TRPM4 Blocking Antibody Protects Cerebral Vasculature in Delayed Stroke Reperfusion
Abstract
1. Introduction
2. Materials and Methods
2.1. Rat Middle Cerebral Artery Occlusion (MCAO) Model and Experimental Protocol
2.2. Cerebral Blood Flow Measurement
2.3. 2,3,5-Triphenyltetrazolium Chloride (TTC) Staining and Evans Blue Extravasation
2.4. Immunofluorescent Staining
2.5. Rotarod Test
2.6. Electrophysiology
2.7. Statistical Analysis
3. Results
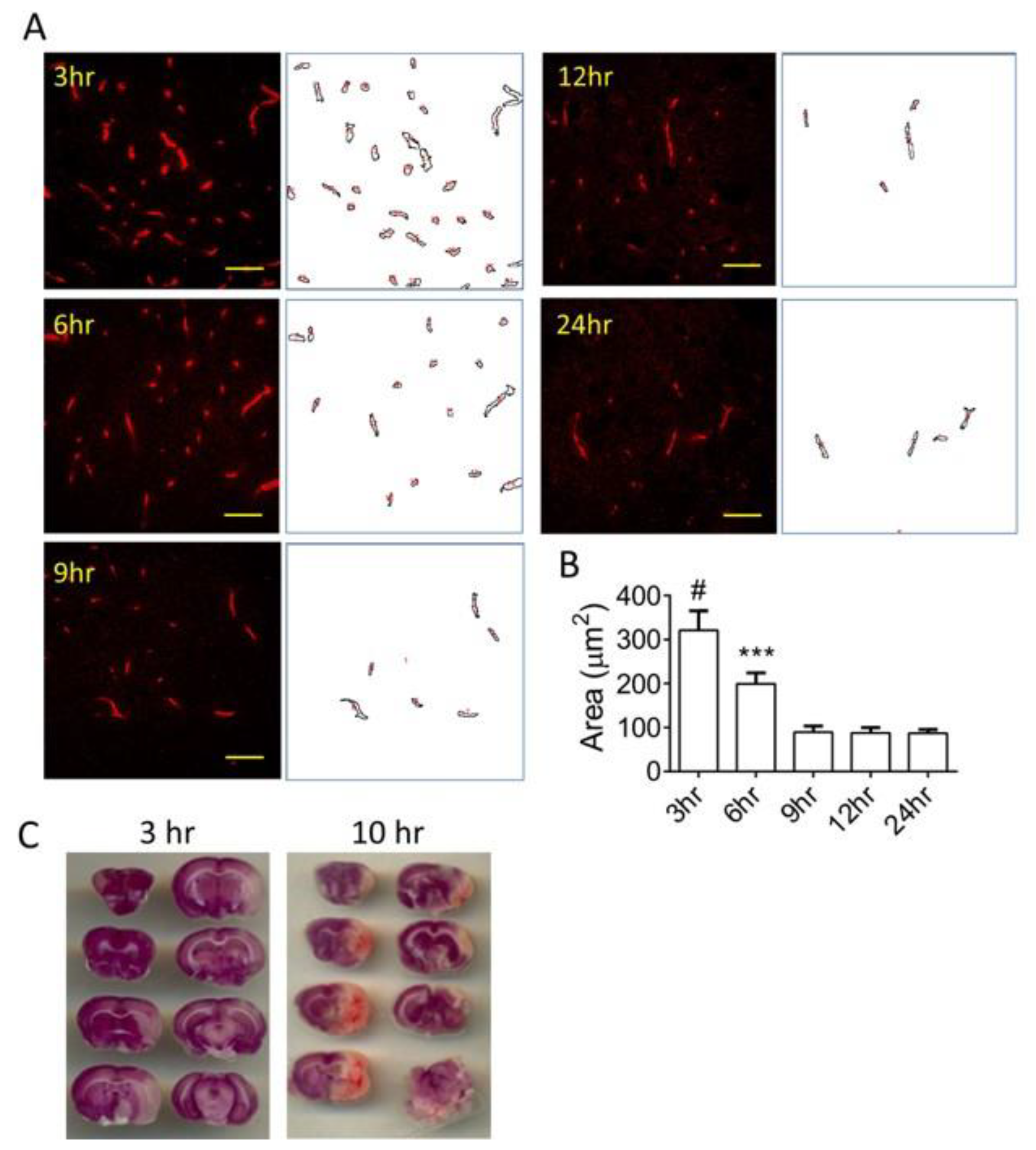
3.1. Time-Dependent Vascular Injury Post MCAO
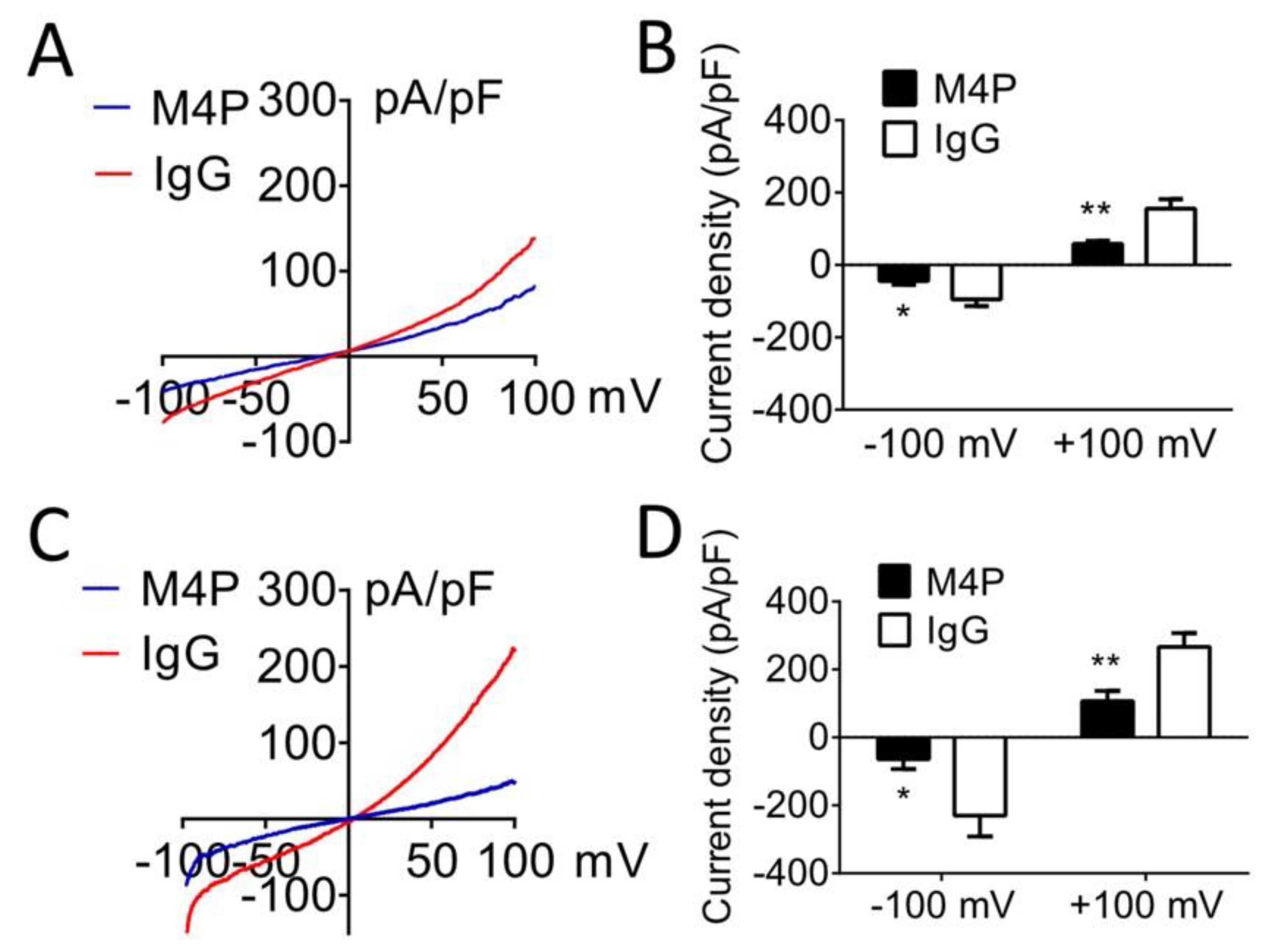
3.2. M4P Inhibits TRPM4 Current
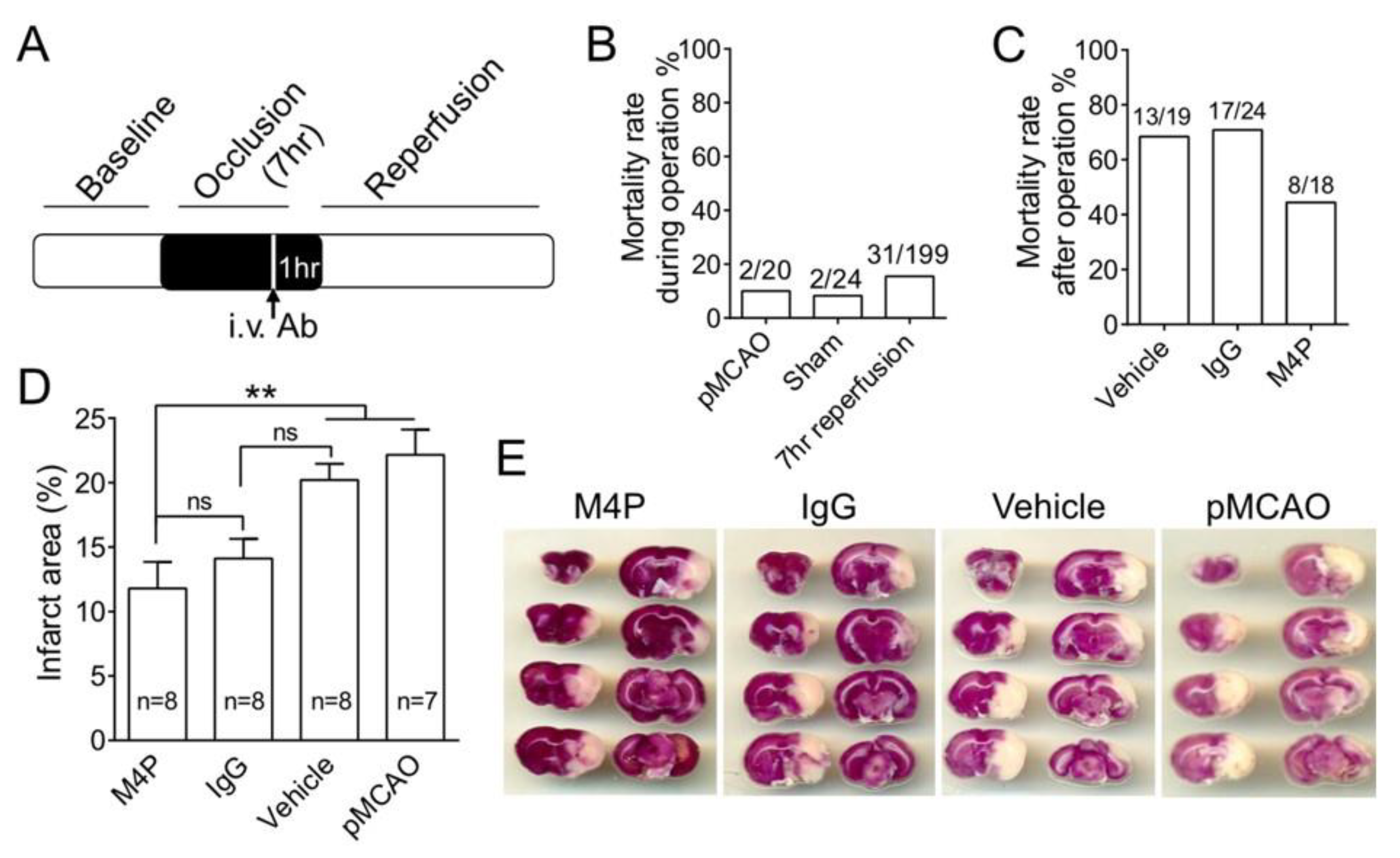
3.3. M4P Reduces Mortality Rate and Infarct Volume in 7-h Stroke Reperfusion
3.4. M4P Improves Vascular Integrity after 7-h Stroke Reperfusion
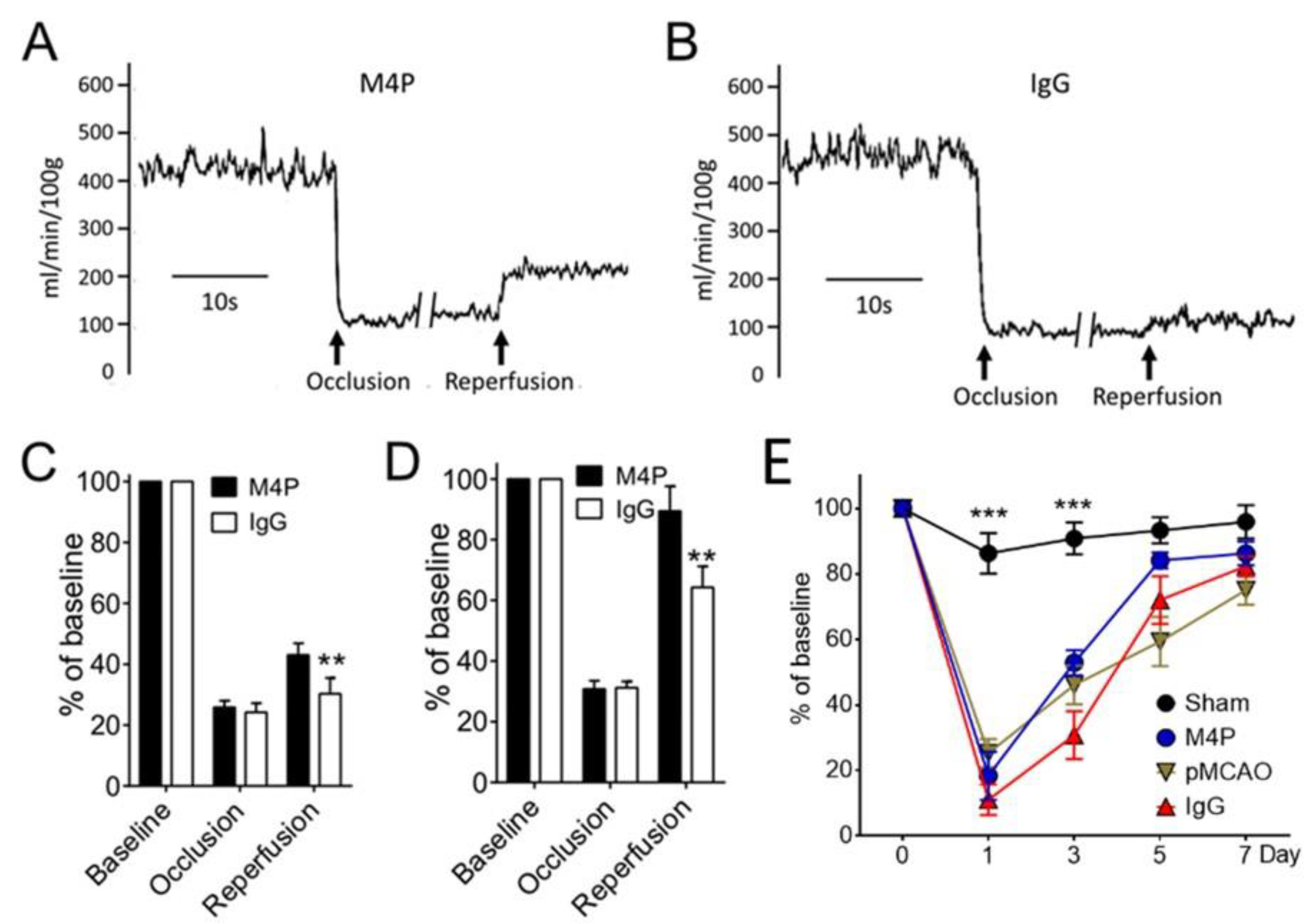
3.5. M4P on Cerebral Blood Flow and Functional Recovery
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahad, M.A.; Kumaran, K.R.; Ning, T.; Mansor, N.I.; Effendy, M.A.; Damodaran, T.; Lingam, K.; Wahab, H.A.; Nordin, N.; Liao, P.; et al. Insights into the neuropathology of cerebral ischemia and its mechanisms. Rev. Neurosci. 2020, 31, 521–538. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2018, 49, e46–e110. [Google Scholar] [CrossRef] [PubMed]
- Nour, M.; Scalzo, F.; Liebeskind, D.S. Ischemia-reperfusion injury in stroke. Interv. Neurol. 2013, 1, 185–199. [Google Scholar] [CrossRef]
- Khatri, R.; McKinney, A.M.; Swenson, B.; Janardhan, V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 2012, 79, S52–S57. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke 1997, 28, 2109–2118. [Google Scholar] [CrossRef]
- Henninger, N.; Fisher, M. Extending the Time Window for Endovascular and Pharmacological Reperfusion. Transl. Stroke Res. 2016, 7, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Liao, P. Brain transient receptor potential channels and stroke. J. Neurosci. Res. 2015, 93, 1165–1183. [Google Scholar] [CrossRef] [PubMed]
- Himmel, N.J.; Cox, D.N. Transient receptor potential channels: Current perspectives on evolution, structure, function and nomenclature. Proc. Biol. Sci. 2020, 287, 20201309. [Google Scholar] [CrossRef]
- Chen, B.; Gao, Y.; Wei, S.; Low, S.W.; Ng, G.; Yu, D.; Tu, T.M.; Soong, T.W.; Nilius, B.; Liao, P. TRPM4-specific blocking antibody attenuates reperfusion injury in a rat model of stroke. Pflugers Arch. 2019, 471, 1455–1466. [Google Scholar] [CrossRef]
- Chen, B.; Ng, G.; Gao, Y.; Low, S.W.; Sandanaraj, E.; Ramasamy, B.; Sekar, S.; Bhakoo, K.; Soong, T.W.; Nilius, B.; et al. Non-Invasive Multimodality Imaging Directly Shows TRPM4 Inhibition Ameliorates Stroke Reperfusion Injury. Transl. Stroke Res. 2019, 10, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Loh, K.Y.; Wang, Z.; Liao, P. Oncotic Cell Death in Stroke. Rev. Physiol. Biochem. Pharmacol. 2019, 176, 37–64. [Google Scholar] [CrossRef]
- Simard, J.M.; Kahle, K.T.; Gerzanich, V. Molecular mechanisms of microvascular failure in central nervous system injury-synergistic roles of NKCC1 and SUR1/TRPM4. J. Neurosurg. 2010, 113, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.M.; Tarasov, K.V.; Gerzanich, V. Non-selective cation channels, transient receptor potential channels and ischemic stroke. Biochim. Biophys. Acta 2007, 1772, 947–957. [Google Scholar] [CrossRef]
- Wei, S.; Low, S.W.; Poore, C.P.; Chen, B.; Gao, Y.; Nilius, B.; Liao, P. Comparison of Anti-oncotic Effect of TRPM4 Blocking Antibody in Neuron, Astrocyte and Vascular Endothelial Cell Under Hypoxia. Front. Cell Dev. Biol. 2020, 8, 562584. [Google Scholar] [CrossRef]
- Low, S.W.; Gao, Y.; Wei, S.; Chen, B.; Nilius, B.; Liao, P. Development and characterization of a monoclonal antibody blocking human TRPM4 channel. Sci. Rep. 2021, 11, 10411. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.M.; Chen, M.; Tarasov, K.V.; Bhatta, S.; Ivanova, S.; Melnitchenko, L.; Tsymbalyuk, N.; West, G.A.; Gerzanich, V. Newly expressed SUR1-regulated NC(Ca-ATP) channel mediates cerebral edema after ischemic stroke. Nat. Med. 2006, 12, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liao, P. TRPM4 channel and cancer. Cancer Lett. 2019, 454, 66–69. [Google Scholar] [CrossRef]
- Gerzanich, V.; Woo, S.K.; Vennekens, R.; Tsymbalyuk, O.; Ivanova, S.; Ivanov, A.; Geng, Z.; Chen, Z.; Nilius, B.; Flockerzi, V.; et al. De novo expression of Trpm4 initiates secondary hemorrhage in spinal cord injury. Nat. Med. 2009, 15, 185–191. [Google Scholar] [CrossRef]
- Schattling, B.; Steinbach, K.; Thies, E.; Kruse, M.; Menigoz, A.; Ufer, F.; Flockerzi, V.; Bruck, W.; Pongs, O.; Vennekens, R.; et al. TRPM4 cation channel mediates axonal and neuronal degeneration in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat. Med. 2012, 18, 1805–1811. [Google Scholar] [CrossRef]
- Vennekens, R.; Nilius, B. Insights into TRPM4 function, regulation and physiological role. In Transient Receptor Potential (TRP) Channels; Flockerzi, V., Nilius, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 269–285. [Google Scholar] [CrossRef]
- Loh, K.P.; Ng, G.; Yu, C.Y.; Fhu, C.K.; Yu, D.; Vennekens, R.; Nilius, B.; Soong, T.W.; Liao, P. TRPM4 inhibition promotes angiogenesis after ischemic stroke. Pflugers Arch. 2014, 466, 563–576. [Google Scholar] [CrossRef]
- Hazalin, N.; Liao, P.; Hassan, Z. TRPM4 inhibition improves spatial memory impairment and hippocampal long-term potentiation deficit in chronic cerebral hypoperfused rats. Behav. Brain Res. 2020, 393, 112781. [Google Scholar] [CrossRef]
- Woo, S.K.; Kwon, M.S.; Ivanov, A.; Gerzanich, V.; Simard, J.M. The sulfonylurea receptor 1 (Sur1)-transient receptor potential melastatin 4 (Trpm4) channel. J. Biol. Chem. 2013, 288, 3655–3667. [Google Scholar] [CrossRef] [PubMed]
- Nichols, C.G. KATP channels as molecular sensors of cellular metabolism. Nature 2006, 440, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Kunte, H.; Busch, M.A.; Trostdorf, K.; Vollnberg, B.; Harms, L.; Mehta, R.I.; Castellani, R.J.; Mandava, P.; Kent, T.A.; Simard, J.M. Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann. Neurol. 2012, 72, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Kimberly, W.T.; Battey, T.W.; Pham, L.; Wu, O.; Yoo, A.J.; Furie, K.L.; Singhal, A.B.; Elm, J.J.; Stern, B.J.; Sheth, K.N. Glyburide is associated with attenuated vasogenic edema in stroke patients. Neurocrit. Care 2014, 20, 193–201. [Google Scholar] [CrossRef]
- Sheth, K.N.; Simard, J.M.; Elm, J.; Kronenberg, G.; Kunte, H.; Kimberly, W.T. Human Data Supporting Glyburide in Ischemic Stroke. Acta Neurochir. Suppl. 2016, 121, 13–18. [Google Scholar] [CrossRef]
- Sheth, K.N.; Kimberly, W.T.; Elm, J.J.; Kent, T.A.; Yoo, A.J.; Thomalla, G.; Campbell, B.; Donnan, G.A.; Davis, S.M.; Albers, G.W.; et al. Exploratory analysis of glyburide as a novel therapy for preventing brain swelling. Neurocrit. Care 2014, 21, 43–51. [Google Scholar] [CrossRef]
- Sheth, K.N.; Kimberly, W.T.; Elm, J.J.; Kent, T.A.; Mandava, P.; Yoo, A.J.; Thomalla, G.; Campbell, B.; Donnan, G.A.; Davis, S.M.; et al. Pilot study of intravenous glyburide in patients with a large ischemic stroke. Stroke 2014, 45, 281–283. [Google Scholar] [CrossRef]
- Horsdal, H.T.; Mehnert, F.; Rungby, J.; Johnsen, S.P. Type of preadmission antidiabetic treatment and outcome among patients with ischemic stroke: A nationwide follow-up study. J. Stroke Cerebrovasc. Dis. 2012, 21, 717–725. [Google Scholar] [CrossRef]
- Weih, M.; Amberger, N.; Wegener, S.; Dirnagl, U.; Reuter, T.; Einhaupl, K. Sulfonylurea drugs do not influence initial stroke severity and in-hospital outcome in stroke patients with diabetes. Stroke 2001, 32, 2029–2032. [Google Scholar] [CrossRef] [PubMed]
- Tziomalos, K.; Bouziana, S.D.; Spanou, M.; Kostaki, S.; Papadopoulou, M.; Giampatzis, V.; Dourliou, V.; Kostourou, D.T.; Savopoulos, C.; Hatzitolios, A.I. Prior treatment with dipeptidyl peptidase 4 inhibitors is associated with better functional outcome and lower in-hospital mortality in patients with type 2 diabetes mellitus admitted with acute ischaemic stroke. Diab. Vasc. Dis. Res. 2015, 12, 463–466. [Google Scholar] [CrossRef]
- Favilla, C.G.; Mullen, M.T.; Ali, M.; Higgins, P.; Kasner, S.E. Sulfonylurea use before stroke does not influence outcome. Stroke 2011, 42, 710–715. [Google Scholar] [CrossRef]
- Liu, R.; Wang, H.; Xu, B.; Chen, W.; Turlova, E.; Dong, N.; Sun, C.L.; Lu, Y.; Fu, H.; Shi, R.; et al. Cerebrovascular safety of sulfonylureas: The role of KATP channels in neuroprotection and the risk of stroke in patients with type 2 diabetes. Diabetes 2016, 65, 2795–2809. [Google Scholar] [CrossRef]
- King, Z.A.; Sheth, K.N.; Kimberly, W.T.; Simard, J.M. Profile of intravenous glyburide for the prevention of cerebral edema following large hemispheric infarction: Evidence to date. Drug Des. Dev. Ther. 2018, 12, 2539–2552. [Google Scholar] [CrossRef]
- Darsalia, V.; Ortsater, H.; Olverling, A.; Darlof, E.; Wolbert, P.; Nystrom, T.; Klein, T.; Sjoholm, A.; Patrone, C. The DPP-4 inhibitor linagliptin counteracts stroke in the normal and diabetic mouse brain: A comparison with glimepiride. Diabetes 2013, 62, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Behn, J.; Poore, C.P.; Low, S.W.; Nilius, B.; Fan, H.; Liao, P. Binding epitope for recognition of human TRPM4 channel by monoclonal antibody M4M. Sci. Rep. 2022, 12, 19562. [Google Scholar] [CrossRef]
- Liao, P.; Yu, D.; Hu, Z.; Liang, M.C.; Wang, J.J.; Yu, C.Y.; Ng, G.; Yong, T.F.; Soon, J.L.; Chua, Y.L.; et al. Alternative splicing generates a novel truncated Cav1.2 channel in neonatal rat heart. J. Biol. Chem. 2015, 290, 9262–9272. [Google Scholar] [CrossRef]
- Yeo, C.; Kim, H.; Song, C. Cerebral Blood Flow Monitoring by Diffuse Speckle Contrast Analysis during MCAO Surgery in the Rat. Curr. Opt. Photonics 2017, 1, 433–439. [Google Scholar] [CrossRef]
- Walberer, M.; Stolz, E.; Muller, C.; Friedrich, C.; Rottger, C.; Blaes, F.; Kaps, M.; Fisher, M.; Bachmann, G.; Gerriets, T. Experimental stroke: Ischaemic lesion volume and oedema formation differ among rat strains (a comparison between Wistar and Sprague-Dawley rats using MRI). Lab Anim. 2006, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Adapala, R.K.; Thoppil, R.J.; Ghosh, K.; Cappelli, H.C.; Dudley, A.C.; Paruchuri, S.; Keshamouni, V.; Klagsbrun, M.; Meszaros, J.G.; Chilian, W.M.; et al. Activation of mechanosensitive ion channel TRPV4 normalizes tumor vasculature and improves cancer therapy. Oncogene 2016, 35, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Mathar, I.; Jacobs, G.; Kecskes, M.; Menigoz, A.; Philippaert, K.; Vennekens, R. Trpm4. Handb. Exp. Pharmacol. 2014, 222, 461–487. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Ng, G.; Liao, P. Therapeutic Antibodies in Stroke. Transl. Stroke Res. 2013, 4, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Christou, I.; Alexandrov, A.V.; Burgin, W.S.; Wojner, A.W.; Felberg, R.A.; Malkoff, M.; Grotta, J.C. Timing of recanalization after tissue plasminogen activator therapy determined by transcranial doppler correlates with clinical recovery from ischemic stroke. Stroke 2000, 31, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; McCullough, L.D. Middle cerebral artery occlusion model in rodents: Methods and potential pitfalls. J. Biomed. Biotechnol. 2011, 2011, 464701. [Google Scholar] [CrossRef]
- Zhou, Y.; He, Y.; Yan, S.; Chen, L.; Zhang, R.; Xu, J.; Hu, H.; Liebeskind, D.S.; Lou, M. Reperfusion Injury Is Associated with Poor Outcome in Patients With Recanalization After Thrombectomy. Stroke 2023, 54, 96–104. [Google Scholar] [CrossRef]
- Hossmann, K.A. Pathophysiological basis of translational stroke research. Folia Neuropathol. 2009, 47, 213–227. [Google Scholar]
- El Amki, M.; Gluck, C.; Binder, N.; Middleham, W.; Wyss, M.T.; Weiss, T.; Meister, H.; Luft, A.; Weller, M.; Weber, B.; et al. Neutrophils Obstructing Brain Capillaries Are a Major Cause of No-Reflow in Ischemic Stroke. Cell Rep. 2020, 33, 108260. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Karimi-Haghighi, S.; Chavoshinezhad, S.; Pandamooz, S.; Belem-Filho, I.J.A.; Keshavarz, S.; Bayat, M.; Hooshmandi, E.; Rahimi Jaberi, A.; Salehi, M.S.; et al. The impacts of anesthetic regimens on the middle cerebral artery occlusion outcomes in male rats. Neuroreport 2022, 33, 561–568. [Google Scholar] [CrossRef]
- Hoffmann, U.; Sheng, H.; Ayata, C.; Warner, D.S. Anesthesia in Experimental Stroke Research. Transl. Stroke Res. 2016, 7, 358–367. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J. Animal models of stroke. Anim. Model. Exp. Med. 2021, 4, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Tornyos, D.; Komocsi, A.; Balint, A.; Kupo, P.; El Abdallaoui, O.E.A.; Szapary, L.; Szapary, L.B. Antithrombotic therapy for secondary prevention in patients with stroke or transient ischemic attack: A multiple treatment network meta-analysis of randomized controlled trials. PLoS ONE 2022, 17, e0273103. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Zhong, Y.B.; Zhao, J.J.; Ren, M.; Zhang, S.C.; Xu, M.S.; Xu, S.T.; Zhang, Y.J.; Shan, C.L. Transcranial pulse current stimulation improves the locomotor function in a rat model of stroke. Neural Regen. Res. 2021, 16, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Sun, F.; Li, Z.; Liu, M.; Tian, X.; Guo, D.; Wei, P.; Shan, Y.; Liu, T.; Guo, M.; et al. Electrical stimulation of the lateral cerebellar nucleus promotes neurogenesis in rats after motor cortical ischemia. Sci. Rep. 2020, 10, 16563. [Google Scholar] [CrossRef]
- Battaglia, S.; Di Fazio, C.; Vicario, C.M.; Avenanti, A. Neuropharmacological Modulation of N-methyl-D-aspartate, Noradrenaline and Endocannabinoid Receptors in Fear Extinction Learning: Synaptic Transmission and Plasticity. Int. J. Mol. Sci. 2023, 24, 5926. [Google Scholar] [CrossRef]
- Damodaran, T.; Tan, B.W.L.; Liao, P.; Ramanathan, S.; Lim, G.K.; Hassan, Z. Clitoria ternatea L. root extract ameliorated the cognitive and hippocampal long-term potentiation deficits induced by chronic cerebral hypoperfusion in the rat. J. Ethnopharmacol. 2018, 224, 381–390. [Google Scholar] [CrossRef]
- Engelhorn, T.; von Kummer, R.; Reith, W.; Forsting, M.; Doerfler, A. What is effective in malignant middle cerebral artery infarction: Reperfusion, craniectomy, or both? An experimental study in rats. Stroke 2002, 33, 617–622. [Google Scholar] [CrossRef]
- Ruan, J.; Yao, Y. Behavioral tests in rodent models of stroke. Brain Hemorrhages 2020, 1, 171–184. [Google Scholar] [CrossRef]
- Rajendram, P.; Ikram, A.; Fisher, M. Combined Therapeutics: Future Opportunities for Co-therapy with Thrombectomy. Neurotherapeutics 2023. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Wei, S.; Low, S.W.; Poore, C.P.; Lee, A.T.-H.; Nilius, B.; Liao, P. TRPM4 Blocking Antibody Protects Cerebral Vasculature in Delayed Stroke Reperfusion. Biomedicines 2023, 11, 1480. https://doi.org/10.3390/biomedicines11051480
Chen B, Wei S, Low SW, Poore CP, Lee AT-H, Nilius B, Liao P. TRPM4 Blocking Antibody Protects Cerebral Vasculature in Delayed Stroke Reperfusion. Biomedicines. 2023; 11(5):1480. https://doi.org/10.3390/biomedicines11051480
Chicago/Turabian StyleChen, Bo, Shunhui Wei, See Wee Low, Charlene Priscilla Poore, Andy Thiam-Huat Lee, Bernd Nilius, and Ping Liao. 2023. "TRPM4 Blocking Antibody Protects Cerebral Vasculature in Delayed Stroke Reperfusion" Biomedicines 11, no. 5: 1480. https://doi.org/10.3390/biomedicines11051480
APA StyleChen, B., Wei, S., Low, S. W., Poore, C. P., Lee, A. T.-H., Nilius, B., & Liao, P. (2023). TRPM4 Blocking Antibody Protects Cerebral Vasculature in Delayed Stroke Reperfusion. Biomedicines, 11(5), 1480. https://doi.org/10.3390/biomedicines11051480






