matowicka-Karna J, Gruchała M, Jankowski M, et al. Lipoprotein(a) As a Potential Predictive Factor for Earlier Aortic Valve Replacement in Patients with Bicuspid Aortic Valve. Biomedicines. 2023; 11(7):1823. https://doi.org/10.3390/biomedicines11071823
Chicago/Turabian StyleKrzesińska, Aleksandra, Maria Nowak, Agnieszka Mickiewicz, Gabriela Chyła-Danił, Agnieszka Ćwiklińska, Olga M. Koper-Lenkiewicz, Joanna Kamińska, Joanna Matowicka-Karna, Marcin Gruchała, Maciej Jankowski, and et al. 2023. "Lipoprotein(a) As a Potential Predictive Factor for Earlier Aortic Valve Replacement in Patients with Bicuspid Aortic Valve" Biomedicines 11, no. 7: 1823. https://doi.org/10.3390/biomedicines11071823
APA StyleKrzesińska, A., Nowak, M., Mickiewicz, A., Chyła-Danił, G., Ćwiklińska, A., Koper-Lenkiewicz, O. M., Kamińska, J., Matowicka-Karna, J., Gruchała, M., Jankowski, M., Fijałkowski, M., & Kuchta, A. (2023). Lipoprotein(a) As a Potential Predictive Factor for Earlier Aortic Valve Replacement in Patients with Bicuspid Aortic Valve. Biomedicines, 11(7), 1823. https://doi.org/10.3390/biomedicines11071823









