Cognition in Patients with Schizophrenia: Interplay between Working Memory, Disorganized Symptoms, Dissociation, and the Onset and Duration of Psychosis, as Well as Resistance to Treatment
Abstract
:1. Introduction
2. Methods
2.1. Clinical Contingent
- Prospective monitoring for at least 12 weeks.
- Administration of at least two antipsychotic medication trials at a dose corresponding to or greater than 600 mg chlorpromazine equivalents.
- Reduction of symptoms when assessed with the PANSS and BPRS scale by less than 20% for the observed period.
- The assessment of social dysfunction using the SOFAS scale is below 60.
- Mental retardation.
- Psychoactive substance abuse.
- Presence of organic brain damage.
- Concomitant progressive neurological or severe somatic diseases.
- Score of MMSE (Mini-Mental State Examination) below 25 points.
- Pregnancy and breastfeeding.
2.2. Assessment
- meat, glass, road, egg, birch, jam, goat, flag, sky, bag
- house, horse, mushroom, honey, brother, forest, chair, bread, labor, oak
2.3. Statistical Analyses
3. Results
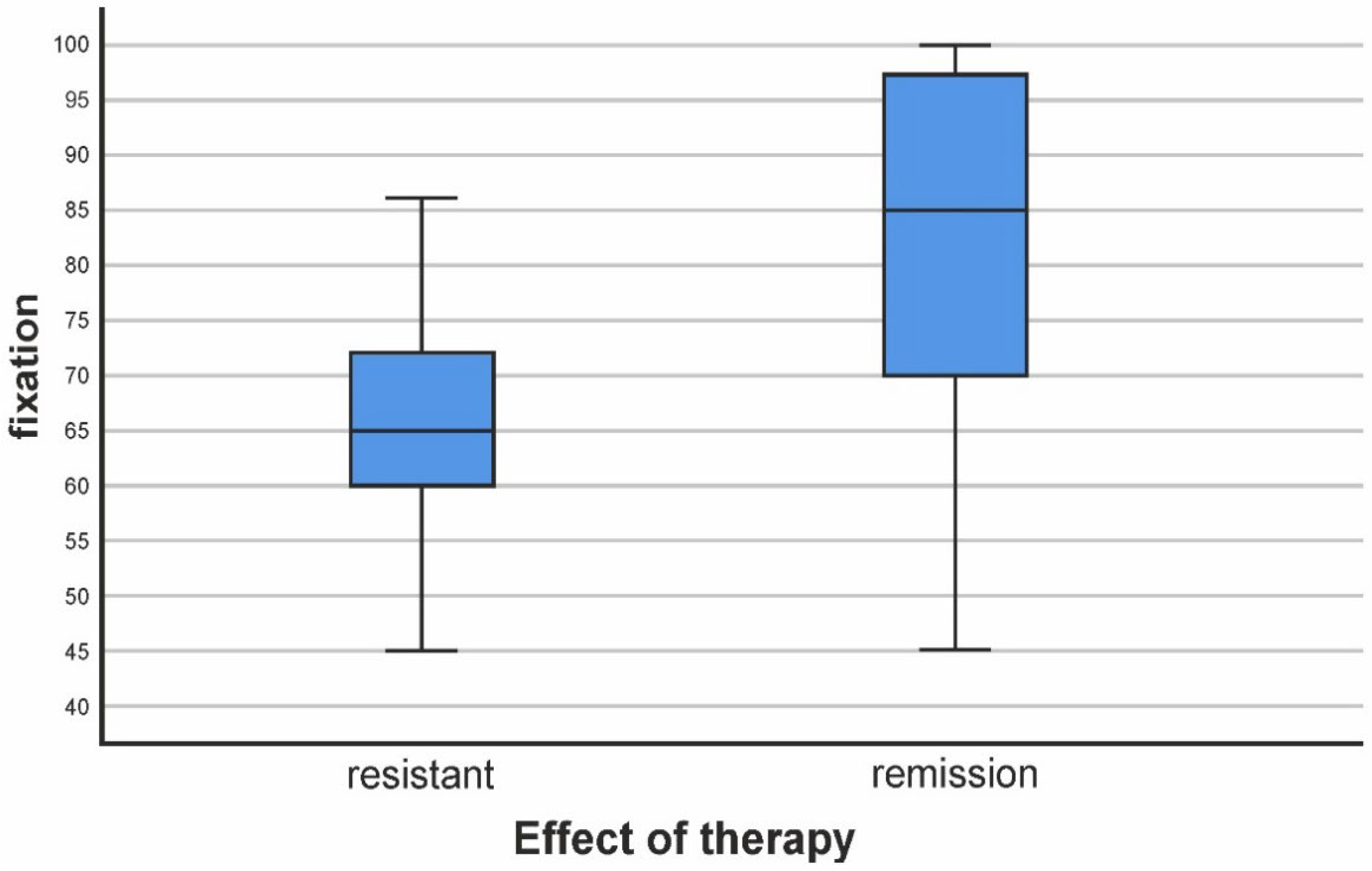
3.1. Assessment of Fixation
3.2. Reproduction
3.3. Attention
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- Application 1: (Memory Impairment Rating Scale)
- Scale of Prof. Dr. K. Mechkov
- Degree Percent Deviation
- I over 84 no/norm/
- II 84–68 slightly
- III 68–51 moderate
- IV 51–34 significantly
- V 34–17 severe
- VI under 17 very heavy
References
- Tanaka, M.; Vécsei, L. Editorial of Special Issue “Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- Bitter, I.; Mohr, P.; Raspopova, N.; Szulc, A.; Samochowiec, J.; Micluia, I.V.; Skugarevsky, O.; Herold, R.; Mihaljevic-Peles, A.; Okribelashvili, N.; et al. Assessment and Treatment of Negative Symptoms in Schizophrenia-A Regional Perspective. Front Psychiatry 2022, 12, 820801. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, D. Advances in the Diagnosis and Management of Psychosis. Diagnostics 2023, 13, 1517. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, D.; Aryutova, K.; Kandilarova, S.; Paunova, R.; Arabadzhiev, Z.; Todeva-Radneva, A.; Kostianev, S.; Borgwardt, S. Diagnostic Task Specific Activations in Functional MRI and Aberrant Connectivity of Insula with Middle Frontal Gyrus Can Inform the Differential Diagnosis of Psychosis. Diagnostics 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Stoyanov, D.; Kandilarova, S.; Borgwardt, S.; Stieglitz, R.D.; Hugdahl, K.; Kostianev, S. Psychopathology Assessment Methods Revisited: On Translational Cross-Validation of Clinical Self-Evaluation Scale and fMRI. Front. Psychiatry 2018, 9, 21. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Moustafa, S.R.; Al-Rawi, K.F.; Stoyanov, D.; Al-Dujaili, A.H.; Supasitthumrong, T.; Al-Hakeim, H.K.; Maes, M. The Endogenous Opioid System in Schizophrenia and Treatment Resistant Schizophrenia: Increased Plasma Endomorphin 2, and κ and μ Opioid Receptors Are Associated with Interleukin-6. Diagnostics 2020, 10, 633. [Google Scholar] [CrossRef]
- Masumo, Y.; Kanahara, N.; Kogure, M.; Yamasaki, F.; Nakata, Y.; Iyo, M. Dopamine supersensitivity psychosis and delay of clozapine treatment in patients with treatment-resistant schizophrenia. Int. Clin. Psychopharmacol. 2022, 38, 102–109. [Google Scholar] [CrossRef]
- Helaly, A.M.N.; Ghorab, D.S.E.D. Schizophrenia as metabolic disease. What are the causes? Metab Brain Dis. 2023, 38, 795–804. [Google Scholar] [CrossRef]
- Stojanov, D. A Pharmacogenetic and Dynamical Model of the Resistance to Antipsychotic Treatment of Schizophrenia. Biotechnol. Biotechnol. Equip. 2006, 20, 169–170. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Editorial of Special Issue ‘Dissecting Neurological and Neuropsychiatric Diseases: Neurodegeneration and Neuroprotection’. Int. J. Mol. Sci. 2022, 23, 6991. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Thase, M.E.; Pillinger, T. Treatment resistance in psychiatry: State of the art and new directions. Mol Psychiatry 2022, 27, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Demjaha, A.; Murray, R.M.; McGuire, P.K.; Kapur, S.; Howes, O.D. Dopamine synthesis capacity in patients with treatment-resistant schizophrenia. Am. J. Psychiatry 2012, 169, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.R. Evidence-based guidelines for the pharmacological treatment of schizophrenia: Recommendations from the British Association for Psychopharmacology. J. Psychopharmacol. 2011, 25, 567–620. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Chan, L.L.; Chee, K.S.; Chen, H.; Chin, S.A.; Chong, S.A.; Chua, W.; Fones, C.; Fung, D.; Khoo, C.L.; et al. Ministry of Health clinical practice guidelines: Schizophrenia. Singap. Med. J. 2011, 52, 521–525, quiz 526. [Google Scholar]
- Howes, O.D.; McCutcheon, R.; Agid, O.; de Bartolomeis, A.; van Beveren, N.J.; Birnbaum, M.L.; Bloomfield, M.A.; Bressan, R.A.; Buchanan, R.W.; Carpenter, W.T.; et al. Treatment-Resistant Schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) Working Group Consensus Guidelines on Diagnosis and Terminology. Am. J. Psychiatry 2017, 174, 216–229. [Google Scholar] [CrossRef]
- de Vries, P.J.; Honer, W.G.; Kemp, P.M.; McKenna, P.J. Dementia as a complication of schizophrenia. J. Neurol. Neurosurg. Psychiatry 2001, 70, 588–596. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Keefe, R.S.E.; McGuire, P.K. Cognitive impairment in schizophrenia: Etiology, pathophysiology, and treatment. Mol. Psychiatry 2023, 28, 1902–1918. [Google Scholar] [CrossRef]
- Richmond-Rakerd, L.S.; D’Souza, S.; Milne, B.J.; Caspi, A.; Moffitt, T.E. Longitudinal associations of mental disorders with dementia: 30-year analysis of 1.7 million New Zealand citizens. JAMA Psychiatry 2022, 48109, 333–340. [Google Scholar] [CrossRef]
- Green, M.F.; Horan, W.P.; Lee, J. Nonsocial and social cognition in schizophrenia: Current evidence and future directions. World Psychiatry 2019, 18, 146–161. [Google Scholar] [CrossRef]
- Javitt, D.; Sweet, R. Auditory dysfunction in schizophrenia: Integrating clinical and basic features. Nat. Rev. Neurosci. 2015, 16, 535–550. [Google Scholar] [CrossRef] [PubMed]
- Keefe, R.S.E.; Fox, K.H.; Harvey, P.D.; Cucchiaro, J.; Siu, C.; Loebel, A. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophr. Res. 2011, 125, 161–168. [Google Scholar] [CrossRef]
- Taylor, S.E. Adjustment to threatening events: A theory of cognitive adaptation. Am. Psychol. 1983, 38, 1161–1173. [Google Scholar] [CrossRef]
- Taylor, S.E.; Brown, J.D. Illusion and well-being: A social psychological perspective on mental health. Psychol. Bull. 1988, 103, 193–210. [Google Scholar] [CrossRef]
- Wójciak, P.; Rybakowski, J. Clinical picture, pathogenesis and psychometric assessment of negative symptoms of schizophrenia. Psychiatr. Pol. 2018, 52, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Bozikas, V.P.; Andreou, C. Longitudinal studies of cognition in first episode psychosis: A systematic review of the literature. Aust. N. Z. J. Psychiatry 2011, 45, 93–108. [Google Scholar] [CrossRef]
- Shah, J.; Eack, S.M.; Montrose, D.M.; Tandon, N.; Miewald, J.M.; Prasad, K.M.; Keshavan, M.S. Multivariate prediction of emerging psychosis in adolescents at high risk for schizophrenia. Schizophr. Res. 2012, 141, 189–196. [Google Scholar] [CrossRef]
- Biedermann, F.; Fleischhacker, W.W. Psychotic disorders in DSM-5 and ICD-11. CNS Spectr. 2016, 21, 349–354. [Google Scholar] [CrossRef]
- Vidailhet, P. Premier épisode psychotique, troubles cognitifs et remédiation [First-episode psychosis, cognitive difficulties and remediation]. Encephale 2013, 39 (Suppl. S2), S83–S92. (In French) [Google Scholar] [CrossRef]
- Hashimoto, K. Recent Advances in the Early Intervention in Schizophrenia: Future Direction from Preclinical Findings. Curr. Psychiatry Rep. 2019, 21, 75. [Google Scholar] [CrossRef]
- Oberauer, K. Working Memory and Attention—A Conceptual Analysis and Review. J. Cogn. 2019, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Oberauer, K. Design for a working memory. Psychol. Learn. Motiv. Adv. Res. Theory 2009, 51, 45–100. [Google Scholar] [CrossRef]
- Oberauer, K. Control of the contents of working memory—A comparison of two paradigms and two age groups. J. Exp. Psychol. Learn. Mem. Cogn. 2005, 31, 714–728. [Google Scholar] [CrossRef]
- Kaar, S.J.; Natesan, S.; McCutcheon, R.; Howes, O.D. Antipsychotics: Mechanisms underlying clinical response and side-effects and novel treatment approaches based on pathophysiology. Neuropharmacology 2020, 172, 107704. [Google Scholar] [CrossRef] [PubMed]
- Hogarty, G.E.; Flesher, S. Developmental theory for a cognitive enhancement therapy of schizophrenia. Schizophr. Bull. 1999, 25, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Bellack, A.S. Cognitive rehabilitation for schizophrenia—Is it possible—Is it necessary. Schizophr. Bull. 1992, 8, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Vita, A.; Barlati, S.; Ceraso, A.; Nibbio, G.; Ariu, C.; Deste, G.; Wykes, T. Effectiveness, Core Elements, and Moderators of Response of Cognitive Remediation for Schizophrenia: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Psychiatry 2021, 78, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, J.A.; Northrop, A.; Kurtz, M.M. A Meta-analysis of Cognitive Remediation for Schizophrenia: Efficacy and the Role of Participant and Treatment Factors. Schizophr. Bull. 2021, 47, 997–1006. [Google Scholar] [CrossRef]
- Vita, A.; Gaebel, W.; Mucci, A.; Sachs, G.; Barlati, S.; Giordano, G.M.; Nibbio, G.; Nordentoft, M.; Wykes, T.; Galderisi, S. European Psychiatric Association guidance on treatment of cognitive impairment in schizophrenia. Eur. Psychiatry 2022, 65, e57. [Google Scholar] [CrossRef]
- Vita, A.; Barlati, S. Recovery from schizophrenia: Is it possible? Curr. Opin. Psychiatry 2018, 31, 246–255. [Google Scholar] [CrossRef]
- Rund, B.R. A review of longitudinal studies of cognitive functions in schizophrenia patients. Schizophr. Bull. 1998, 24, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Rund, B.R. Is there a degenerative process going on in the brain of people with Schizophrenia? Front. Hum. Neurosci. 2009, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Rund, B.R.; Sundet, K.; Asbjørnsen, A.; Egeland, J.; Landrø, N.I.; Lund, A.; Roness, A.; Stordal, K.I.; Hugdahl, K. Neuropsychological test profiles in schizophrenia and non-psychotic depression. Acta Psychiatr. Scand. 2006, 113, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Stirling, J.; White, C.; Lewis, S.; Hopkins, R.; Tantam, D.; Huddy, A.; Montague, L. Neurocognitive function and outcome in first-episode schizophrenia: A 10-year follow-up of an epidemiological cohort. Schizophr Res. 2003, 65, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Hoff, A.L.; Svetina, C.; Shields, G.; Stewart, J.; DeLisi, L.E. Ten year longitudinal study of neuropsychological functioning subsequent to a first episode of schizophrenia. Schizophr. Res. 2005, 78, 27–34. [Google Scholar] [CrossRef]
- Øie, M.; Sundet, K.; Ueland, T. Neurocognition and functional outcome in early-onset schizophrenia and attention-deficit/hyperactivity disorder: A 13-year follow-up. Neuropsychology 2011, 25, 25–35. [Google Scholar] [CrossRef]
- Heilbronner, U.; Samara, M.; Leucht, S.; Falkai, P.; Schulze, T.G. The Longitudinal Course of Schizophrenia Across the Lifespan: Clinical, Cognitive, and Neurobiological Aspects. Harv. Rev. Psychiatry 2016, 24, 118–128. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Smieskova, R.; Kempton, M.J.; Ho, B.C.; Andreasen, N.C.; Borgwardt, S. Progressive brain changes in schizophrenia related to antipsychotic treatment? A meta-analysis of longitudinal MRI studies. Neurosci. Biobehav. Rev. 2013, 37, 1680–1691. [Google Scholar] [CrossRef]
- Cowan, N. What are the differences between long-term, short-term, and working memory? Prog. Brain Res. 2008, 169, 323–338. [Google Scholar] [CrossRef]
- Heinrichs, R.W.; Zakzanis, K.K. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology 1998, 12, 426–445. [Google Scholar] [CrossRef]
- Guo, J.Y.; Ragland, J.D.; Carter, C.S. Memory and cognition in schizophrenia. Mol. Psychiatry 2019, 24, 633–642. [Google Scholar] [CrossRef]
- Lee, J.; Park, S. Working memory impairments in schizophrenia: A meta-analysis. J. Abnorm. Psychol. 2005, 114, 599–611. [Google Scholar] [CrossRef]
- Saykin, A.J.; Gur, R.C.; Gur, R.E.; Mozley, P.D.; Mozley, L.H.; Resnick, S.M.; Kester, D.B.; Stafiniak, P. Neuropsychological function in schizophrenia. Selective impairment in memory and learning. Arch. Gen. Psychiatr. 1991, 48, 618–624. [Google Scholar] [CrossRef]
- Budson, A.E.; Richman, K.A.; Kensinger, E.A. Consciousness as a Memory System. Cogn. Behav. Neurol. 2022, 35, 263–297. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, M.; Carlone, O.; Vitale, B.; Cinti, M.E.; Clare, L. A meta-analysis of cognitive deficits in adults with a diagnosis of schizophrenia. Neuropsychol. Rev. 2005, 15, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J.; Gold, J.M. The construct of attention in schizophrenia. Biol. Psychiatry 2008, 64, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Luria, A.R. Higher Cortical Functions in Man; Haigh, B., Ed.; Basic Books; Moscow University Press: New York, NY, USA, 1962. [Google Scholar]
- Mechkov, K. Medical Psychology; Izdatelstvo “Pik”: Veliko Tarnovo, Bulgaria, 1995. [Google Scholar]
- 59. Özdemir, O.; Güzel Özdemir, P.; Boysan, M.; Yilmaz, E. The Relationships Between Dissociation, Attention, and Memory Dysfunction. Noro Psikiyatr Ars. 2015, 52, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Gregory, J.; Oakley, S.; Jr Bloch, R.; Gardner, M. Reduction in dissociation due to aging and cognitive deficit. Compr. Psychiatry 1996, 37, 31–36. [Google Scholar] [CrossRef]
- Overall, J.E.; Gorham, D.R. The Brief Psychiatric Rating Scale. Psychol. Rep. 1962, 10, 799–812. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Andrean, N.C.; Carpenter, W.T., Jr.; Kane, J.M.; Lasser, R.A.; Marder, S.R.; Weinberger, D.R. Remission in schizophrenia: Proposed criteria and rationale for consensus. Am. J. Psychiatry 2005, 162, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Edition, F. Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- World Health Organization. Division of Mental Health; The ICD-10; WHO: Geneva, Switzerland, 1994.
- Lezak, M.D. Neuropsychological Assessment, 3rd ed.; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Mann, H.B.; Whitney, D.R. On a Test of Whether One of Two Random Variables Is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Panov, G.; Panova, P. Obsessive-compulsive symptoms in patient with schizophrenia: The influence of disorganized symptoms, duration of schizophrenia, and drug resistance. Front. Psychiatry 2023, 14, 1120974. [Google Scholar] [CrossRef] [PubMed]
- Panov, G. Dissociative Model in Patients with Resistant Schizophrenia. Front. Psychiatry 2022, 13, 845493. [Google Scholar] [CrossRef] [PubMed]
- Panov, G. Comparative Analysis of Lateral Preferences in Patients with Resistant Schizophrenia. Front. Psychiatry 2022, 13, 868285. [Google Scholar] [CrossRef]
- Panov, G. Gender-associated role in patients with schizophrenia. Is there a connection with the resistance? Front. Psychiatry 2022, 13, 845493. [Google Scholar] [CrossRef]
- Panov, G. Higher Depression Scores in Patients with Drug-Resistant Schizophrenia. J. Integr. Neurosci. 2022, 21, 126. [Google Scholar] [CrossRef] [PubMed]
- Panov, G.P. Early Markers in Resistant Schizophrenia: Effect of the First Antipsychotic Drug. Diagnostics 2022, 12, 803. [Google Scholar] [CrossRef]
- Panov, G.; Djulgerova, S.; Panova, P. The effect of education level and sex differences on resistance to treatment in patients with schizophrenia. Bulg. Med. 2022, 12, 22–29. [Google Scholar]
- Panov, G.; Djulgerova, S.; Panova, P. Comparative anthropometric criteria in patients with resistant schizophrenia. Bulg. Med. 2022, 12, 30–39. [Google Scholar]
- Chen, M.; Zhang, L.; Jiang, Q. Gender Difference in Cognitive Function Among Stable Schizophrenia: A Network Perspective. Neuropsychiatr. Dis. Treat. 2022, 18, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Huang, X.F.; Chen, D.C.; Xiu, M.H.; Hui, L.; Liu, H.; Kosten, T.R.; Zhang, X.Y. Gender differences in cognitive function of patients with chronic schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2012, 39, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.M.; Bucci, P.; Mucci, A.; Pezzella, P.; Galderisi, S. Gender Differences in Clinical and Psychosocial Features Among Persons with Schizophrenia: A Mini Review. Front. Psychiatry 2021, 12, 789179. [Google Scholar] [CrossRef] [PubMed]
- McEvoy, J.P. The costs of schizophrenia. J. Clin. Psychiatry 2007, 68, 4–7. [Google Scholar]
- Harvey, P.D.; Bosia, M.; Cavallaro, R.; Howes, O.D.; Kahn, R.S.; Leucht, S.; Müller, D.R.; Penadés, R.; Vita, A. Cognitive dysfunction in schizophrenia: An expert group paper on the current state of the art. Schizophr. Res. Cogn. 2022, 29, 100249. [Google Scholar] [CrossRef]
- Kucharska-Mazur, J.; Podwalski, P.; Rek-Owodziń, K.; Waszczuk, K.; Sagan, L.; Mueller, S.T.; Michalczyk, A.; Misiak, B.; Samochowiec, J. Executive Functions and Psychopathology Dimensions in Deficit and Non-Deficit Schizophrenia. J. Clin. Med. 2023, 12, 1998. [Google Scholar] [CrossRef]
- Green, M.F.; Kern, R.S.; Heaton, R.K. Longitudinal studies of cognition and functional outcome in schizophrenia: Implications for MATRICS. Schizophr. Res. 2004, 72, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, J.L.; Giedd, J.N.; Gogtay, N. Neurodevelopmental model of schizophrenia: Update 2012. Mol. Psychiatry 2012, 17, 1228–1238. [Google Scholar] [CrossRef]
- Davidson, M.; Reichenberg, A.; Rabinowitz, J.; Weiser, M.; Kaplan, Z.; Mark, M. Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am. J. Psychiatry 1999, 156, 1328–1335. [Google Scholar] [CrossRef]
- van Haren, N.E.M.; Pol, H.E.H.; Schnack, H.G.; Cahn, W.; Brans, R.; Carati, I.; Rais, M.; Kahn, R.S. Progressive brain volume loss in schizophrenia over the course of the illness: Evidence of maturational abnormalities in early adulthood. Biol. Psychiatry 2008, 63, 106–113. [Google Scholar] [CrossRef]
- Barone, A.; De Prisco, M.; Altavilla, B.; Avagliano, C.; Balletta, R.; Buonaguro, E.F.; Ciccarelli, M.; D’Ambrosio, L.; Giordano, S.; Latte, G.; et al. Disorganization domain as a putative predictor of Treatment Resistant Schizophrenia (TRS) diagnosis: A machine learning approach. J. Psychiatr. Res. 2022, 155, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Metsanen, M.; Wahlberg, K.E.; Hakko, H.; Saarento, O.; Tienari, P. Thought disorder index: A longitudinal study of severity levels and schizophrenia factors. J. Psychiatr. Res. 2006, 40, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Reed, R.A.; Harrow, M.; Herbener, E.S.; Martin, E.M. Executive function in schizophrenia: Is it linked to psychosis and poor life functioning? J. Nerv. Ment. Dis. 2002, 190, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Shenton, M.E.; Kikinis, R.; Jolesz, F.A.; Pollak, S.D.; LeMay, M.; Wible, C.G.; Hokama, H.; Martin, J.; Metcalf, D.; Coleman, M.; et al. l. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. Aquantitative magnetic resonance imaging study. N. Engl. J. Med. 1992, 327, 604–612. [Google Scholar] [CrossRef]
- O’Leary, D.S.; Flaum, M.; Kesler, M.L.; Flashman, L.A.; Arndt, S.; Andreasen, N.C. Cognitive correlates of the negative, disorganized, and psychotic symptom dimensions of schizophrenia. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 4–15. [Google Scholar] [CrossRef] [PubMed]
- 91. Ruben, C.; Gur, R.C.; Gur, R.E. Memory in health and in schizophrenia. Dialogues Clin. Neurosci. 2013, 15, 399–410. [Google Scholar] [CrossRef]
- González-Andrade, A.; López-Luengo, B.; Álvarez, M.M.R.; Santiago-Ramajo, S. Divided Attention in Schizophrenia: A Dual Task Paradigm. Am. J. Psychol. 2021, 134, 187–200. [Google Scholar] [CrossRef]
- Ivanova, E.; Panayotova, T.; Grechenliev, I.; Peshev, B.; Kolchakova, P.; Milanova, V. A Complex Combination Therapy for a Complex Disease-Neuroimaging Evidence for the Effect of Music Therapy in Schizophrenia. Front. Psychiatry 2022, 13, 795344. [Google Scholar] [CrossRef]
- Younes, K.; Miller, B.L. Frontotemporal Dementia: Neuropathology, Genetics, Neuroimaging, and Treatments. Psychiatr. Clin. North Am. 2020, 43, 331–344. [Google Scholar] [CrossRef]
- Bang, J.; Spina, S.; Miller, B.L. Frontotemporal dementia. Lancet 2015, 386, 1672–1682. [Google Scholar] [CrossRef]
- Laskaris, L.; Mancuso, S.; Shannon Weickert, C.; Zalesky, A.; Chana, G.; Wannan, C.; Bousman, C.; Baune, B.T.; McGorry, P.; Pantelis, C.; et al. Brain morphology is differentially impacted by peripheral cytokines in schizophrenia-spectrum disorder. Brain Behav. Immun. 2021, 95, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Bortolon, C.; Macgregor, A.; Capdevielle, D.; Raffard, S. Apathy in schizophrenia: A review of neuropsychological and neuroanatomical studies. Neuropsychologia 2018, 118 Pt B, 22–33. [Google Scholar] [CrossRef]
- Nyatega, C.O.; Qiang, L.; Adamu, M.J.; Kawuwa, H.B. Gray matter, white matter and cerebrospinal fluid abnormalities in Parkinson’s disease: A voxel-based morphometry study. Front. Psychiatry 2022, 13, 1027907. [Google Scholar] [CrossRef] [PubMed]
- Cowan, N. George Miller’s magical number of immediate memory in retrospect: Observations on the faltering progression of science. Psychol. Rev. 2015, 122, 536–541. [Google Scholar] [CrossRef] [PubMed]
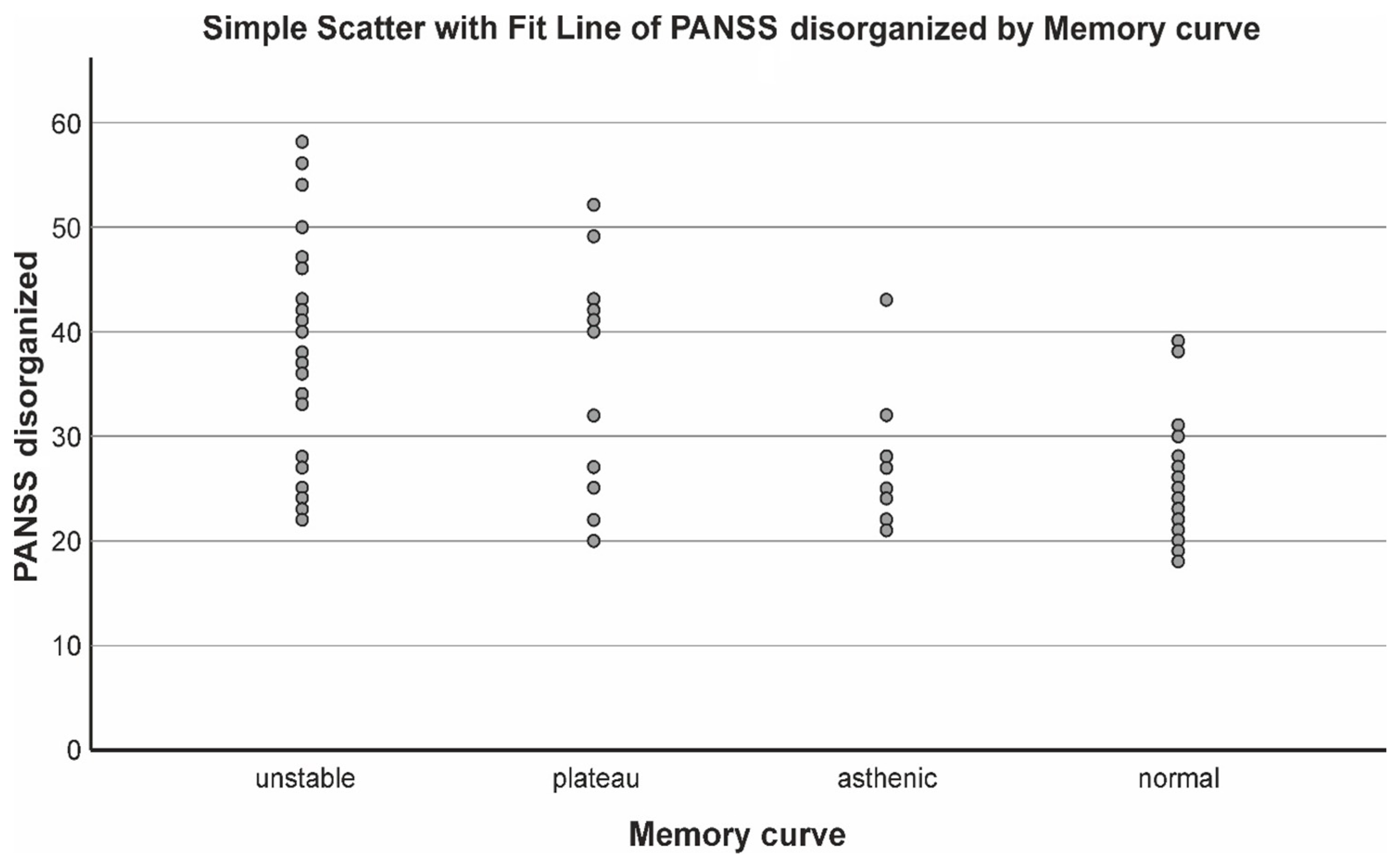
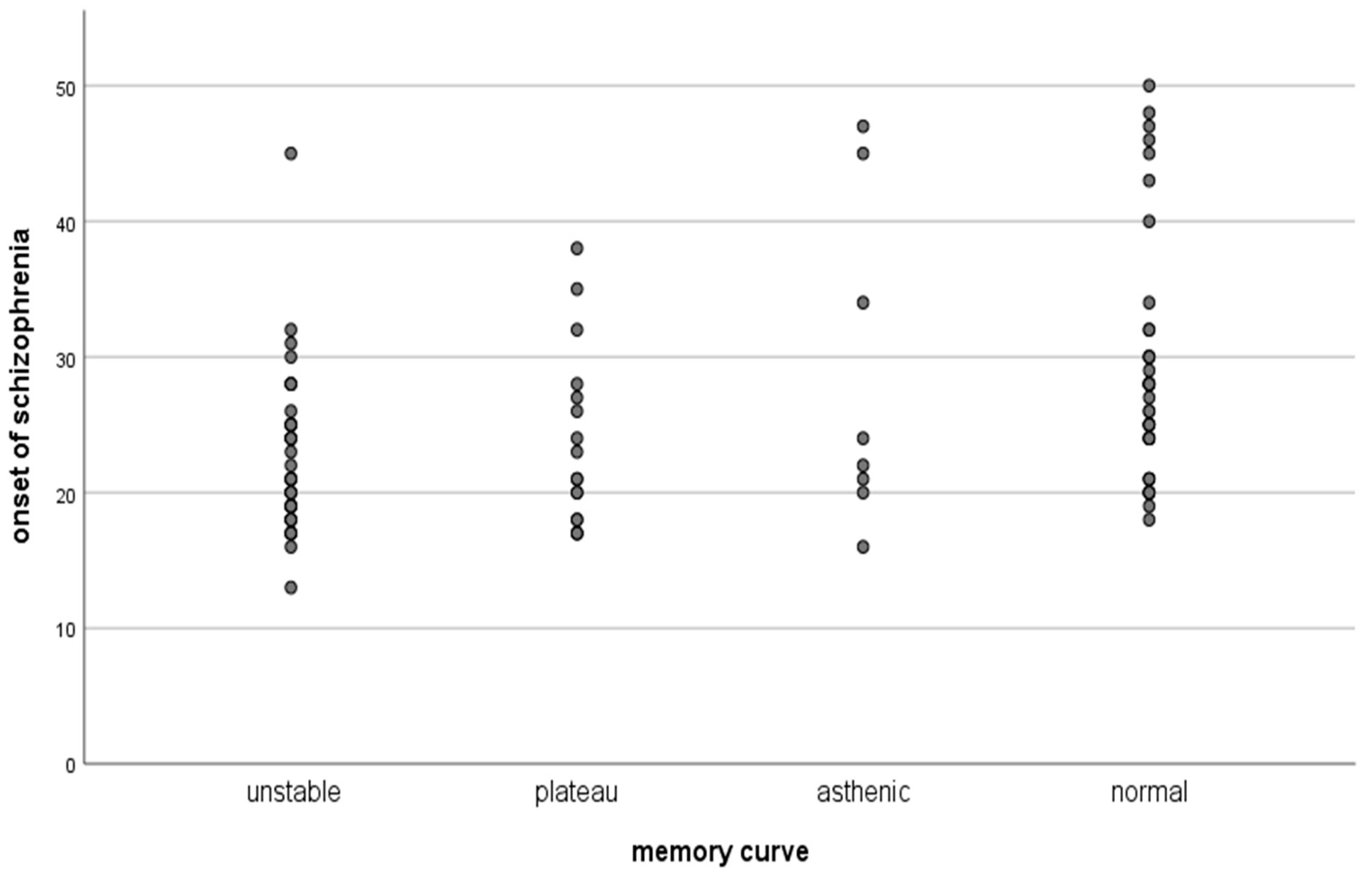
| Resistant SZ | Clinical Remission | Resistant SZ |
|---|---|---|
| Age (years) | 36.98 | 37.25 |
| Age of onset of SZ (years) | 23.04 | 27.37 |
| Duration of SZ (years) | 14.31 | 9.87 |
| BMI | 26.6022 | 27.2217 |
| Height | 170.11 | 167.38 |
| Education (years) | 11.33 | 11.60 |
| Handedness (right/left) | 42/3 | 56/4 |
| Sex (M/F) | 20/25 | 19/41 |
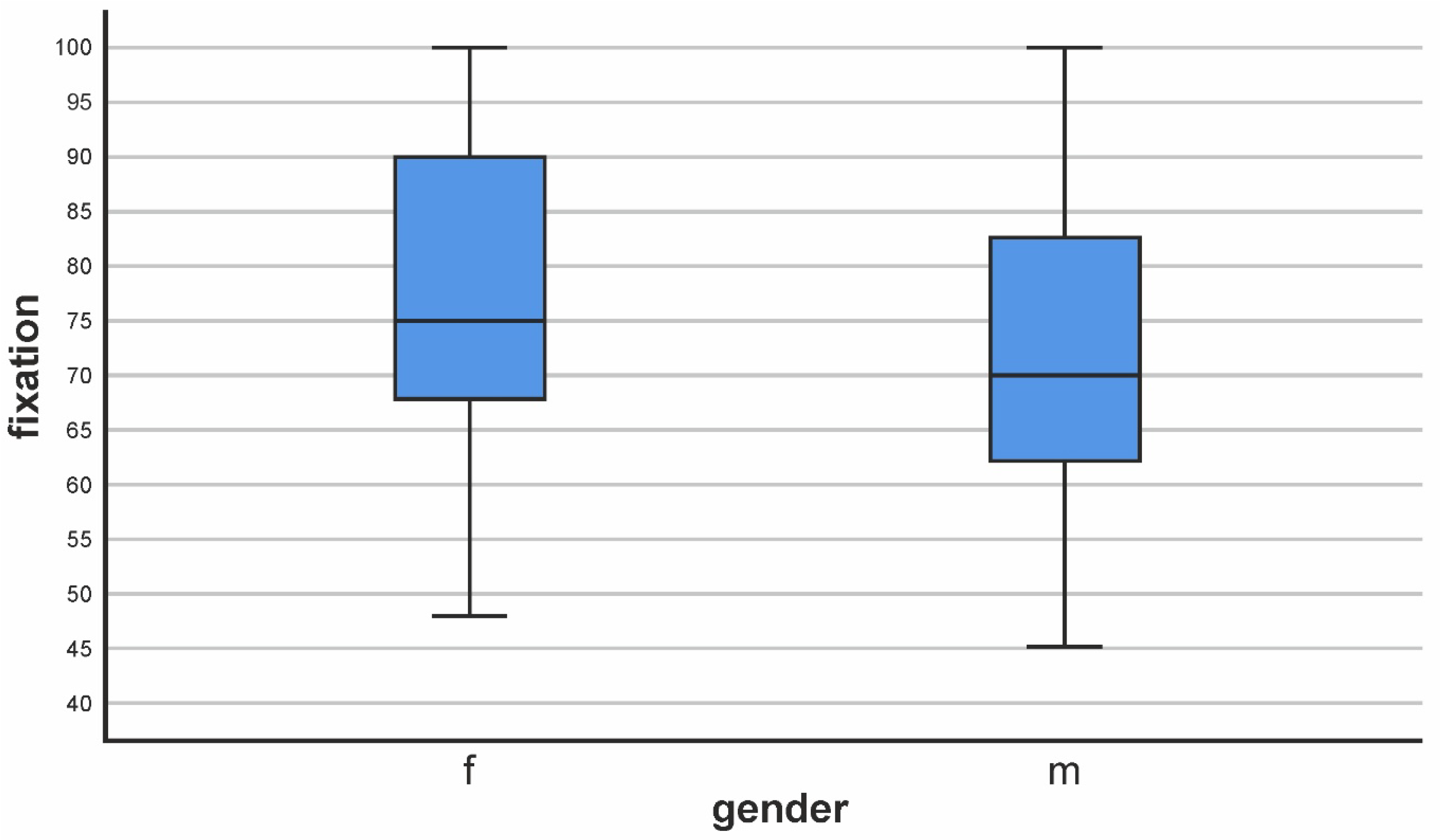
| Fixation Score | |||
|---|---|---|---|
| Gender | Mean | N | Std. Deviation |
| f | 77,33 | 66 | 15,472 |
| m | 71,23 | 39 | 15,141 |
| Total | 75,07 | 105 | 15,561 |
| Mann–Whitney U | 467,000 |
| Asymp. Sig. (2-tailed) | 0.000 (***) |
| R2 | β | t | p (Sig) | |
|---|---|---|---|---|
| Step 1 PANSS disorganized | 0.338 | 0.889 | 7.259 | 0.000 (***) |
| Step 2 PANSS disorganized Duration of Sch Step 3 PANSS disorganized Duration of Sch Dissociation score | 0.388 0.411 | 0.350 0.130 | 6.865 2.880 4.869 2.646 1.988 | 0.000 (***) 0.000 (***) |
| Onset of the Sch | Duration of Sch | ||
|---|---|---|---|
| Fixation | Pearson Correlation | −0.274 ** | −0.325 ** |
| Sig. (2-tailed) | 0.005 | 0.001 | |
| R2 | β | T | p (sig) | |
|---|---|---|---|---|
| Step 1 PANSS disorganized | 0.321 | 0.889 | 6.972 | 0.000 (***) |
| Step 2 | 0.351 | 0.350 | 0.000 (***) | |
| PANSS disorganized | 3.251 | |||
| PANSS negative | 2.190 |
| Reproduction | Onset of the Sch | Duration of Sch | ||
|---|---|---|---|---|
| Reproduction | Pearson Correlation | 1 | 0.153 | −0.199 * |
| Sig. (2-tailed) | 0.120 | 0.041 | ||
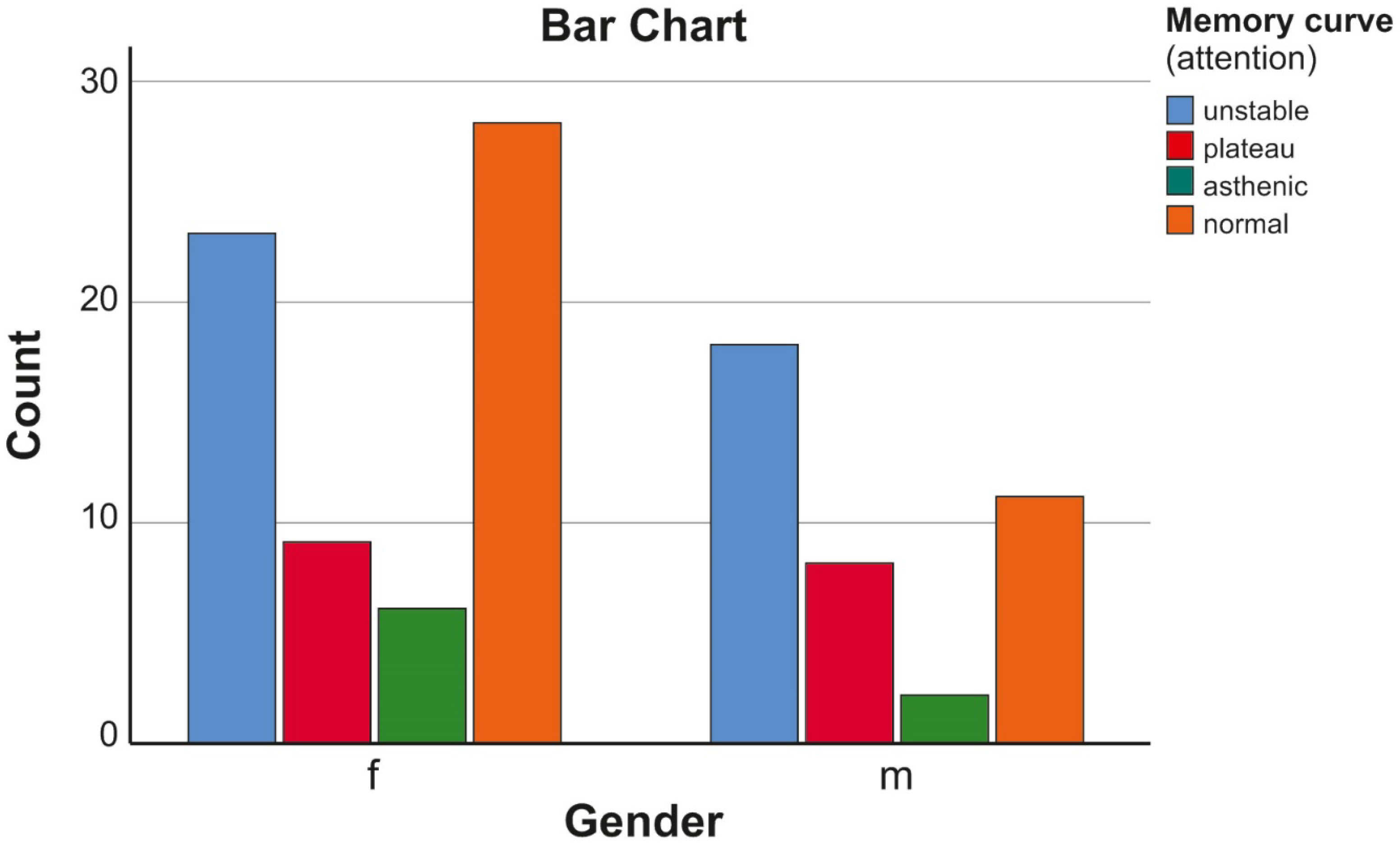
| Assessed Memory Curve (Attention) | ||||||
|---|---|---|---|---|---|---|
| Unstable | Plateau | Asthenic | Normal | Total | ||
| Gender | f | 23 | 9 | 6 | 28 | 66 |
| m | 18 | 8 | 2 | 11 | 39 | |
| Total | 41 | 17 | 8 | 39 | 105 | |
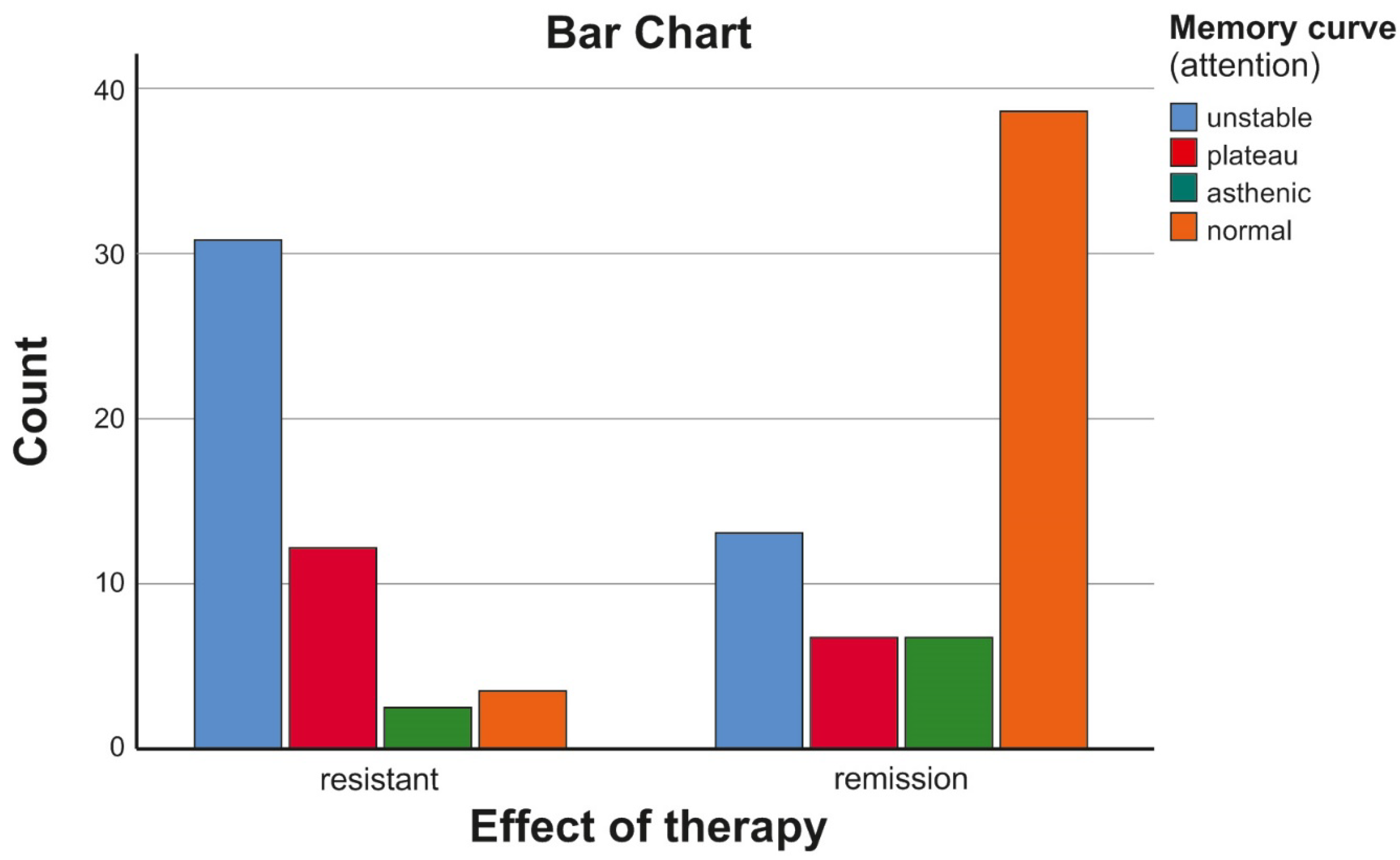
| Assessed Memory Curve | ||||||
|---|---|---|---|---|---|---|
| Unstable | Plateau | Asthenic | Normal | Total | ||
| Treatment effect | resistant | 29 | 11 | 2 | 3 | 45 |
| remission | 12 | 6 | 6 | 36 | 60 | |
| Total | 41 | 17 | 8 | 39 | 105 | |
| R2 | β | t | p (Sig) | |
|---|---|---|---|---|
| Step 1 PANSS disorganized | 0.375 | 0.613 | 7.865 | 0.000 (***) |
| Step 2 | 0.445 | 0.561 0.268 | 0.000 (***) | |
| PANSS disorganized | 7.469 | |||
| Onset of Sch | 3.587 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panov, G.; Dyulgerova, S.; Panova, P. Cognition in Patients with Schizophrenia: Interplay between Working Memory, Disorganized Symptoms, Dissociation, and the Onset and Duration of Psychosis, as Well as Resistance to Treatment. Biomedicines 2023, 11, 3114. https://doi.org/10.3390/biomedicines11123114
Panov G, Dyulgerova S, Panova P. Cognition in Patients with Schizophrenia: Interplay between Working Memory, Disorganized Symptoms, Dissociation, and the Onset and Duration of Psychosis, as Well as Resistance to Treatment. Biomedicines. 2023; 11(12):3114. https://doi.org/10.3390/biomedicines11123114
Chicago/Turabian StylePanov, Georgi, Silvana Dyulgerova, and Presyana Panova. 2023. "Cognition in Patients with Schizophrenia: Interplay between Working Memory, Disorganized Symptoms, Dissociation, and the Onset and Duration of Psychosis, as Well as Resistance to Treatment" Biomedicines 11, no. 12: 3114. https://doi.org/10.3390/biomedicines11123114
APA StylePanov, G., Dyulgerova, S., & Panova, P. (2023). Cognition in Patients with Schizophrenia: Interplay between Working Memory, Disorganized Symptoms, Dissociation, and the Onset and Duration of Psychosis, as Well as Resistance to Treatment. Biomedicines, 11(12), 3114. https://doi.org/10.3390/biomedicines11123114









