Move Your Body, Boost Your Brain: The Positive Impact of Physical Activity on Cognition across All Age Groups
Abstract
1. Introduction
2. Acute Exercise
3. Chronic Exercise
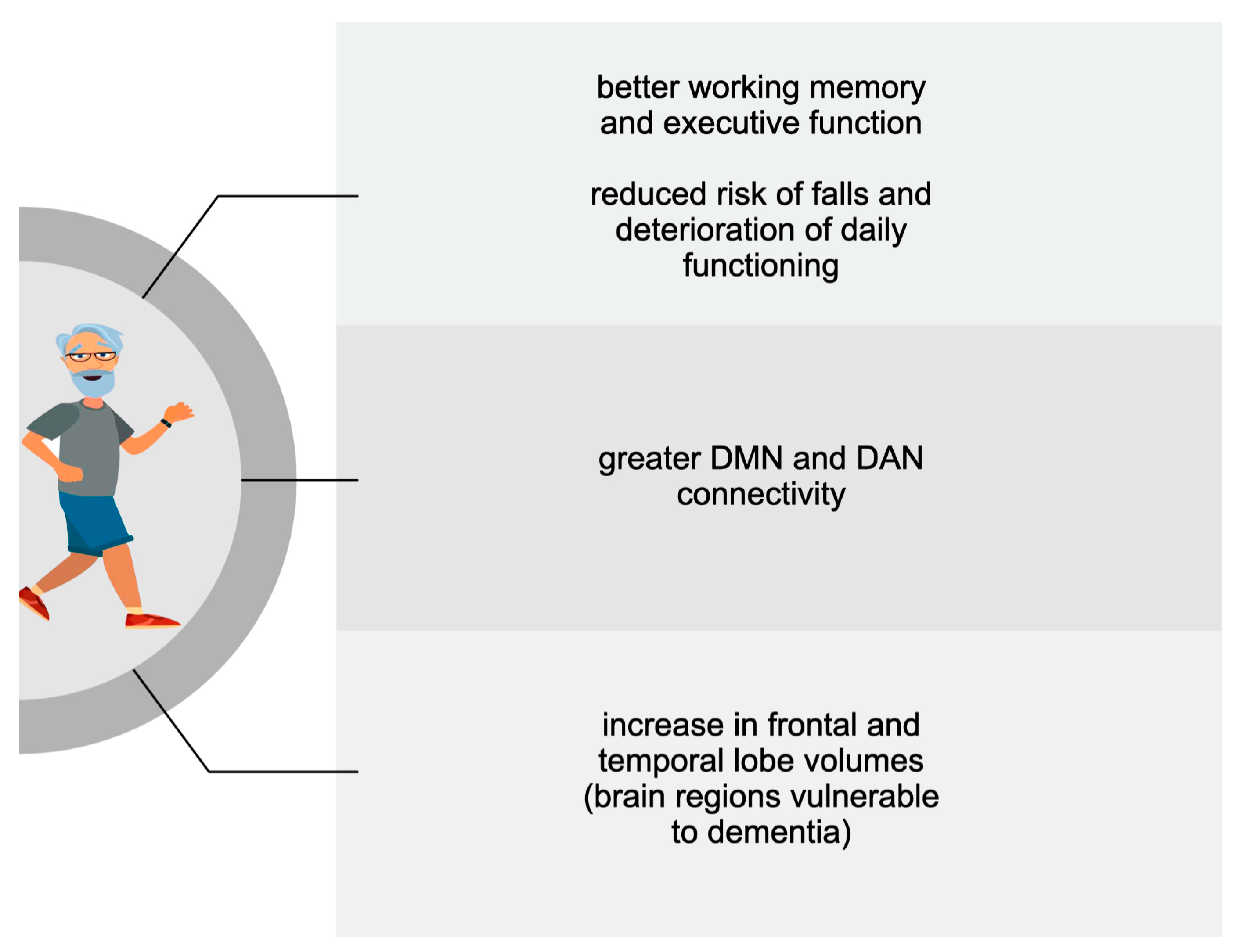
3.1. Older Adults
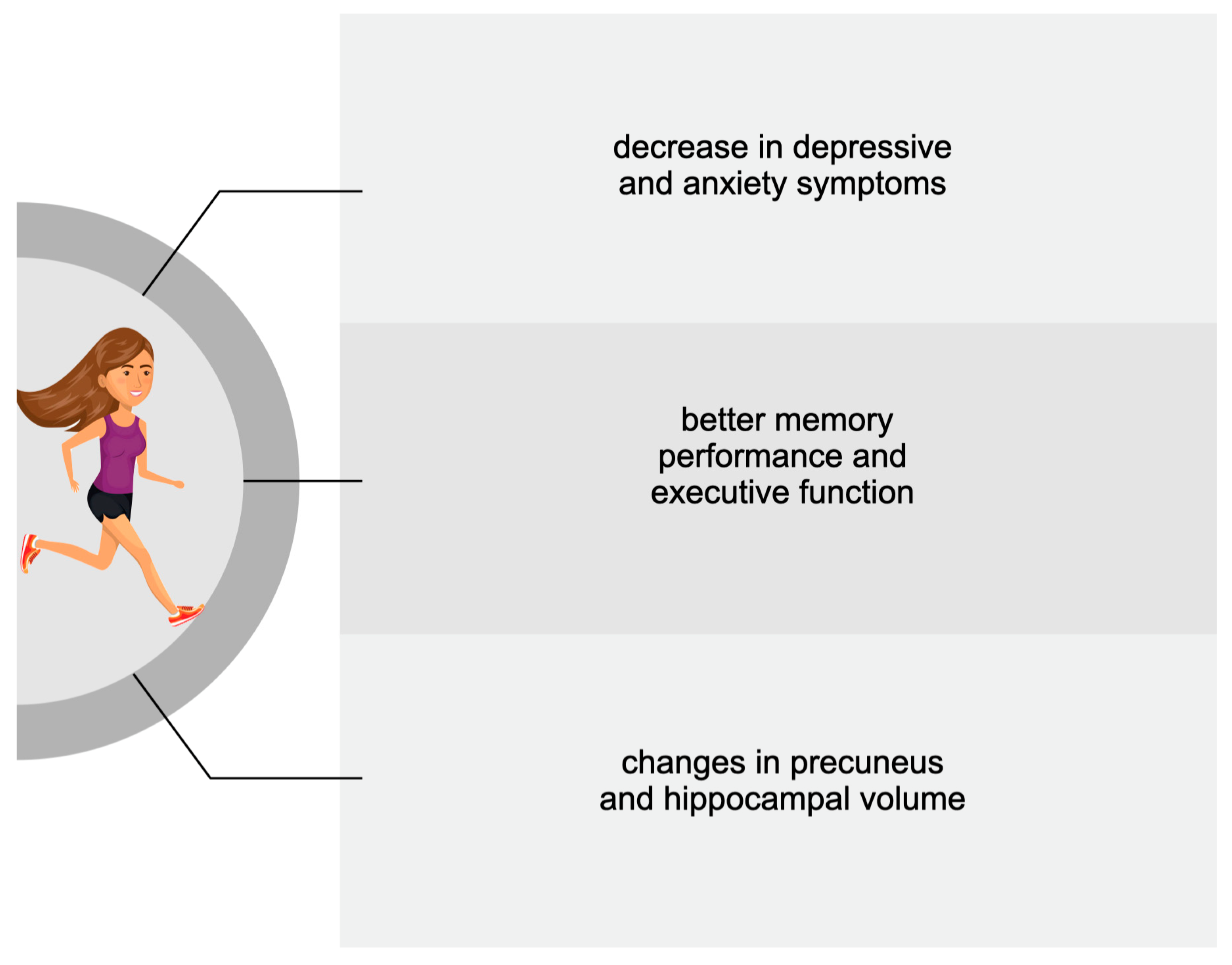
3.2. Adults
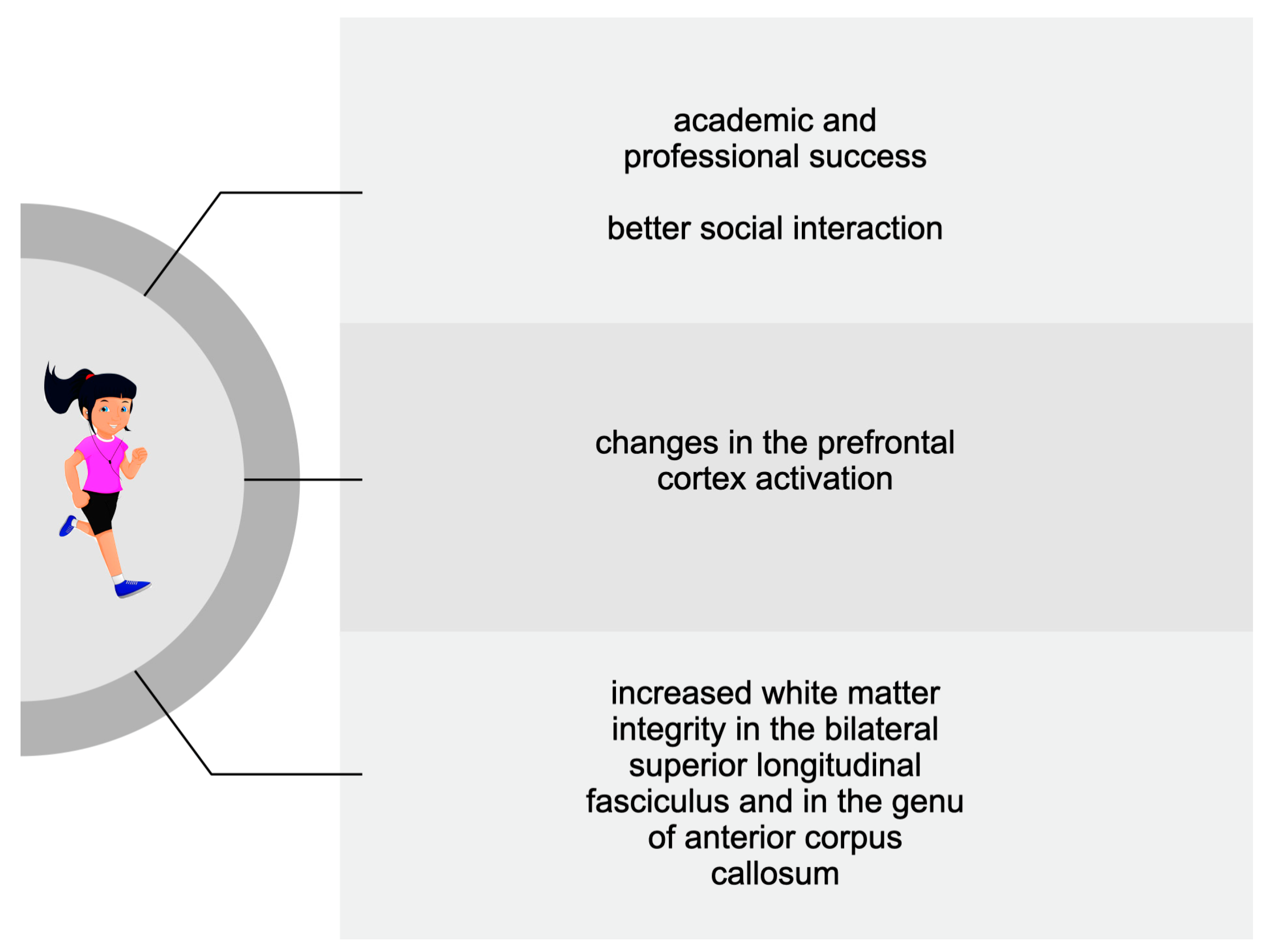
3.3. Adolescents and Children
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 100, 126–131. [Google Scholar] [PubMed]
- World Health Organization. Global Status Report on Physical Activity. 2022. Available online: https://www.who.int/publications/i/item/9789240059153 (accessed on 19 October 2022).
- Hillman, C.H.; Erickson, K.I.; Kramer, A.F. Be smart, exercise your heart: Exercise effects on brain and cognition. Nat. Rev. Neurosci. 2008, 9, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Lipowski, M.; Zaleski, Z. Inventory of Physical Activity Objectives—A new method of measuring motives for physical activity and sport. Health Psychol. Rep. 2015, 3, 47–58. [Google Scholar] [CrossRef]
- Lee, I.M.; Shiroma, E.J.; Lobelo, F.; Puska, P.; Blair, S.N.; Katzmarzyk, P.T. Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: An analysis of disease burden and life expectancy. Lancet 2012, 380, 219–229. [Google Scholar] [CrossRef]
- Sanad, E.A.; El-Shinnawy, H.A.E.H.; Hebah, H.A.; Farrag, D.A.; Soliman, E.R.A.; Abdelgawad, M.A. Effect of intra-dialytic physical exercise on depression in prevalent hemodialysis patients. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 124. [Google Scholar] [CrossRef]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychol. Sci. 2003, 14, 125–130. [Google Scholar] [CrossRef]
- Allen, C. On (not) defining cognition. Synthese 2017, 194, 4233–4249. [Google Scholar] [CrossRef]
- van Praag, H.; Kempermann, G.; Gage, F.H. Neural consequences of environmental enrichment. Nat. Rev. Neurosci. 2000, 1, 191–198. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Kramer, A.F.; Erickson, K.I.; Scalf, P.; McAuley, E.; Cohen, N.J.; Webb, A.; Jerome, G.J.; Marquez, D.X.; Elavsky, S. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. USA 2004, 101, 3316–3321. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef]
- Pignataro, P.; Dicarlo, M.; Zerlotin, R.; Storlino, G.; Oranger, A.; Sanesi, L.; Lovero, R.; Buccoliero, C.; Mori, G.; Colaianni, G.; et al. Antidepressant Effect of Intermittent Long-Term Systemic Administration of Irisin in Mice. Int. J. Mol. Sci. 2022, 23, 7596. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D. Time to move beyond a brainless exercise physiology: The evidence for complex regulation of human exercise performance. Appl. Physiol. Nutr. Metab. 2011, 36, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Törpel, A.; Herold, F.; Hamacher, D.; Müller, N.G.; Schega, L. Strengthening the Brain—Is Resistance Training with Blood Flow Restriction an Effective Strategy for Cognitive Improvement? J. Clin. Med. 2018, 7, 377. [Google Scholar] [CrossRef]
- Herold, F.; Wiegel, P.; Scholkmann, F.; Müller, N.G. Applications of Functional Near-Infrared Spectroscopy (fNIRS) Neuroimaging in Exercise—Cognition Science: A Systematic, Methodology-Focused Review. J. Clin. Med. 2018, 7, 466. [Google Scholar] [CrossRef]
- Boecker, H.; Hillman, C.H.; Scheef, L.; Strüder, H.K. Functional Neuroimaging in Exercise and Sport Sciences; Springer: New York, NY, USA, 2012; pp. 419–446. ISBN 978-1-4614-3292-0. [Google Scholar]
- Ciccarelli, O.; Toosy, A.T.; Marsden, J.F.; Wheeler-Kingshott, C.M.; Sahyoun, C.; Matthews, P.M.; Miller, D.H.; Thompson, A.J. Identifying brain regions for integrative sensorimotor processing with ankle movements. Exp. Brain Res. 2005, 166, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Glover, G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef]
- Hahn, A.; Lanzenberger, R.; Kasper, S. Making Sense of Connectivity. Int. J. Neuropsychopharmacol. 2019, 22, 194–207. [Google Scholar] [CrossRef]
- Festa, F.; Rotelli, C.; Scarano, A.; Navarra, R.; Caulo, M.; Macrì, M. Functional Magnetic Resonance Connectivity in Patients with Temporomadibular Joint Disorders. Front. Neurol. 2021, 12, 629211. [Google Scholar] [CrossRef]
- Kawata, K.H.D.S.; Hirano, K.; Hamamoto, Y.; Oi, H.; Kanno, A.; Kawashima, R.; Sugiura, M. Motivational decline and proactive response under thermal environmental stress are related to emotion- and problem-focused coping, respectively: Questionnaire construction and fMRI study. Front. Behav. Neurosci. 2023, 17, 1143450. [Google Scholar] [CrossRef]
- Damoiseaux, J.S.; Beckmann, C.F.; Arigita, E.J.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Rombouts, S.A. Reduced resting-state brain activity in the “default network” in normal aging. Cereb. Cortex 2008, 18, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Preclinical modelling in depression and anxiety: Current challenges and future research directions. Adv. Clin. Exp. Med. 2023, 32, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szabó, Á.; Spekker, E.; Polyák, H.; Tóth, F.; Vécsei, L. Mitochondrial Impairment: A Common Motif in Neuropsychiatric Presentation? The Link to the Tryptophan—Kynurenine Metabolic System. Cells 2022, 11, 2607. [Google Scholar] [CrossRef] [PubMed]
- Stillman, C.M.; Donofry, S.D.; Erickson, K.I. Exercise, fitness and the aging brain: A Review of functional connectivity in aging. Arch. Psychol. 2019, 3. [Google Scholar] [CrossRef]
- Eckstrom, E.; Neukam, S.; Kalin, L.; Wright, J. Physical Activity and Healthy Aging. Clin. Geriatr. Med. 2020, 36, 671–683. [Google Scholar] [CrossRef]
- Won, J.; Callow, D.D.; Pena, G.S.; Gogniat, M.A.; Kommula, Y.; Arnold-Nedimala, N.A.; Jordan, L.S.; Smith, J.C. Evidence for exercise-related plasticity in functional and structural neural network connectivity. Neurosci. Biobehav. Rev. 2021, 131, 923–940. [Google Scholar] [CrossRef]
- Haeger, A.; Costa, A.S.; Schulz, J.B.; Reetz, K. Cerebral changes improved by physical activity during cognitive decline: A systematic review on MRI studies. Neuroimage Clin. 2019, 23, 101933. [Google Scholar] [CrossRef]
- Li, M.Y.; Huang, M.M.; Li, S.Z.; Tao, J.; Zheng, G.H.; Chen, L.D. The effects of aerobic exercise on the structure and function of DMN-related brain regions: A systematic review. Int. J. Neurosci. 2017, 127, 634–649. [Google Scholar] [CrossRef]
- Teixeira-Machado, L.; Arida, R.M.; de Jesus Mari, J. Dance for neuroplasticity: A descriptive systematic review. Neurosci. Biobehav. Rev. 2019, 96, 232–240. [Google Scholar] [CrossRef]
- Sigmundsson, H.; Dybendal, B.H.; Grassini, S. Motion, Relation, and Passion in Brain Physiological and Cognitive Aging. Brain Sci. 2022, 12, 1122. [Google Scholar] [CrossRef]
- Turrini, S.; Bevacqua, N.; Cataneo, A.; Chiappini, E.; Fiori, F.; Battaglia, S.; Romei, V.; Avenanti, A. Neurophysiological Markers of Premotor–Motor Network Plasticity Predict Motor Performance in Young and Older Adults. Biomedicines 2023, 11, 1464. [Google Scholar] [CrossRef] [PubMed]
- Gunnell, K.E.; Poitras, V.J.; LeBlanc, A.; Schibli, K.; Barbeau, K.; Hedayati, N.; Ponitfex, M.B.; Goldfied, G.S.; Dunlap, C.; Lehan, E.; et al. Physical activity and brain structure, brain function, and cognition in children and youth: A systematic review of randomized controlled trials. Ment. Health Phys. Act. 2019, 16, 105–127. [Google Scholar] [CrossRef]
- Valkenborghs, S.R.; Noetel, M.; Hillman, C.H.; Nilsson, M.; Smith, J.J.; Ortega, F.B.; Lubans, D.R. The Impact of Physical Activity on Brain Structure and Function in Youth: A Systematic Review. Pediatrics 2019, 144, e20184032. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Hillman, C.; Stillman, C.M.; Ballard, R.M.; Bloodgood, B.; Conroy, D.E.; Macko, R.; Marquez, D.X.; Petruzzello, S.J.; Powell, K.E.; et al. Physical Activity, Cognition, and Brain Outcomes: A Review of the 2018 Physical Activity Guidelines. Med. Sci. Sports Exerc. 2019, 51, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef]
- Emery, C.F.; Honn, V.J.; Frid, D.J.; Lebowitz, K.R.; Diaz, P.T. Acute effects of exercise on cognition in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2001, 164, 1624–1627. [Google Scholar] [CrossRef] [PubMed]
- Pontifex, M.B.; Hillman, C.H.; Fernhall, B.; Thompson, K.M.; Valentini, T.A. The effect of acute aerobic and resistance exercise on working memory. Med. Sci. Sports Exerc. 2009, 41, 927–934. [Google Scholar] [CrossRef]
- Coelho, F.G.; Vital, T.M.; Stein, A.M.; Arantes, F.J.; Rueda, A.V.; Camarini, R.; Teodorov, E.; Santos-Galduróz, R.F. Acute aerobic exercise increases brain-derived neurotrophic factor levels in elderly with Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 39, 401–408. [Google Scholar] [CrossRef]
- Won, J.; Alfini, A.J.; Weiss, L.R.; Michelson, C.S.; Callow, D.D.; Ranadive, S.M.; Gentili, R.J.; Smith, J.C. Semantic Memory Activation After Acute Exercise in Healthy Older Adults. J. Int. Neuropsychol. Soc. 2019, 25, 557–568. [Google Scholar] [CrossRef]
- Won, J.; Alfini, A.J.; Weiss, L.R.; Callow, D.D.; Smith, J.C. Brain activation during executive control after acute exercise in older adults. Int. J. Psychophysiol. 2019, 146, 240–248. [Google Scholar] [CrossRef]
- Voss, M.W.; Wenig, T.B.; Narayana-Kumanan, K.; Cole, R.C.; Wharff, C.; Reist, L.; Dubose, L.; Sigurdsson, G.; Mills, J.A.; Long, J.D.; et al. Acute Exercise Effects Predict Training Change in Cognition and Connectivity. Med. Sci. Sports Exerc. 2020, 52, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Suwabe, K.; Byun, K.; Hyodo, K.; Reagh, Z.M.; Roberts, J.M.; Matsushita, A.; Saotome, K.; Ochi, G.; Fukuie, T.; Suzuki, K.; et al. Rapid stimulation of human dentate gyrus function with acute mild exercise. Proc. Natl. Acad. Sci. USA 2018, 115, 10487–10492. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Men, W.W.; Chang, Y.K.; Fan, M.X.; Ji, L.; Wei, G.X. Acute aerobic exercise increases cortical activity during working memory: A functional MRI study in female college students. PLoS ONE 2014, 9, e99222. [Google Scholar] [CrossRef] [PubMed]
- Marin Bosch, B.; Bringard, A.; Logrieco, M.G.; Lauer, E.; Imobersteg, N.; Thomas, A.; Ferretti, G.; Schwartz, S.; Igloi, K. Effect of acute physical exercise on motor sequence memory. Sci. Rep. 2020, 10, 15322. [Google Scholar] [CrossRef]
- Perini, R.; Bortoletto, M.; Capogrosso, M.; Fertonani, A.; Miniussi, C. Acute effects of aerobic exercise promote learning. Sci. Rep. 2016, 6, 25440. [Google Scholar] [CrossRef]
- Mehren, A.; Özyurt, J.; Lam, A.P.; Brandes, M.; Müller, H.H.O.; Thiel, C.M.; Philipsen, A. Acute Effects of Aerobic Exercise on Executive Function and Attention in Adult Patients with ADHD. Front. Psychiatry 2019, 10, 132. [Google Scholar] [CrossRef]
- Mehren, A.; Diaz Luque, C.; Brandes, M.; Lam, A.P.; Thiel, C.M.; Philipsen, A.; Özyurt, J. Intensity-Dependent Effects of Acute Exercise on Executive Function. Neural Plast. 2019, 2019, 8608317. [Google Scholar] [CrossRef]
- Schmitt, A.; Martin, J.A.; Rojas, S.; Vafa, R.; Scheef, L.; Strüder, H.K.; Boecker, H. Effects of low- and high-intensity exercise on emotional face processing: An fMRI face-matching study. Soc. Cogn. Affect. Neurosci. 2019, 14, 657–665. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Cui, J.; Chen, L.Z.; Wang, X.; Fan, M.; Wei, G.X. Fitness-Dependent Effect of Acute Aerobic Exercise on Executive Function. Front. Physiol. 2019, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Park, S.; Kim, K.K.; Lee, K.; Rhyu, H.S. Acute effects of aerobic stretching, health and happiness improving movement exercise on cortical activity of children. J. Exerc. Rehabil. 2016, 12, 320–327. [Google Scholar] [CrossRef]
- Chen, A.G.; Zhu, L.N.; Yan, J.; Yin, H.C. Neural Basis of Working Memory Enhancement after Acute Aerobic Exercise: fMRI Study of Preadolescent Children. Front. Psychol. 2016, 7, 1804. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.G.; Zhu, L.N.; Xiong, X.; Li, Y. Acute aerobic exercise alters executive control network in preadolescent children. J. Sport Psychol. 2017, 26, 132–137. [Google Scholar]
- Metcalfe, A.W.; MacIntosh, B.J.; Scavone, A.; Ou, X.; Korczak, D.; Goldstein, B.I. Effects of acute aerobic exercise on neural correlates of attention and inhibition in adolescents with bipolar disorder. Transl. Psychiatry 2016, 6, e814. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.M.; Staines, W.R. The effects of acute aerobic exercise on the primary motor cortex. J. Mot. Behav. 2015, 47, 328–339. [Google Scholar] [CrossRef]
- Herold, F.; Aye, N.; Lehmann, N.; Taubert, M.; Müller, N.G. The Contribution of Functional Magnetic Resonance Imaging to the Understanding of the Effects of Acute Physical Exercise on Cognition. Brain Sci. 2020, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Ageing and Health. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 1 October 2022).
- Heger, I.; Deckers, K.; van Boxtel, M.; de Vugt, M.; Hajema, K.; Verhey, F.; Köhler, S. Dementia awareness and risk perception in middle-aged and older individuals: Baseline results of the MijnBreincoach survey on the association between lifestyle and brain health. BMC Public Health 2019, 19, 678. [Google Scholar] [CrossRef]
- Dedeyne, L.; Deschodt, M.; Verschueren, S.; Tournoy, J.; Gielen, E. Effects of multi-domain interventions in (pre)frail elderly on frailty, functional, and cognitive status: A systematic review. Clin. Interv. Aging 2017, 12, 873–896. [Google Scholar] [CrossRef]
- Karssemeijer, E.G.A.; Aaronson, J.A.; Bossers, W.J.; Smits, T.; Olde Rikkert, M.G.M.; Kessels, R.P.C. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: A meta-analysis. Ageing Res. Rev. 2017, 40, 75–83. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef]
- Kerr, J.; Marshall, S.J.; Patterson, R.E.; Marinac, C.R.; Natarajan, L.; Rosenberg, D.; Wasilenko, K.; Crist, K. Objectively measured physical activity is related to cognitive function in older adults. J. Am. Geriatr. Soc. 2013, 61, 1927–1931. [Google Scholar] [CrossRef]
- Zhu, W.; Howard, V.J.; Wadley, V.G.; Hutto, B.; Blair, S.N.; Vena, J.E.; Colabianchi, N.; Rhodes, D.; Hooker, S.P. Association Between Objectively Measured Physical Activity and Cognitive Function in Older Adults-The Reasons for Geographic and Racial Differences in Stroke Study. J. Am. Geriatr. Soc. 2015, 63, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Blackwell, T.; Stone, K.L.; Goldman, S.E.; Hillier, T.; Yaffe, K.; Study of Osteoporotic Fractures. Cognition in older women: The importance of daytime movement. J. Am. Geriatr. Soc. 2008, 56, 1658–1664. [Google Scholar] [CrossRef]
- Buchman, A.S.; Wilson, R.S.; Bennett, D.A. Total daily activity is associated with cognition in older persons. Am. J. Geriatr. Psychiatry 2008, 16, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, B.; Bergland, A.; Rydwik, E. The Importance of Physical Activity Exercise among Older People. Biomed. Res. Int. 2018, 2018, 7856823. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D. Physical activity is medicine for older adults. Postgrad. Med. J. 2014, 90, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Pietrelli, A.; Lopez-Costa, J.; Goñi, R.; Brusco, A.; Basso, N. Aerobic exercise prevents age-dependent cognitive decline and reduces anxiety-related behaviors in middle-aged and old rats. Neuroscience 2012, 202, 252–266. [Google Scholar] [CrossRef]
- Klimova, B.; Dostalova, R. The Impact of Physical Activities on Cognitive Performance among Healthy Older Individuals. Brain Sci. 2020, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.; Choi, M.; Chun, B.O.; Sun, K.; Kim, K.S.; Kang, S.W.; Song, H.S.; Moon, S.Y. Effects of a High-Intensity Interval Physical Exercise Program on Cognition, Physical Performance, and Electroencephalogram Patterns in Korean Elderly People: A Pilot Study. Dement. Neurocogn. Disord. 2022, 21, 93–102. [Google Scholar] [CrossRef]
- De la Rosa, A.; Olaso-Gonzalez, G.; Arc-Chagnaud, C.; Millan, F.; Salvador-Pascual, A.; García-Lucerga, C.; Blasco-Lafarga, C.; Garcia-Dominguez, E.; Carretero, A.; Correas, A.G.; et al. Physical exercise in the prevention and treatment of Alzheimer’s disease. J. Sport Health Sci. 2020, 9, 394–404. [Google Scholar] [CrossRef]
- Engeroff, T.; Füzéki, E.; Vogt, L.; Fleckenstein, J.; Schwarz, S.; Matura, S.; Pilatus, U.; Deichmann, R.; Hellweg, R.; Pantel, J.; et al. Is Objectively Assessed Sedentary Behavior, Physical Activity and Cardiorespiratory Fitness Linked to Brain Plasticity Outcomes in Old Age? Neuroscience 2018, 388, 384–392. [Google Scholar] [CrossRef]
- Wheeler, M.J.; Green, D.J.; Ellis, K.A.; Cerin, E.; Heinonen, I.; Naylor, L.H.; Larsen, R.; Wennberg, P.; Boraxbekk, C.J.; Lewis, J.; et al. Distinct effects of acute exercise and breaks in sitting on working memory and executive function in older adults: A three-arm, randomised cross-over trial to evaluate the effects of exercise with and without breaks in sitting on cognition. Br. J. Sports Med. 2020, 54, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Gaitán, J.M.; Moon, H.Y.; Stremlau, M.; Dubal, D.B.; Cook, D.B.; Okonkwo, O.C.; van Praag, H. Effects of Aerobic Exercise Training on Systemic Biomarkers and Cognition in Late Middle-Aged Adults at Risk for Alzheimer’s Disease. Front. Endocrinol. 2021, 12, 660181. [Google Scholar] [CrossRef]
- Neale, C.; Aspinall, P.; Roe, J.; Tilley, S.; Mavros, P.; Cinderby, S.; Coyne, R.; Thin, N.; Bennett, G.; Thompson, C.W. The Aging Urban Brain: Analyzing Outdoor Physical Activity Using the Emotiv Affectiv Suite in Older People. J. Urban Health 2017, 94, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Gogniat, M.A.; Robison, T.L.; Jean, K.R.; Stephen Miller, L. Physical activity moderates the association between executive function and functional connectivity in older adults. Aging Brain 2022, 2, 100036. [Google Scholar] [CrossRef] [PubMed]
- Gogniat, M.A.; Robison, T.L.; Jean, K.R.; Stephen Miller, L. Physical activity and fitness moderate the association between executive function and anti-correlated networks in the aging brain. Sport Sci. Health 2022, 18, 1021–1031. [Google Scholar] [CrossRef]
- Pieramico, V.; Esposito, R.; Sensi, F.; Cilli, F.; Mantini, D.; Mattei, P.A.; Frazzini, V.; Ciavardelli, D.; Gatta, V.; Ferretti, A.; et al. Combination training in aging individuals modifies functional connectivity and cognition, and is potentially affected by dopamine-related genes. PLoS ONE 2012, 7, e43901. [Google Scholar] [CrossRef]
- Li, R.; Zhu, X.; Yin, S.; Niu, Y.; Zheng, Z.; Huang, X.; Wang, B.; Li, J. Multimodal intervention in older adults improves resting-state functional connectivity between the medial prefrontal cortex and medial temporal lobe. Front. Aging Neurosci. 2014, 6, 39. [Google Scholar] [CrossRef]
- Tao, J.; Liu, J.; Egorova, N.; Chen, X.; Sun, S.; Xue, X.; Huang, J.; Zheng, G.; Wang, Q.; Chen, L.; et al. Increased Hippocampus-Medial Prefrontal Cortex Resting-State Functional Connectivity and Memory Function after Tai Chi Chuan Practice in Elder Adults. Front. Aging Neurosci. 2016, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Dorsman, K.A.; Weiner-Light, S.; Staffaroni, A.M.; Brown, J.A.; Wolf, A.; Cobigo, Y.; Walters, S.; Kramer, J.H.; Casaletto, K.B. Get Moving! Increases in Physical Activity Are Associated with Increasing Functional Connectivity Trajectories in Typically Aging Adults. Front. Aging Neurosci. 2020, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zhang, H.; Potter, G.G.; Zang, Y.F.; Steffens, D.C.; Guo, H.; Wang, L. Multiple Neuroimaging Measures for Examining Exercise-induced Neuroplasticity in Older Adults: A Quasi-experimental Study. Front. Aging Neurosci. 2017, 9, 102. [Google Scholar] [CrossRef]
- Voss, M.W.; Prakash, R.S.; Erickson, K.I.; Basak, C.; Chaddock, L.; Kim, J.S.; Alves, H.; Heo, S.; Szabo, A.N.; White, S.M.; et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Front. Aging Neurosci. 2010, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Flodin, P.; Jonasson, L.S.; Riklund, K.; Nyberg, L.; Boraxbekk, C.J. Does Aerobic Exercise Influence Intrinsic Brain Activity? An Aerobic Exercise Intervention among Healthy Old Adults. Front. Aging Neurosci. 2017, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Voss, M.W.; Sutterer, M.; Weng, T.B.; Burzynska, A.Z.; Fanning, J.; Salerno, E.; Gothe, N.P.; Ehlers, D.K.; McAuley, E.; Kramer, A.F. Nutritional supplementation boosts aerobic exercise effects on functional brain systems. J. Appl. Physiol. 2019, 126, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Yasunaga, A.; Wang, L.Q. Correlation between moderate daily physical activity and neurocognitive variability in healthy elderly people. Arch. Gerontol. Geriatr. 2013, 56, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bugg, J.M.; Head, D. Exercise moderates age-related atrophy of the medial temporal lobe. Neurobiol. Aging 2011, 32, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Papenberg, G.; Ferencz, B.; Mangialasche, F.; Mecocci, P.; Cecchetti, R.; Kalpouzos, G.; Fratiglioni, L.; Bäckman, L. Physical activity and inflammation: Effects on gray-matter volume and cognitive decline in aging. Hum. Brain Mapp. 2016, 37, 3462–3473. [Google Scholar] [CrossRef] [PubMed]
- Wells, R.E.; Yeh, G.Y.; Kerr, C.E.; Wolkin, J.; Davis, R.B.; Tan, Y.; Spaeth, R.; Wall, R.B.; Walsh, J.; Kaptchuk, T.J.; et al. Meditation’s impact on default mode network and hippocampus in mild cognitive impairment: A pilot study. Neurosci. Lett. 2013, 556, 15–19. [Google Scholar] [CrossRef]
- Eyre, H.A.; Acevedo, B.; Yang, H.; Siddarth, P.; van Dyk, K.; Ercoli, L.; Leaver, A.M.; Cyr, N.S.; Narr, K.; Baune, B.T.; et al. Changes in Neural Connectivity and Memory Following a Yoga Intervention for Older Adults: A Pilot Study. J. Alzheimers Dis. 2016, 52, 673–684. [Google Scholar] [CrossRef]
- Tao, J.; Liu, J.; Chen, X.; Xia, R.; Li, M.; Huang, M.; Li, S.; Park, J.; Wilson, G.; Lang, C.; et al. Mind-body exercise improves cognitive function and modulates the function and structure of the hippocampus and anterior cingulate cortex in patients with mild cognitive impairment. Neuroimage Clin. 2019, 23, 101834. [Google Scholar] [CrossRef]
- Suo, C.; Singh, M.F.; Gates, N.; Wen, W.; Sachdev, P.; Brodaty, H.; Saigal, N.; Wilson, G.C.; Meiklejohn, J.; Singh, N.; et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol. Psychiatry 2016, 21, 1633–1642. [Google Scholar] [CrossRef]
- Hsu, C.L.; Best, J.R.; Wang, S.; Voss, M.W.; Hsiung, R.G.Y.; Munkacsy, M.; Cheung, W.; Handy, T.C.; Liu-Ambrose, T. The Impact of Aerobic Exercise on Fronto-Parietal Network Connectivity and Its Relation to Mobility: An Exploratory Analysis of a 6-Month Randomized Controlled Trial. Front. Hum. Neurosci. 2017, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Veldsman, M.; Churilov, L.; Werden, E.; Li, Q.; Cumming, T.; Brodtmann, A. Physical activity after stroke is associated with increased interhemispheric connectivity of the dorsal attention network. Neurorehabilit. Neural Repair 2017, 31, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Corbetta, M.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. USA 2006, 103, 10046–10051. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Kelly, A.M.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009, 30, 625–637. [Google Scholar] [CrossRef]
- Churchwell, J.C.; Kesner, R.P. Hippocampal-prefrontal dynamics in spatial working memory: Interactions and independent parallel processing. Behav. Brain Res. 2011, 225, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.; Bush, D.; Bonnefond, M.; Bandettini, P.A.; Barnes, G.R.; Doeller, C.F.; Burgess, N. Medial prefrontal theta phase coupling during spatial memory retrieval. Hippocampus 2014, 24, 656–665. [Google Scholar] [CrossRef]
- Dougherty, R.J.; Ellingson, L.D.; Schultz, S.A.; Boots, E.A.; Meyer, J.D.; Lindheimer, J.B.; van Riper, S.; Stegner, A.J.; Edwards, D.F.; Oh, J.M.; et al. Meeting physical activity recommendations may be protective against temporal lobe atrophy in older adults at risk for Alzheimer’s disease. Alzheimers Dement. 2016, 4, 14–17. [Google Scholar] [CrossRef]
- Vogt, B.A.; Vogt, L.J.; Vrana, K.E.; Gioia, L.; Meadows, R.S.; Challa, V.R.; Hof, P.R.; van Hoesen, G.W. Multivariate analysis of laminar patterns of neurodegeneration in posterior cingulate cortex in Alzheimer’s disease. Exp. Neurol. 1998, 153, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhou, W.; Xia, R.; Tao, J.; Chen, L. Aerobic Exercises for Cognition Rehabilitation following Stroke: A Systematic Review. J. Stroke Cerebrovasc. Dis. 2016, 25, 2780–2789. [Google Scholar] [CrossRef]
- Oberlin, L.E.; Waiwood, A.M.; Cumming, T.B.; Marsland, A.L.; Bernhardt, J.; Erickson, K.I. Effects of Physical Activity on Poststroke Cognitive Function: A Meta-Analysis of Randomized Controlled Trials. Stroke 2017, 48, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Li, X.; Zhu, Z.; Li, T.; Li, W.; Huang, P.; Gong, W. Effects of Combined Cognitive and Exercise Interventions on Poststroke Cognitive Function: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2021, 2021, 4558279. [Google Scholar] [CrossRef] [PubMed]
- Goldin, P.; Ziv, M.; Jazaieri, H.; Gross, J.J. Randomized controlled trial of mindfulness-based stress reduction versus aerobic exercise: Effects on the self-referential brain network in social anxiety disorder. Front. Hum. Neurosci. 2012, 6, 295. [Google Scholar] [CrossRef]
- Goldin, P.; Ziv, M.; Jazaieri, H.; Hahn, K.; Gross, J.J. MBSR vs aerobic exercise in social anxiety: fMRI of emotion regulation of negative self-beliefs. Soc. Cogn. Affect. Neurosci. 2013, 8, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Gourgouvelis, J.; Yielder, P.; Murphy, B. Exercise Promotes Neuroplasticity in Both Healthy and Depressed Brains: An fMRI Pilot Study. Neural Plast. 2017, 2017, 8305287. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Huang, G.; Ding, Q.; Liang, P.; Hu, C.; Zhang, H.; Zhan, L.; Wang, Q.; Cao, Y.; Zhang, J.; et al. Amplitude of low-frequency fluctuation (ALFF) alterations in adults with subthreshold depression after physical exercise: A resting-state fMRI study. J. Affect. Disord. 2021, 295, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Stern, Y.; MacKay-Brandt, A.; Lee, S.; McKinley, P.; McIntyre, K.; Razlighi, Q.; Agarunov, E.; Bartels, M.; Sloan, R.P. Effect of aerobic exercise on cognition in younger adults: A randomized clinical trial. Neurology 2019, 92, e905–e916. [Google Scholar] [CrossRef]
- Bashir, S.; Al-Sultan, F.; Jamea, A.A.; Almousa, A.; Alzahrani, M.S.; Alhargan, F.A.; Abualait, T.; Yoo, W.K. Physical exercise and cortical thickness in healthy controls: A pilot study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7375–7379. [Google Scholar] [CrossRef]
- Kaiser, A.; Reneman, L.; Solleveld, M.M.; Coolen, B.F.; Scherder, E.J.A.; Knutsson, L.; Bjørnerud, A.; van Osch, M.J.P.; Wijnen, J.P.; Lucassen, P.J.; et al. A Randomized Controlled Trial on the Effects of a 12-Week High- vs. Low-Intensity Exercise Intervention on Hippocampal Structure and Function in Healthy, Young Adults. Front. Psychiatry 2022, 12, 780095. [Google Scholar] [CrossRef]
- Fontes, E.B.; Okano, A.H.; De Guio, F.; Schabort, E.J.; Min, L.L.; Basset, F.A.; Stein, D.J.; Noakes, T.D. Brain activity and perceived exertion during cycling exercise: An fMRI study. Br. J. Sports Med. 2015, 49, 556–560. [Google Scholar] [CrossRef]
- Ishihara, T.; Miyazaki, A.; Tanaka, H.; Matsuda, T. Identification of the brain networks that contribute to the interaction between physical function and working memory: An fMRI investigation with over 1,000 healthy adults. Neuroimage 2020, 221, 117152. [Google Scholar] [CrossRef]
- Nakagawa, T.; Koan, I.; Chen, C.; Matsubara, T.; Hagiwara, K.; Lei, H.; Hirotsu, M.; Yamagata, H.; Nakagawa, S. Regular Moderate- to Vigorous-Intensity Physical Activity Rather Than Walking Is Associated with Enhanced Cognitive Functions and Mental Health in Young Adults. Int. J. Environ. Res. Public Health 2020, 17, 614. [Google Scholar] [CrossRef] [PubMed]
- Bezzola, L.; Mérillat, S.; Jäncke, L. The effect of leisure activity golf practice on motor imagery: An fMRI study in middle adulthood. Front. Hum. Neurosci. 2012, 6, 67. [Google Scholar] [CrossRef] [PubMed]
- Wadden, K.; Brown, K.; Maletsky, R.; Boyd, L.A. Correlations between brain activity and components of motor learning in middle-aged adults: An fMRI study. Front. Hum. Neurosci. 2013, 7, 169. [Google Scholar] [CrossRef] [PubMed]
- Pensel, M.C.; Daamen, M.; Scheef, L.; Knigge, H.U.; Rojas Vega, S.; Martin, J.A.; Schild, H.H.; Strüder, H.K.; Boecker, H. Executive control processes are associated with individual fitness outcomes following regular exercise training: Blood lactate profile curves and neuroimaging findings. Sci. Rep. 2018, 8, 4893. [Google Scholar] [CrossRef]
- De Greeff, J.W.; Bosker, R.J.; Oosterlaan, J.; Visscher, C.; Hartman, E. Effects of physical activity on executive functions, attention and academic performance in preadolescent children: A meta-analysis. J. Sci. Med. Sport 2018, 21, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ayllon, M.; Plaza-Florido, A.; Mendez-Gutierrez, A.; Altmäe, S.; Solis-Urra, P.; Aguilera, C.M.; Catena, A.; Ortega, F.B.; Esteban-Cornejo, I. The effects of a 20-week exercise program on blood-circulating biomarkers related to brain health in overweight or obese children: The ActiveBrains project. J. Sport Health Sci. 2023, 12, 175–185. [Google Scholar] [CrossRef]
- De Menezes-Junior, F.J.; Jesus, Í.C.; Brand, C.; Mota, J.; Leite, N. Physical Exercise and Brain-Derived Neurotrophic Factor Concentration in Children and Adolescents: A Systematic Review with Meta-Analysis. Pediatr. Exerc. Sci. 2022, 34, 44–53. [Google Scholar] [CrossRef]
- Davis, C.L.; Tomporowski, P.D.; McDowell, J.E.; Austin, B.P.; Miller, P.H.; Yanasak, N.E.; Allison, J.D.; Naglieri, J.A. Exercise improves executive function and achievement and alters brain activation in overweight children: A randomized, controlled trial. Health Psychol. 2011, 30, 91–98. [Google Scholar] [CrossRef]
- Jackson, W.M.; Davis, N.; Sands, S.A.; Whittington, R.A.; Sun, L.S. Physical Activity and Cognitive Development: A Meta-Analysis. J. Neurosurg. Anesthesiol. 2016, 28, 373–380. [Google Scholar] [CrossRef]
- Kjellenberg, K.; Ekblom, O.; Ahlen, J.; Helgadóttir, B.; Nyberg, G. Cross-sectional associations between physical activity pattern, sports participation, screen time and mental health in Swedish adolescents. BMJ Open 2022, 12, e061929. [Google Scholar] [CrossRef]
- Kamijo, K.; Pontifex, M.B.; O’Leary, K.C.; Scudder, M.R.; Wu, C.T.; Castelli, D.M.; Hillman, C.H. The effects of an afterschool physical activity program on working memory in preadolescent children. Dev. Sci. 2011, 14, 1046–1058. [Google Scholar] [CrossRef]
- Quinzi, F.; Modica, M.; Berchicci, M.; Bianco, V.; Perri, R.L.; Di Russo, F. Does sport type matter? The effect of sport discipline on cognitive control strategies in preadolescents. Int. J. Psychophysiol. 2022, 177, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Hillman, C.H.; Pontifex, M.B.; Castelli, D.M.; Khan, N.A.; Raine, L.B.; Scudder, M.R.; Drollette, E.S.; Moore, R.D.; Wu, C.T.; Kamijo, K. Effects of the FITKids randomized controlled trial on executive control and brain function. Pediatrics 2014, 134, e1063–e1071. [Google Scholar] [CrossRef] [PubMed]
- Chaddock-Heyman, L.; Erickson, K.I.; Voss, M.W.; Knecht, A.M.; Pontifex, M.B.; Castelli, D.M.; Hillman, C.H.; Kramer, A.F. The effects of physical activity on functional MRI activation associated with cognitive control in children: A randomized controlled intervention. Front. Hum. Neurosci. 2013, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Chaddock-Heyman, L.; Erickson, K.I.; Kienzler, C.; Drollette, E.S.; Raine, L.B.; Kao, S.C.; Bensken, J.; Weisshappel, R.; Castelli, D.M.; Hillman, C.H.; et al. Physical Activity Increases White Matter Microstructure in Children. Front. Neurosci. 2018, 12, 950. [Google Scholar] [CrossRef] [PubMed]
- Logan, N.E.; Raine, L.B.; Drollette, E.S.; Castelli, D.M.; Khan, N.A.; Kramer, A.F.; Hillman, C.H. The differential relationship of an afterschool physical activity intervention on brain function and cognition in children with obesity and their normal weight peers. Pediatr. Obes. 2021, 16, e12708. [Google Scholar] [CrossRef]
- Ortega, F.B.; Mora-Gonzalez, J.; Cadenas-Sanchez, C.; Esteban-Cornejo, I.; Migueles, J.H.; Solis-Urra, P.; Verdejo-Román, J.; Rodriguez-Ayllon, M.; Molina-Garcia, P.; Ruiz, J.R.; et al. Effects of an Exercise Program on Brain Health Outcomes for Children with Overweight or Obesity: The ActiveBrains Randomized Clinical Trial. JAMA Netw. Open 2022, 5, e2227893. [Google Scholar] [CrossRef] [PubMed]
- Krafft, C.E.; Schwarz, N.F.; Chi, L.; Weinberger, A.L.; Schaeffer, D.J.; Pierce, J.E.; Rodrigue, A.L.; Yanasak, N.E.; Miller, P.H.; Tomporowski, P.D.; et al. An 8-month randomized controlled exercise trial alters brain activation during cognitive tasks in overweight children. Obesity 2014, 22, 232–242. [Google Scholar] [CrossRef]
- Krafft, C.E.; Pierce, J.E.; Schwarz, N.F.; Chi, L.; Weinberger, A.L.; Schaeffer, D.J.; Rodrigue, A.L.; Camchong, J.; Allison, J.D.; Yanasak, N.E.; et al. An eight month randomized controlled exercise intervention alters resting state synchrony in overweight children. Neuroscience 2014, 256, 445–455. [Google Scholar] [CrossRef]
- Krafft, C.E.; Schaeffer, D.J.; Schwarz, N.F.; Chi, L.; Weinberger, A.L.; Pierce, J.E.; Rodrigue, A.L.; Allison, J.D.; Yanasak, N.E.; Liu, T.; et al. Improved frontoparietal white matter integrity in overweight children is associated with attendance at an after-school exercise program. Dev. Neurosci. 2014, 36, 1–9. [Google Scholar] [CrossRef]
- Schaeffer, D.J.; Krafft, C.E.; Schwarz, N.F.; Chi, L.; Rodrigue, A.L.; Pierce, J.E.; Allison, J.D.; Yanasak, N.E.; Liu, T.; Davis, C.L.; et al. An 8-month exercise intervention alters frontotemporal white matter integrity in overweight children. Psychophysiology 2014, 51, 728–733. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Regional Office for Europe. WHO European Regional Obesity Report. 2022. Available online: https://apps.who.int/iris/handle/10665/353747 (accessed on 3 May 2022).
- Bauer, C.C.; Moreno, B.; González-Santos, L.; Concha, L.; Barquera, S.; Barrios, F.A. Child overweight and obesity are associated with reduced executive cognitive performance and brain alterations: A magnetic resonance imaging study in Mexican children. Pediatr. Obes. 2015, 10, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, K.; Maruyama, T.; Murota, M.; Nakahara, Y. Positive effects of acute and moderate physical exercise on cognitive function. J. Physiol. Anthropol. 2009, 28, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Stillman, C.M.; Cohen, J.; Lehman, M.E.; Erickson, K.I. Mediators of Physical Activity on Neurocognitive Function: A Review at Multiple Levels of Analysis. Front. Hum. Neurosci. 2016, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Mottola, L.; Crisostomi, S.; Ferrari, M.; Quaresima, V. Relationship between handgrip sustained submaximal exercise and prefrontal cortex oxygenation. Adv. Exp. Med. Biol. 2006, 578, 305–309. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, Z.; Zhu, L.; Huang, G.; Li, B.; Chen, C.; Huang, J.; Ma, F.; Liu, T.C. Effects of different physical activities on brain-derived neurotrophic factor: A systematic review and bayesian network meta-analysis. Front. Aging Neurosci. 2022, 14, 981002. [Google Scholar] [CrossRef]
- Moore, D.; Jung, M.; Hillman, C.H.; Kang, M.; Loprinzi, P.D. Interrelationships between exercise, functional connectivity, and cognition among healthy adults: A systematic review. Psychophysiology 2022, 59, e14014. [Google Scholar] [CrossRef]
- Domingos, C.; Pêgo, J.M.; Santos, N.C. Effects of physical activity on brain function and structure in older adults: A systematic review. Behav. Brain Res. 2021, 402, 113061. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.F.; Kim, C.; Clasey, J.L.; Bailey, A.; Gold, B.T. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage 2012, 59, 1514–1523. [Google Scholar] [CrossRef]
- Voss, M.W.; Heo, S.; Prakash, R.S.; Erickson, K.I.; Alves, H.; Chaddock, L.; Szabo, A.N.; Mailey, E.L.; Wójcicki, T.R.; White, S.M.; et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Hum. Brain Mapp. 2013, 34, 2972–2985. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, H.I.; van Boxtel, M.P.; Jolles, J.; Verhey, F.R.; Uylings, H.B. Parietal cortex matters in Alzheimer’s disease: An overview of structural, functional and metabolic findings. Neurosci. Biobehav. Rev. 2012, 36, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Kivipelto, M.; Mangialasche, F.; Ngandu, T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Alty, J.; Farrow, M.; Lawler, K. Exercise and dementia prevention. Pract. Neurol. 2020, 20, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Heinze, K.; Cumming, J.; Dosanjh, A.; Palin, S.; Poulton, S.; Bagshaw, A.P.; Broome, M.R. Neurobiological evidence of longer-term physical activity interventions on mental health outcomes and cognition in young people: A systematic review of randomised controlled trials. Neurosci. Biobehav. Rev. 2021, 120, 431–441. [Google Scholar] [CrossRef]
- Birren, J.E.; Schaie, K.W. Handbook of the Psychology of Aging, 4th ed.; Academic Press: San Diego, CA, USA, 1996; ISBN 0-121-01260-3. [Google Scholar]
- Voss, M.W.; Weng, T.B.; Burzynska, A.Z.; Wong, C.N.; Cooke, G.E.; Clark, R.; Fanning, J.; Awick, E.; Gothe, N.P.; Olson, E.A.; et al. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. Neuroimage 2016, 131, 113–125. [Google Scholar] [CrossRef]
- Fox, M.D.; Greicius, M. Clinical applications of resting state functional connectivity. Front. Syst. Neurosci. 2010, 4, 19. [Google Scholar] [CrossRef]
- Fair, D.A.; Cohen, A.L.; Power, J.D.; Dosenbach, N.U.; Church, J.A.; Miezin, F.M.; Schlaggar, B.L.; Petersen, S.E. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009, 5, e1000381. [Google Scholar] [CrossRef]
- Bruke, T.M.; Scheer, F.A.J.L.; Ronda, J.M.; Czeisler, C.A.; Wright, K.P. Sleep inertia, sleep homeostatic and circadian influences on higher-order cognitive functions. J. Sleep Res. 2015, 24, 364–371. [Google Scholar] [CrossRef]
- Anderson, J.A.E.; Campbell, K.L.; Amer, T.; Grady, C.L.; Hasher, L. Timing is everything: Age differences in the cognitive control network are modulated by time of day. Psychol. Aging 2014, 29, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Hodyl, N.A.; Schneider, L.; Vallence, A.M.; Clow, A.; Ridding, M.C.; Pitcher, J.B. The cortisol awakening response is associated with performance of a serial sequence reaction time task. Int. J. Psychophysiol. 2016, 100, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Abe, T.; Morishita, S.; Inagaki, Y.; Qin, W.; Hotta, K.; Tsubaki, A. Acute moderate-intensity exercise improves 24-h sleep deprivation-induced cognitive decline and cerebral oxygenation: A near-infrared spectroscopy study. Respir. Physiol. Neurobiol. 2019, 274, 103354. [Google Scholar] [CrossRef]
- Wilckens, K.A.; Erickson, K.I.; Wheeler, M.E. Physical Activity and Cognition: A Mediating Role of Efficient Sleep. Behav. Sleep Med. 2018, 16, 569–586. [Google Scholar] [CrossRef] [PubMed]
- Macrì, M.; Flores, N.V.G.; Stefanelli, R.; Pegreffi, F.; Festa, F. Interpreting the prevalence of musculoskeletal pain impacting Italian and Peruvian dentists likewise: A cross-sectional study. Front. Public Health 2023, 11, 1090683. [Google Scholar] [CrossRef] [PubMed]
- Macrì, M.; Murmura, G.; Scarano, A.; Festa, F. Prevalence of temporomandibular disorders and its association with malocclusion in children: A transversal study. Front. Public Health 2022, 10, 860833. [Google Scholar] [CrossRef] [PubMed]
| Study | Sample | Physical Activity | Methods | Main Findings |
|---|---|---|---|---|
| Coelho et al., 2014 [40] | 21 AD * patients (76.3 ± 6.2 yrs) 18 healthy controls (74.6 ± 4.7 yrs) | Treadmill | Treadmill grade, time to exhaustion, VO2, maximal lactate, Baecke Questionnaire | Increase in BDNF * plasma levels |
| Won et al., 2019 [41] | 26 healthy older adults (65.9 ± 7.2 yrs) | 30 min of cycling | fMRI * scan | Activation of semantic memory |
| Won et al., 2019 [42] | 32 healthy older adults (66.2 ± 7.3 yrs) | 30 min cycling | fMRI scan | Activation in the left inferior frontal gyrus and inferior parietal lobule |
| Voss et al., 2020 [43] | 34 healthy older adults (67.1 ± 4.3 yrs) | 20 min of light and moderate cycling | fMRI scan | Improvements in hippocampal–cortical connections and working memory |
| Suwabe et al., 2018 [44] | 36 healthy young adults (20.9 ± 1.8 yrs) | 10 min of light exercise | fMRI scan | Increase in connectivity between dentate gyrus and cortical regions |
| Li et al., 2014 [45] | 15 female students (19–22 yrs) | 20 min of moderate exercise | fMRI scan | Activation in the right middle prefrontal gyrus, right lingual gyrus, and left fusiform gyrus; deactivations in anterior cingulate cortex: left inferior frontal gyrus and right paracentral lobule |
| Marian Bosch et al., 2020 [46] | 15 healthy male young adults (23.7 ± 4.02 yrs) | 30 min of moderate exercise/15 min of vigorous exercise | fMRI scan | Significant improvements in motor sequence memory (high intensity)/improvements tending towards significance (moderate intensity) |
| Perini et al., 2016 [47] | 84 healthy male young adults: 44 (23.0 ± 2.2 yrs) in the orientation discrimination task group and 40 (22.9 ± 2.2 yrs) in the motor task group | 30 min of exercise | Physiological and behavioural tests | Gradual up-regulation of a functional network |
| Mehren et al., 2019 [48] | 20 ADHD * patients (29.9 ± 9.5 yrs) 20 healthy controls (29.0 ± 7.4 yrs) | 30 min of moderate cycling | fMRI scan | Improvements in reaction times and attention in ADHD patients; activation in frontal and sensorimotor regions in patients and controls |
| Mehren et al., 2019 [49] | 64 healthy young adults: 32 participants (29.3 ± 8.5 yrs) in the moderate-intensity group; 32 participants (28.6 ± 7.7 yrs) in the high-intensity group | Moderate- and high-intensity cycling | fMRI scan | Activation in the insula, superior frontal gyrus, precentral gyrus, and supplementary motor area in the moderate-intensity group |
| Schmitt et al., 2019 [50] | 21 male athletes (27.2 ± 4.2 yrs) | 30 min of low- and high-intensity exercise | fMRI scan | Reduced activation in posterior cingulate cortex/precuneus for low intensity; reduced activation in the caudate nucleus and ventral anterior putamen for high intensity |
| Li et al., 2019 [51] | 12 healthy high-fit and 12 healthy low-fit female students | 30 min of aerobic exercise | fMRI scan | Activation in the right cerebellum and subcortical regions |
| Choi et al., 2016 [52] | 37 ADHD children; 18 healthy controls (12.8 ± 0.79 yrs) | 13 min of aerobic stretching and moderate exercise | EEG * | Increased alpha activity; decreased theta activity |
| Chen et al., 2016 [53] | 9 healthy children (10 yrs) | 30 min of moderate cycling | fMRI scan | Activation in bilateral parietal cortex: left hippocampus and bilateral cerebellum |
| Chen et al., 2017 [54] | 9 healthy children (10 yrs) | 30 min of moderate cycling | fMRI scan | Connectivity between left cerebellum and right inferior frontal gyrus |
| Metcalfe et al., 2016 [55] | 30 BD * adolescents (16.8 ± 1.4 yrs); 20 healthy controls (16.1 ± 1.5 yrs) | 20 min of recumbent cycling | fMRI scan | Deactivation in the left inferior frontal gyrus, right frontal pole, temporal pole, hippocampus, and right amygdala |
| Study | Sample | Physical Activity | Methods | Main Findings |
|---|---|---|---|---|
| Engeroff et al., 2018 [73] | Healthy older adults (>65 yrs) | Moderate-to-vigorous exercise | MRS *-based marked | Increase in BDNF * and hippocampus volume |
| Wheeler et al., 2020 [74] | 67 healthy older adults (67 ± 7 yrs) | 6 days of moderate exercise | Cognitive testing | Increase in BDNF, working memory, and executive function |
| Gaitán et al., 2021 [75] | 23 late middle-aged adults (mean age 65 yrs) | 26 weeks of treadmill training | Cognitive function test and Enzyme-Linked Immunosorbent Assay (ELISA) | Increased plasma Cathepsin B; unchanged serum klotho |
| Neale et al., 2017 [76] | 95 healthy older adults (65–92 yrs) | Light exercise | EEG * | Association between neural signature and environment |
| Gogniat et al., 2022 [77] | 47 healthy older adults (>65 yrs) | 7 days of exercise | Neuropsychological tests and fMRI * scan | Increased DMN * and DAN * functional connectivity |
| Gogniat et al., 2022 [78] | 51 healthy older adults (>65 yrs) | Light exercise | Neuropsychological tests and fMRI scan | Relationship between low DMN/DAN anti-correlations levels and better executive function |
| Pieramico et al., 2012 [79] | 30 healthy older adults (60–75 yrs): 15 participants; 15 controls | 6 months of structured multimodal activities (cognitive, aerobic, and sensorial stimuli and fun recreational activities) | fMRI scan | Improvements in cognitive performance and reorganization of functional connectivity |
| Li et al., 2014 [80] | 34 healthy male older adults: 17 (68.6 ± 5.7 yrs) participants; 17 (71.7 ± 4.0) controls | 6 weeks of multimodal activities (Tai Chi and counselling group); lecture for the control group | Cognitive tests and fMRI scan | Improved functional connectivity between the medial prefrontal cortex and medial temporal lobe |
| Tao et al., 2016 [81] | 62 older adults: 21 in the Tai Chin Chaun group; 16 in the Baduanjin group; 25 in the control group | 12 weeks of Tai Chin Chuan or Baduanjin exercise | Memory function measurement and fMRI scan | Increased memory quotient; improved functional connectivity between the hippocampus and medial prefrontal cortex |
| Dorsman et al., 2020 [82] | 212 older adults (73.3 ± 6.2 yrs) | 7 days of exercise | fMRI scan | Greater frontal-subcortical and within-subcortical network synchrony |
| Ji et al., 2017 [83] | 24 older adults: 12 (67.0 ± 6.40 yrs) participants; 12 (73.0 ± 8.0 yrs) controls | 6 weeks of exercise | Cognitive tests, and MRI * and fMRI scans | Improved memory and executive function; increased posterior cingulate volume; higher connectivity between the striatum and cingulate, temporal, parietal, and occipital regions |
| Voss et al., 2010 [84] | 65 older adults: 30 (67.3 ± 5.8 yrs) participants; 35 (65.4 ± 5.2 yrs) controls | 6 and 12 months of moderate aerobic exercise | Cognitive tests and fMRI scan | Improved executive function; increased functional connectivity within DMN and FEN * |
| Flodin et al., 2017 [85] | 47 older adults: 22 (68.4 ± 2.6 yrs) participants; 25 (69.16 ± 3.0 yrs) controls | 6 months of aerobic exercise | fMRI scan | Decreased connectivity between left hippocampus and contralateral precentral gyrus; Better connectivity between right mid-temporal areas and frontal and parietal region |
| Voss et al., 2019 [86] | 189 healthy older adults (65.4 ± 4.4 yrs) | 6 months of aerobic exercise (dance and walk) | fMRI scan | Increased salience network connectivity via nutritional supplementation |
| Kimura et al., 2013 [87] | 72 healthy older adults (70.3 ± 4.0) | 3 months of short and long brisk walking | fMRI scan | Activation in left prefrontal and parietal regions and in dorsolateral prefrontal cortex |
| Bugg et al., 2011 [88] | 52 healthy older adults (69.0 ± 6.7 yrs) | exercise (running or walking) practiced over the last 10 yrs | MRI scan | Larger frontal volume and medial temporal lobule |
| Papenberg et al., 2016 [89] | 414 healthy older adults | exercise practiced in the last 12 months | MRI scan | Correlation between physical inactivity and small grey matter volume |
| Well et al., 2013 [90] | 14 MCI patients: 9 participants; 5 controls | 8 weeks of mindfulness | fMRI scan | Increased connectivity between posterior cingulate cortex, bilateral medial prefrontal cortex, and left hippocampus |
| Eyre et al., 2016 [91] | 25 MCI * patients: 14 in the yoga group; 11 in the memory enhancement training group | 12 weeks of yoga | fMRI scan | Greater connectivity between the DMN and medial frontal cortex, pregenual anterior cingulate cortex, right middle frontal cortex, posterior cingulate cortex, and left lateral occipital cortex |
| Tao et al., 2019 [92] | 47 MCI patients: 20 (66.17 ± 4.17 yrs) in the Baduanjin group; 17 (64.32 ± 2.60 yrs) in the brisk walking group; 20 (65.97 ± 5.66 yrs) in the control group | 24 weeks of exercise (Baduanjin and brisk walking) | Montreal cognitive test, and MRI and fMRI scans | Improved cognitive function; greater hippocampus grey matter volume; higher connectivity between the hippocampus and right angular gyrus |
| Suo et al., 2016 [93] | 100 MCI older adults (70.1 ± 6.7 yrs) | 6 months of resistance training | Neuropsychological tests, and MRI and fMRI scans | Better global cognition; greater cortical thickness in the posterior cingulate; improved connectivity between the hippocampus and superior frontal cortex |
| Hsu et al., 2017 [94] | 21 SIVCI * older adults | 6 months of aerobic exercise | fMRI scan | FPN * linked to better mobility performance |
| Veldsman et al., 2017 [95] | 62 stroke patients (67 ± 12.6 yrs) 27 healthy controls (68.0 ± 5.94 yrs) | 3 months of exercise | fMRI scan | Increased connectivity of superior parietal lobule in DAN |
| Study | Sample | Physical Activity | Methods | Main Findings |
|---|---|---|---|---|
| Goldin et al., 2012 [105] | 42 adults with SAD * (32.88 ± 7.97 yrs): 24 in the MBSR group; 18 in the aerobic group | 8 sessions of weekly MBSR; 8 weeks of aerobic exercise | fMRI * scan | Greater brain responses in the posterior cingulate cortex in the MBSR group |
| Goldin et al., 2013 [106] | 42 adults with SAD (32.88 ± 7.97 yrs): 24 in the MBSR * group; 18 in the aerobic group | 8 sessions of weekly MBSR; 8 weeks of aerobic exercise | fMRI scan | Reduced negative emotions; increase in attention-related parietal cortical regions in the MBSR group |
| Gourgouvelis et al., 2017 [107] | 16 adults: 8 patients with depression and anxiety (37.25 ± 8.0 yrs); 8 healthy controls (20.63 ± 1.19 yrs) | 8 weeks of moderate intervention: resistance training, and mild to vigorous aerobic session | fMRI scan | Reduced hippocampal activity in patients |
| Huang et al., 2021 [108] | 70 adults (18–50 yrs): 38 StD patients; 32 healthy controls | 8 weeks of moderate aerobic exercise | fMRI scan | Reduced right inferior parietal lobule activity in StD * patients |
| Stern et al., 2019 [109] | 132 healthy adults (20–67 yrs) | 6 months of aerobic exercise/stretching and toning | MRI scan | Increased cortical thickness in the left caudal middle frontal cortex Brodmann area in the aerobic group |
| Bashir et., 2021 [110] | 45 healthy adults (19–27 yrs): 25 in the exercise group; 20 in the control group | 6 months of aerobic and anaerobic exercise | MRI scan | Increased cortical thickness in left peri calcarine area, left superior parietal area, right rostral middle frontal, and right lateral occipital gyrus |
| Kaiser et al., 2022 [111] | 45 healthy adults (18–30 yrs) | 12 weeks of high- vs. low-intensity exercise | MRI scan | Increased left hippocampal and decreased right hippocampal volume after vigorous exercise |
| Fontes et al., 2013 [112] | 7 healthy male adults (26.6 ± 4.0 yrs) | 6 sessions of cycling | fMRI scan | Relation between posterior cingulate cortex and precuneus and higher levels of perceived exertion |
| Ishihara et al., 2020 [113] | 1033 healthy adults (22–37 yrs) | Not specified | fMRI scan | Increased functional connectivity within DMN and FPN * |
| Nakagawa et al., 2020 [114] | 58 healthy adults (22.4 ± 2.4) | Moderate-to-vigorous exercise vs. low-to-moderate exercise | Cognitive tests | Better cognitive performance in moderate-to-vigorous group |
| Bezzola et al., 2012 [115] | 32 healthy middle-aged adults (51.2 ± 7.2 yrs): 11 in the golf group; 11 in the control group | 40 h of golf training | fMRI scan | Reduction in neuronal recruitment in the right and left dorsal premotor cortex |
| Wadden et al., 2013 [116] | 10 healthy middle-aged adults (64.7 ± 8.5 yrs) | 7 days of exercise | fMRI scan | Bilateral cerebellar activation |
| Pensel et al., 2018 [117] | 25 healthy middle-aged adults (52.21 ± 6.39 yrs) | 6 months of aerobic exercise | fMRI scan | Bilateral frontal activation |
| Study | Sample | Physical Activity | Methods | Main Findings |
|---|---|---|---|---|
| Kjellenberg et al., 2022 [123] | 1139 adolescent (13.4 ± 0.3 yrs) | 7 days of moderate-to-vigorous exercise | Cognitive tests | Better cognitive function |
| Kamijo et al., 2011 [124] | 43 children (7–9 yrs): 22 in the exercise group; 21 in the control group | 9 months of aerobic exercise | Cognitive tests | Improved working memory |
| Quinzi et al., 2022 [125] | 64 children | Racket sport, martial arts, and indoor climbing | Cognitive tests | Improved specific domains related to exercise type |
| Hillmann et al., 2014 [126] | 221 children (7–9 yrs): 109 in the exercise group; 112 in the control group | 9 months of aerobic exercise | fMRI * scan | Improved executive control and brain activity |
| Chaddock-Heyman et al., 2013 [127] | 23 children (8.9 ± 5.8) | 9 months of moderate-to-vigorous aerobic exercise | fMRI scan | Reduced activation in the right anterior prefrontal cortex |
| Chaddock-Heyman et al., 2013 [128] | 143 children (8.7 ± 0.55) | 9 months of aerobic exercise | MRI * scan | Improved white matter microstructure in the genu of the anterior corpus callosum |
| Logan et al., 2021 [129] | 206 children (8–10 yrs): 103 normal weight; 103 obese | 9 months of aerobic exercise | EEG * | Reduced neuroleptic indices in obese |
| Ortega et al., 2022 [130] | 90 overweight children (8–10 yrs) | 20 weeks of high-intensity aerobic exercise | Standardised tests and MRI scan | Improved intelligence and cognitive flexibility; unidentified structural changes |
| Davis et al., 2011 [121] | 19 overweight children (9.8 ± 1.0 yrs) | 3 months of regular aerobic exercise | fMRI scan | Increased bilateral prefrontal cortex activity. Reduced bilateral posterior parietal cortex activity |
| Kraff et al., 2014 [131] | 43 overweight children (9.8 ± 0.8 yrs) | 8 months of aerobic exercise | fMRI scan | Reduced activation in prefrontal and parietal areas; increased activation in the frontal gyrus and anterior cingulate |
| Kraff et al., 2014 [132] | 22 overweight children (9.5 ± 0.7 yrs): 13 in the exercise group; 9 in the control group | 8 months of aerobic exercise | fMRI scan | Reduced synchrony in motor, default mode, and cognitive control networks; increased synchrony only between the motor network and frontal regions |
| Kraff et al., 2014 [133] | 18 overweight children (9.7 ± 0.7 yrs): 10 in the exercise group; 8 in the control group | 8 months of aerobic exercise | DTI * | Increased white matter integrity in the bilateral superior longitudinal fasciculus |
| Schaeffer et al., 2014 [134] | 18 overweight children (9.7 ± 0.7 yrs): 10 in the exercise group; 8 in the control group | 8 months of aerobic exercise | DTI | Positive change in bilateral uncinate fasciculus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Festa, F.; Medori, S.; Macrì, M. Move Your Body, Boost Your Brain: The Positive Impact of Physical Activity on Cognition across All Age Groups. Biomedicines 2023, 11, 1765. https://doi.org/10.3390/biomedicines11061765
Festa F, Medori S, Macrì M. Move Your Body, Boost Your Brain: The Positive Impact of Physical Activity on Cognition across All Age Groups. Biomedicines. 2023; 11(6):1765. https://doi.org/10.3390/biomedicines11061765
Chicago/Turabian StyleFesta, Felice, Silvia Medori, and Monica Macrì. 2023. "Move Your Body, Boost Your Brain: The Positive Impact of Physical Activity on Cognition across All Age Groups" Biomedicines 11, no. 6: 1765. https://doi.org/10.3390/biomedicines11061765
APA StyleFesta, F., Medori, S., & Macrì, M. (2023). Move Your Body, Boost Your Brain: The Positive Impact of Physical Activity on Cognition across All Age Groups. Biomedicines, 11(6), 1765. https://doi.org/10.3390/biomedicines11061765







