Cardiovascular Remodeling Post-Ischemia: Herbs, Diet, and Drug Interventions
Abstract
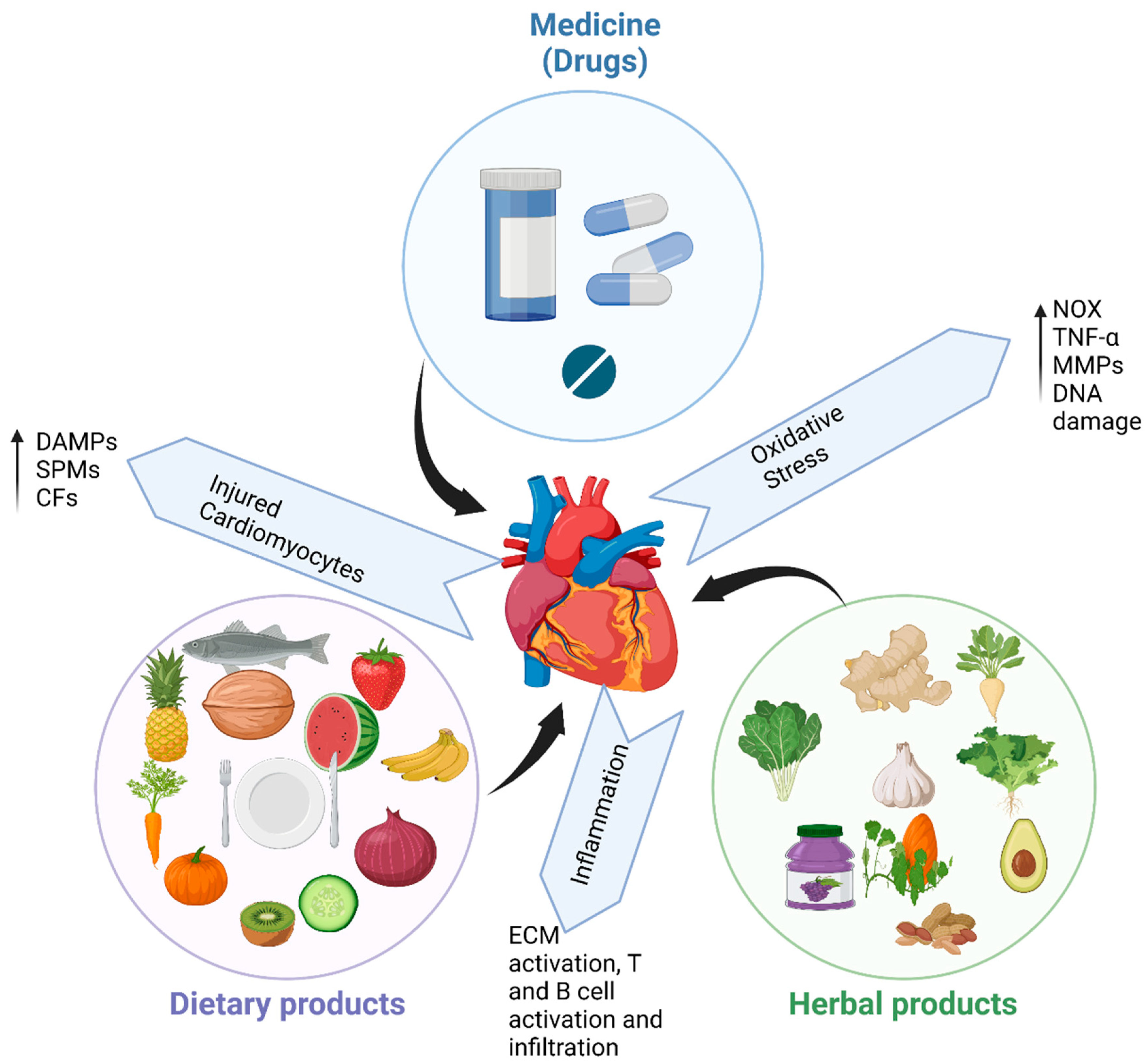
1. Introduction
2. Cardiovascular Health and Drugs Treatment
3. Cardiovascular Health and Diet
3.1. Mediterranean Diet
3.2. Paleo Diet
3.3. Keto Diet
3.4. DASH
4. Cardiovascular Health and Herbal Medicine
| Herbs | Forms of CVDs | Reference |
|---|---|---|
| Ginseng | Oxidative stress, hypertension, cardiac disease, hyperlipidemia and ion regulation | [93,106] |
| Ginkgo biloba | Cardiac activity, vasorelaxant and vasoconstriction activity, hypertension | [93] |
| Ganoderma lucidum | Atherosclerosis, hyperlipidemia | [107] |
| Gnostemma pentaphyllum | Hyperlipidemia | [92] |
| Rosemary oficinalis L. | Cardiac dysfunction and fibrosis | [95] |
| Cocus sativus L. | Systolic hypertension, oxidative stress, inflammation | [86,92] |
| Citrus medica L. | Ischemia heart disease | [100] |
| Crataegus monogyna | Congestive heart failure | [101] |
| Elettaria cardamom | Hypercholesterolemia | [104] |
| Terminalia arjuna | Cardiotoxicity | [105] |
| Punica granatum L. | Blood pressure, inflammation | [108,109] |
| Apple (Malus pumila) | Blood lipid levels | [110,111] |
| Watermelon (Citrullus lanatus) | Heart attacks, ischemic strokes, atherosclerosis | [112,113,114] |
| Berries | Myocardial infarction, oxidative stress, inflammation, platelet aggregation | [115,116,117] |
| Grapes (Vitis vinifera L.) | Cardiac fibrosis, hyperlipidemia | [118,119] |
| Garlic (Allium satinum L.) | Hypertension, hypercholesterolemia | [120] |
| Cinnamon (Cinnamomum verum) | Oxidative stress, inflammation, artherosclerosis | [121] |
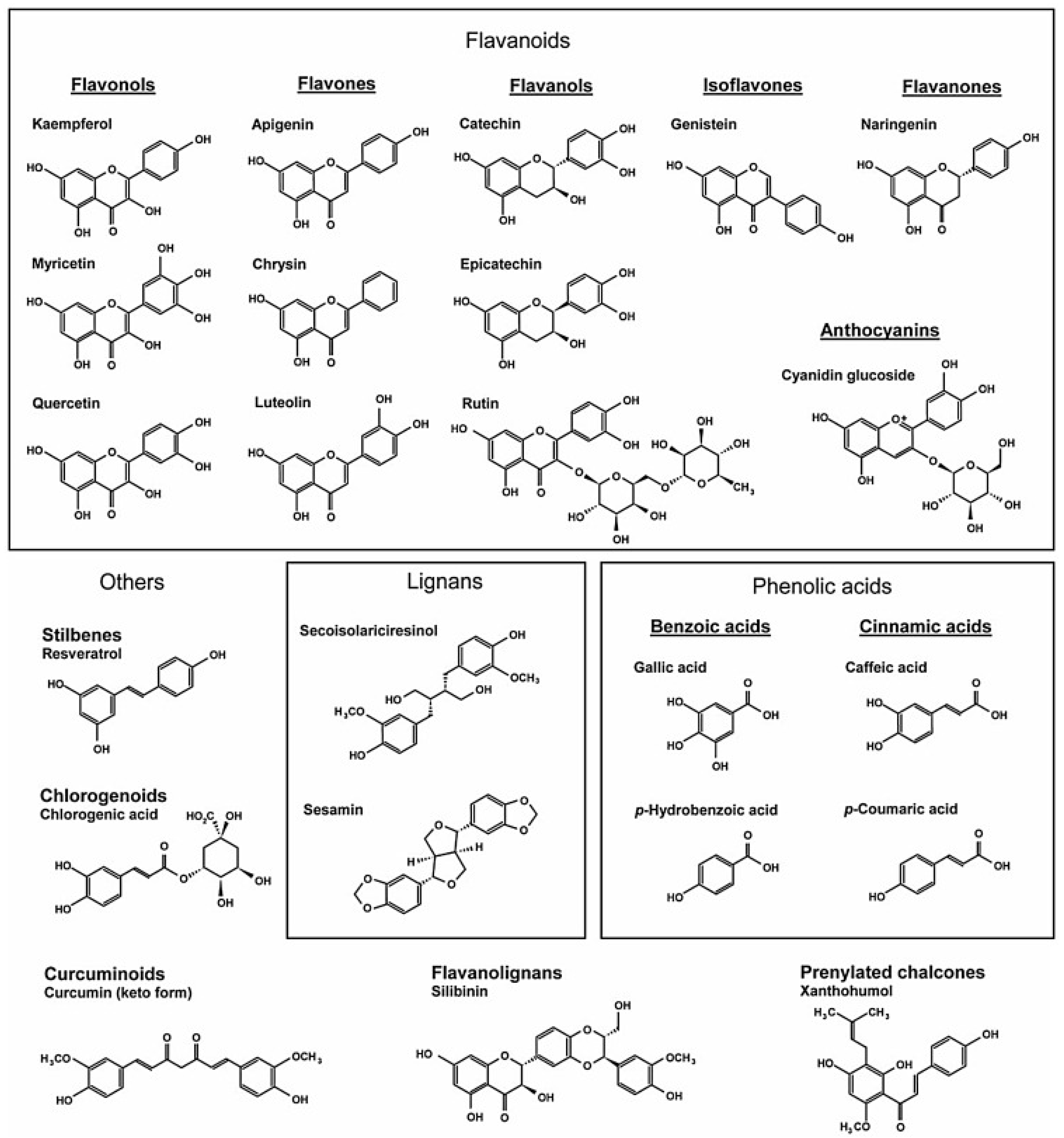
5. Polyphenols and Cardiovascular Health
5.1. Phenolic Acids
5.2. Stilbenes
5.3. Lignans
5.4. Flavonoids
6. Food-Drug and Herb-Drug Interactions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs) (World Health Organization). 2017. Available online: https://www.who.int/news-room/factsheets/detail/cardiovascular-diseases-(cvds) (accessed on 18 October 2020).
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Serhan CN Novel lipid mediators and resolution mechanisms in acute inflammation: To resolve or not? Am. J. Pathol. 2010, 177, 1576–1591. [CrossRef] [PubMed]
- Janicki, J.S.; Brower, G.L.; Henegar, J.R.; Wang, L. Ventricular remodeling in heart failure: The role of myocardial collagen. Adv. Exp. Med. Biol. 1995, 382, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, L.; Neskovic, A.N.; Parodi, G.; Cerisano, G.; Buonamici, P.; Santoro, G.M.; Antoniucci, D. Left ventricular remodeling after primary coronary angioplasty: Patterns of left ventricular dilation and long-term prognostic implications. Circulation 2002, 106, 2351–2357. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics—2020 update: A report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Conrad, N.; Judge, A.; Tran, J.; Mohseni, H.; Hedgecott, D.; Crespillo, A.P.; Allison, M.; Hemingway, H.; Cleland, J.G.; McMurray, J.J.; et al. Temporal trends and patterns in heart failure incidence: A population-based study of 4 million individuals. Lancet 2018, 391, 572–580. [Google Scholar] [CrossRef]
- Störk, S.; Hense, H.W.; Zentgraf, C.; Uebelacker, I.; Jahns, R.; Ertl, G.; Angermann, C.E. Pharmacotherapy according to treatment guidelines is associated with lower mortality in a community-based sample of patients with chronic heart failure A prospective cohort study. Eur. J. Heart Fail. 2008, 10, 1236–1245. [Google Scholar] [CrossRef]
- Desta, L.; Jernberg, T.; Spaak, J.; Hofman-Bang, C.; Persson, H. Risk and predictors of readmission for heart failure following a myocardial infarction between 2004 and 2013: A Swedish nationwide observational study. Int. J. Cardiol. 2017, 248, 221–226. [Google Scholar] [CrossRef]
- Romiti, G.F.; Recchia, F.; Zito, A.; Visioli, G.; Basili, S.; Raparelli, V. Sex and Gender-Related Issues in Heart Failure. Cardiol. Clin. 2022, 40, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Salton, C.J.; Chuang, M.L.; O’Donnell, C.J.; Kupka, M.J.; Larson, M.G.; Kissinger, K.V.; Edelman, R.R.; Levy, D.; Manning, W.J. Gender differences and normal left ventricular anatomy in an adult population free of hypertension: A cardiovascular magnetic resonance study of the Framingham Heart Study Offspring cohort. J. Am. Coll. Cardiol. 2002, 39, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Laufer, E.M.; Mingels, A.M.; Winkens, M.H.; Joosen, I.A.; Schellings, M.W.; Leiner, T.; Wildberger, J.E.; Narula, J.; Van Dieijen-Visser, M.P. and Hofstra, L. The extent of coronary atherosclerosis is associated with increasing circulating levels of high sensitive cardiac troponin T. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1269–1275. [Google Scholar] [CrossRef] [PubMed]
- Piro, M.; Della Bona, R.; Abbate, A.; Biasucci, L.M.; Crea, F. Sex-related differences in myocardial remodeling. J. Am. Coll. Cardiol. 2010, 55, 1057–1065. [Google Scholar] [CrossRef]
- Westerman, S.; Wenger, N.K. Women and heart disease, the underrecognized burden: Sex differences, biases, and unmet clinical and research challenges. Clin. Sci. 2016, 130, 551–563. [Google Scholar] [CrossRef]
- Maggioni, A.A.; Maseri, A.; Fresco, C.; Franzosi, M.G.; Mauri, F.; Santoro, E.; Tognoni, G. Age-related increase in mortality among patients with first myocardial infarctions treated with thrombolysis. N. Engl. J. Med. 1993, 329, 1442–1448. [Google Scholar] [CrossRef]
- Bujak, M.; Kweon, H.J.; Chatila, K.; Li, N.; Taffet, G.; Frangogiannis, N.G. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J. Am. Coll. Cardiol. 2008, 51, 1384–1392. [Google Scholar] [CrossRef]
- Koitabashi, N.; Kass, D.A. Reverse remodeling in heart failure--mechanisms and therapeutic opportunities. Nat. Rev. Cardiol. 2011, 9, 147–157. [Google Scholar] [CrossRef]
- Nisar, B.; Sultan, A.; Rubab, S.L. Comparison of medicinally important natural products versus synthetic drugs-a short commentary. Nat. Prod. Chem. Res. 2018, 6, 308. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Fernández-Jarne, E.; Serrano-Martínez, M.; Wright, M.; Gomez-Gracia, E. Development of a short dietary intake questionnaire for the quantitative estimation of adherence to a cardioprotective Mediterranean diet. Eur. J. Clin. Nutr. 2004, 58, 1550–1552. [Google Scholar] [CrossRef]
- Goszcz, K.; Deakin, S.J.; Duthie, G.G.; Stewart, D.; Megson, I.L. Bioavailable concentrations of delphinidin and its metabolite, gallic acid, induce antioxidant protection associated with increased intracellular glutathione in cultured endothelial cells. Oxidative Med. Cell. Longev. 2017, 2017, 9260701. [Google Scholar] [CrossRef]
- Nabel, E.G.; Braunwald, E.A. Tale of coronary artery disease and myocardial infarction. N. Engl. J. Med. 2012, 366, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, I.; Torp-Pedersen, C.; Køber, L.; Gustafsson, F.; Hildebrandt, P.; Trace Study Group. Effect of the angiotensin-converting enzyme inhibitor trandolapril on mortality and morbidity in diabetic patients with left ventricular dysfunction after acute myocardial infarction. J. Am. Coll. Cardiol. 1999, 34, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Quinones, M.A.; Koilpillai, C.; Limacher, M.; Shindler, D.; Benedict, C.; Shelton, B. Effects of long-term enalapril therapy on cardiac structure and function in patients with left ventricular dysfunction: Results of the SOLVD echocardiography substudy. Circulation 1995, 91, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, M.A.; Greenberg, B.H.; Kopelen, H.A.; Koilpillai, C.; Limacher, M.C.; Shindler, D.M.; Shelton, B.J.; Weiner, D.H.; SOLVD Investigators∗∗. Echocardiographic predictors of clinical outcome in patients with left ventricular dysfunction enrolled in the SOLVD registry and trials: Significance of left ventricular hypertrophy. J. Am. Coll. Cardiol. 2000, 35, 1237–1244. [Google Scholar] [CrossRef]
- Anand, I.S.; Florea, V.G. Structural Remodeling in the Development of Chronic Systolic Heart Failure: Implication for Treatment. In Congestive Heart Failure and Cardiac Transplantation; Springer: Cham, Switzerland, 2017; pp. 247–265. [Google Scholar]
- Pfeffer, M.A.; Braunwald, E.; Moyé, L.A.; Basta, L.; Brown, E.J., Jr.; Cuddy, T.E.; Davis, B.R.; Geltman, E.M.; Goldman, S.; Flaker, G.C.; et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: Results of the Survival and Ventricular Enlargement Trial. N. Engl. J. Med. 1992, 327, 669–677. [Google Scholar] [CrossRef]
- Lopez-Sendon, J.; Swedberg, K.; McMurray, J.; Tamargo, J.; Maggioni, A.P.; Dargie, H.; Tendera, M.; Waagstein, F.; Kjekshus, J.; Lechat, P.; et al. Expert consensus document on angiotensin converting enzyme inhibitors in cardiovascular disease: The Task Force on ACE-inhibitors of the European Society of Cardiology. Eur. Heart J. 2004, 25, 1454–1470. [Google Scholar]
- Fedak, P.W.; Verma, S.; Weisel, R.D.; Li, R.K. Cardiac remodeling and failure: From molecules to man (Part I). Cardiovasc. Pathol. 2005, 14, 1–11. [Google Scholar] [CrossRef]
- Azizi, M.; Ménard, J.; Bissery, A.; Guyenne, T.T.; Bura-Rivière, A.; Vaidyanathan, S.; Camisasca, R.P. Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II–renin feedback interruption. J. Am. Soc. Nephrol. 2004, 15, 3126–3133. [Google Scholar] [CrossRef]
- King, M.K.; Coker, M.L.; Goldberg, A.; McElmurray, J.H.; Gunasinghe, H.R., 3rd; Mukherjee, R.; Zile, M.R.; O’Neill, T.P.; Spinale, F.G. Selective matrix metalloproteinase inhibition with developing heart failure: Effects on left ventricular function and structure. Circ. Res. 2003, 92, 177–185. [Google Scholar] [CrossRef]
- Ikonomidis, J.S.; Hendrick, J.W.; Parkhurst, A.M.; Herron, A.R.; Escobar, P.G.; Dowdy, K.B.; Stroud, R.E.; Hapke, E.; Zile, M.R.; Spinale, F.G. Accelerated LV remodeling after myocardial infarction in TIMP-1-deficient mice: Effects of exogenous MMP inhibition. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H149–H158. [Google Scholar] [CrossRef]
- Matsumura, S.; Iwanaga, S.; Mochizuki, S.; Okamoto, H.; Ogawa, S.; Okada, Y. Targeted deletion or pharmacological inhibition of MMP-2 prevents cardiac rupture after myocardial infarction in mice. J. Clin. Investig. 2005, 115, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Van den Steen, P.E.; Sang, Q.X.A.; Opdenakker, G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat. Rev. Drug Discov. 2007, 6, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, R.E.; Libert, C. Is there new hope for therapeutic matrix metalloproteinase inhibition? Nat. Rev. Drug Discov. 2014, 13, 904–927. [Google Scholar] [CrossRef]
- Fields, G.B. The rebirth of matrix metalloproteinase inhibitors: Moving beyond the dogma. Cells 2019, 8, 984. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: A comprehensive review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef]
- van Abeelen, A.F.; Elias, S.G.; Bossuyt, P.M.; Grobbee, D.E.; van der Schouw, Y.T.; Roseboom, T.J.; Uiterwaal, C.S. Cardiovascular consequences of famine in the young. Eur. Heart J. 2012, 33, 538–545. [Google Scholar] [CrossRef]
- Ferguson, D.P.; Monroe, T.O.; Heredia, C.P.; Fleischmann, R.; Rodney, G.G.; Taffet, G.E.; Fiorotto, M.L. Postnatal undernutrition alters adult female mouse cardiac structure and function leading to limited exercise capacity. J. Physiol. 2019, 597, 1855–1872. [Google Scholar] [CrossRef]
- Bensley, J.G.; Stacy, V.K.; De Matteo, R.; Harding, R.; Black, M.J. Cardiac remodelling as a result of pre-term birth: Implications for future cardiovascular disease. Eur. Heart J. 2010, 31, 2058–2066. [Google Scholar] [CrossRef]
- Anatskaya, O.V.; Sidorenko, N.V.; Beyer, T.V.; Vinogradov, A.E. Neonatal cardiomyocyte ploidy reveals critical windows of heart development. Int. J. Cardiol. 2010, 141, 81–91. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Ludwig, D.S. Dietary guidelines in the 21st century—A time for food. JAMA 2010, 304, 681–682. [Google Scholar] [CrossRef] [PubMed]
- LaCroix, A.Z.; Rillamas-Sun, E.; Buchner, D.; Evenson, K.R.; Di, C.; Lee, I.M.; Marshall, S.; LaMonte, M.J.; Hunt, J.; Tinker, L.F.; et al. The objective physical activity and cardiovascular disease health in older women (OPACH) study. BMC Public Health 2017, 17, 192. [Google Scholar] [CrossRef]
- Esposito, K.; Ciotola, M.; Giugliano, D. Mediterranean diet, endothelial function and vascular inflammatory markers. Public Health Nutr. 2006, 9, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Sacanella, E.; Urpi-Sarda, M.; Chiva-Blanch, G.; Ros, E.; Martinez-Gonzalez, M.A.; Estruch, R. The effects of the mediterranean diet on biomarkers of vascular wall inflammation and plaque vulnerability in subjects with high risk for cardiovascular disease. A randomized trial. PLoS ONE 2014, 9, e100084. [Google Scholar] [CrossRef] [PubMed]
- Casas, R.; Urpi-Sardà, M.; Sacanella, E.; Arranz, S.; Corella, D.; Castañer, O.; Lamuela-Raventós, R.M.; Salas-Salvadó, J.; Lapetra, J.; Portillo, M.P.; et al. Anti-inflammatory effects of the Mediterranean diet in the early and late stages of atheroma plaque development. Mediat. Inflamm. 2017, 2017, 3674390. [Google Scholar] [CrossRef] [PubMed]
- Dauchet, L.; Amouyel, P.; Dallongeville, J. Fruits, vegetables and coronary heart disease. Nat. Rev. Cardiol. 2009, 6, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Dauchet, L.; Amouyel, P.; Hercberg, S.; Dallongeville, J. Fruit and vegetable consumption and risk of coronary heart disease: A meta-analysis of cohort studies. J. Nutr. 2006, 136, 2588–2593. [Google Scholar] [CrossRef]
- Corley, J.; Kyle, J.A.; Starr, J.M.; McNeill, G.; Deary, I.J. Dietary factors and biomarkers of systemic inflammation in older people: The Lothian Birth Cohort 1936. Br. J. Nutr. 2015, 114, 1088–1098. [Google Scholar] [CrossRef]
- Hosseini, B.; Berthon, B.S.; Saedisomeolia, A.; Starkey, M.R.; Collison, A.; Wark, P.A.; Wood, L.G. Effects of fruit and vegetable consumption on inflammatory biomarkers and immune cell populations: A systematic literature review and meta-analysis. Am. J. Clin. Nutr. 2018, 108, 136–155. [Google Scholar] [CrossRef]
- Arouca, A.; Michels, N.; Moreno, L.A.; González-Gil, E.M.; Marcos, A.; Gómez, S.; Díaz, L.E.; Widhalm, K.; Molnár, D.; Manios, Y.; et al. Associations between a Mediterranean diet pattern and inflammatory biomarkers in European adolescents. Eur. J. Nutr. 2018, 57, 1747–1760. [Google Scholar] [CrossRef]
- Wongwarawipat, T.; Papageorgiou, N.; Bertsias, D.; Siasos, G.; Tousoulis, D. Olive oil-related anti-inflammatory effects on atherosclerosis: Potential clinical implications. Endocr. Metab. Immune Disord.-Drug Targets 2018, 18, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Schwingshackl, L.; Christoph, M.; Hoffmann, G. Effects of olive oil on markers of inflammation and endothelial function—A systematic review and meta-analysis. Nutrients 2015, 7, 7651–7675. [Google Scholar] [CrossRef]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Bulló, M.; Martínez-González, M.Á.; Ros, E.; Corella, D.; Estruch, R.; Fitó, M.; Arós, F.; Wärnberg, J.; Fiol, M.; et al. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013, 11, 164. [Google Scholar] [CrossRef]
- Olabiyi, A.A.; Carvalho, F.B.; Bottari, N.B.; Lopes, T.F.; da Costa, P.; Stefanelo, N.; Morsch, V.M.; Akindahunsi, A.A.; Oboh, G.; Schetinger, M.R. Dietary supplementation of tiger nut alters biochemical parameters relevant to erectile function in L-NAME treated rats. Food Res. Int. 2018, 109, 358–367. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Del Gobbo, L.C.; Falk, M.C.; Feldman, R.; Lewis, K.; Mozaffarian, D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: Systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am. J. Clin. Nutr. 2015, 102, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Martini, D.; Godos, J.; Marventano, S.; Tieri, M.; Ghelfi, F.; Titta, L.; Lafranconi, A.; Trigueiro, H.; Gambera, A.; Alonzo, E.; et al. Nut and legume consumption and human health: An umbrella review of observational studies. Int. J. Food Sci. Nutr. 2021, 72, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yu, H.; He, F.; Reilly, K.H.; Zhang, J.; Li, S.; Zhang, T.; Wang, B.; Ding, Y.; Xi, B. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: A systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2014, 100, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Srichana, T. Monitoring of inflammatory mediators induced by silk sericin. J. Biosci. Bioeng. 2009, 107, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Teng, H.; Jia, Z.; Battino, M.; Miron, A.; Yu, Z.; Cao, H. and Xiao, J. Intracellular signaling pathways of inflammation modulated by dietary flavonoids: The most recent evidence. Crit. Rev. Food Sci. Nutr. 2018, 58, 2908–2924. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, T.; Granfeldt, Y.; Ahrén, B.; Branell, U.C.; Pålsson, G.; Hansson, A.; Söderström, M.; Lindeberg, S. Beneficial effects of a Paleolithic diet on cardiovascular risk factors in type 2 diabetes: A randomized cross-over pilot study. Cardiovasc. Diabetol. 2009, 8, 35. [Google Scholar] [CrossRef]
- Jew, S.; AbuMweis, S.S.; Jones, P.J. Evolution of the human diet: Linking our ancestral diet to modern functional foods as a means of chronic disease prevention. J. Med. Food 2009, 12, 925–934. [Google Scholar] [CrossRef]
- Cordain, L. The nutritional characteristics of a contemporary diet based upon Paleolithic food groups. J. Am. Nutraceutical. Assoc. 2002, 5, 15–24. [Google Scholar]
- Österdahl, M.; Kocturk, T.; Koochek, A.; Wändell, P. Effects of a short-term intervention with a paleolithic diet in healthy volunteers. Eur. J. Clin. Nutr. 2008, 62, 682–685. [Google Scholar] [CrossRef]
- Ghaedi, E.; Mohammadi, M.; Mohammadi, H.; Ramezani-Jolfaie, N.; Malekzadeh, J.; Hosseinzadeh, M.; Salehi-Abargouei, A. Effects of a Paleolithic Diet on Cardiovascular Disease Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2019, 10, 634–646, Erratum in Adv. Nutr. 2020, 11, 1054. [Google Scholar] [CrossRef]
- Manheimer, E.W.; van Zuuren, E.J.; Fedorowicz, Z.; Pijl, H. Paleolithic nutrition for metabolic syndrome: Systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 922–932. [Google Scholar] [CrossRef]
- Owen, O.E.; Morgan, A.P.; Kemp, H.G.; Sullivan, J.M.; Herrera, M.G.; Cahill, G.F. Brain metabolism during fasting. J. Clin. Investig. 1967, 46, 1589–1595. [Google Scholar] [CrossRef]
- Santos, F.L.; Esteves, S.S.; da Costa Pereira, A.; Yancy, W.S.; Nunes, J.P., Jr. Systematic review and meta-analysis of clinical trials of the effects of low carbohydrate diets on cardiovascular risk factors: Low carbohydrate diets and cardiovascular risk factors. Obes. Rev. 2012, 13, 1048–1066. [Google Scholar] [CrossRef] [PubMed]
- Bueno, N.B.; de Melo, I.S.; de Oliveira, S.L.; da Rocha Ataide, T. Very-low-carbohydrate ketogenic diet v. low-fat diet for long-term weight loss: A meta-analysis of randomised controlled trials. Br. J. Nutr. 2013, 110, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Naude, C.E.; Schoonees, A.; Senekal, M.; Young, T.; Garner, P.; Volmink, J. Low carbohydrate versus isoenergetic balanced diets for reducing weight and cardiovascular risk: A systematic review and meta-analysis. PLoS ONE 2014, 9, e100652. [Google Scholar] [CrossRef]
- Kosinski, C.; Jornayvaz, F.R. Effects of Ketogenic Diets on Cardiovascular Risk Factors: Evidence from Animal and Human Studies. Nutrients 2017, 9, 517. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, B.; Raggi, P. The ketogenic diet: Pros and cons. Atherosclerosis 2020, 292, 119–126. [Google Scholar] [CrossRef]
- Xu, S.; Tao, H.; Cao, W.; Cao, L.; Lin, Y.; Zhao, S.M.; Xu, W.; Cao, J.; Zhao, J.Y. Ketogenic diets inhibit mitochondrial biogenesis and induce cardiac fibrosis. Signal Transduct. Target Ther. 2021, 6, 54. [Google Scholar] [CrossRef]
- Gao, J.W.; Hao, Q.Y.; Zhang, H.F.; Li, X.Z.; Yuan, Z.M.; Guo, Y.; Wang, J.F.; Zhang, S.L.; Liu, P.M. Low-carbohydrate diet score and coronary artery calcium progression: Results from the CARDIA Study. Arterioscler. Thromb. Vasc. Biol. 2020, 41, 491–500. Available online: https://www.ahajournals.org/doi/10.1161/ATVBAHA.120.314838 (accessed on 29 October 2020). [CrossRef]
- Garcia-Rios, A.; Ordovas, J.M.; Lopez-Miranda, J.; Perez-Martinez, P. New diet trials and cardiovascular risk. Curr. Opin. Cardiol. 2018, 33, 423–428. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Miller III, E.R.; Chang, A.R.; Anderson, C.A.; Hall, J.E.; Appel, L.J. Effects of sodium reduction on energy, metabolism, weight, thirst, and urine volume: Results from the DASH (dietary approaches to stop hypertension)-sodium trial. Hypertension 2020, 75, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Kerley, C.P. Dietary patterns and components to prevent and treat heart failure: A comprehensive review of human studies. Nutr. Res. Rev. 2019, 32, 1–27. [Google Scholar] [CrossRef]
- Kerley, C.P. A review of plant-based diets to prevent and treat heart failure. Card. Fail. Rev. 2018, 4, 54. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Olabiyi, A.A.; Akinyemi, A.J. Inhibitory effect of aqueous extract of different parts of unripe pawpaw (Carica papaya) fruit on Fe2+-induced oxidative stress in rat pancreas in vitro. Pharm. Biol. 2013, 51, 1165–1174. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Ray, S.; Saini, M.K. Cure and prevention of cardiovascular diseases: Herbs for heart. Clin. Phytoscience 2021, 7, 5741198. [Google Scholar] [CrossRef]
- Patwardhan, B.; Mashelkar, R.A. Traditional medicine-inspired approaches to drug discovery: Can Ayurveda show the way forward? Drug Discov. Today 2009, 14, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Olabiyi, A.A.; Afolabi, B.A.; Reichert, K.P.; Palma, T.V.; Morsch, V.M.; Oboh, G.; Schetinger, M.R.C. Assessment of sexual behavior and neuromodulation of Cyperus esculentus L. and Tetracarpidium conophorum Müll. Arg dietary supplementation regulating the purinergic system in the cerebral cortex of L-NAME-challenged rats. J. Food Biochem. 2021, 45, e13862. [Google Scholar] [CrossRef] [PubMed]
- Badole, S.L.; Bodhankar, S.L.; Patel, N.M.; Bhardwaj, S. Acute and chronic diuretic effect of ethanolic extract of leaves of Cocculus hirsutus (L.) Diles in normal rats. J. Pharm. Pharmacol. 2009, 61, 387–393. [Google Scholar] [CrossRef]
- Davison, E.K.; Brimble, M.A. Natural product derived privileged scaffolds in drug discovery. Curr. Opin. Chem. Biol. 2019, 52, 1–8. [Google Scholar] [CrossRef]
- Olabiyi, A.A.; Ajayi, K. Diet, herbs and erectile function: A good friendship! Andrologia 2022, 54, e14424. [Google Scholar] [CrossRef]
- Shaito, A.; Thuan, D.T.B.; Phu, H.T.; Nguyen, T.H.D.; Hasan, H.; Halabi, S.; Abdelhady, S.; Nasrallah, G.K.; Eid, A.H.; Pintus, G. Herbal medicine for cardiovascular diseases: Efficacy, mechanisms, and safety. Front. Pharmacol. 2020, 11, 422. [Google Scholar] [CrossRef]
- Silva, H.; Martins, F.G. Cardiovascular Activity of Ginkgo biloba—An Insight from Healthy Subjects. Biology 2022, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dixit, M. Role of polyphenols and other phytochemicals on molecular signaling. Oxidative Med. Cell. Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef]
- Murino Rafacho, B.P.; Portugal dos Santos, P.; Goncalves, A.D.F.; Fernandes, A.A.H.; Okoshi, K.; Chiuso-Minicucci, F.; Azevedo, P.S.; Mamede Zornoff, L.A.; Minicucci, M.F.; Wang, X.D.; et al. Rosemary supplementation (Rosmarinus oficinallis L.) attenuates cardiac remodeling after myocardial infarction in rats. PLoS ONE 2017, 12, e0177521. [Google Scholar] [CrossRef]
- Butnariu, M.; Quispe, C.; Herrera-Bravo, J.; Sharifi-Rad, J.; Singh, L.; Aborehab, N.M.; Bouyahya, A.; Venditti, A.; Sen, S.; Acharya, K.; et al. The pharmacological activities of Crocus sativus L.: A review based on the mechanisms and therapeutic opportunities of its phytoconstituents. Oxidative Med. Cell. Longev. 2022, 2022, 8214821. [Google Scholar] [CrossRef] [PubMed]
- Khazdair, M.R.; Boskabady, M.H.; Hosseini, M.; Rezaee, R.; Tsatsakis, A.M. The effects of Crocus sativus (saffron) and its constituents on nervous system: A review. Avicenna J. Phytomedicine 2015, 5, 376. [Google Scholar]
- Mzabri, I.; Addi, M.; Berrichi, A. Traditional and modern uses of saffron (Crocus sativus). Cosmetics 2019, 6, 63. [Google Scholar] [CrossRef]
- Sargolzaei, J.; Shabestari, M.M. The effects of Crocus Sativus, L. and its main constituents against cardiovascular diseases. Der Pharm. Lett. 2016, 8, 38–41. [Google Scholar]
- Al-Yahya, M.A.; Mothana, R.A.; Al-Said, M.S.; El-Tahir, K.E.; Al-Sohaibani, M.; Rafatullah, S. Citrus medica “Otroj”: Attenuates oxidative stress and cardiac dysrhythmia in isoproterenol-induced cardiomyopathy in rats. Nutrients 2013, 5, 4269–4283. [Google Scholar] [CrossRef]
- Tassell, M.C.; Kingston, R.; Gilroy, D.; Lehane, M.; Furey, A. Hawthorn (Crataegus spp.) in the treatment of cardiovascular disease. Pharmacogn. Rev. 2010, 4, 32. [Google Scholar]
- Altinterim, B. Cardiovascular effects of Hawthorn (Crataegus monogyna). KSÜ Doğa Bilim. Derg. 2012, 15, 16–18. [Google Scholar]
- Verma, S.K.; Jain, V.; Katewa, S.S. Blood pressure lowering, fibrinolysis enhancing and antioxidant activities of cardamom (Elettaria cardamomum). Indian J. Biochem. Biophys. 2009, 46, 503–506. [Google Scholar]
- Nagashree, S.; Archana, K.K.; Srinivas, P.; Srinivasan, K.; Sowbhagya, H.B. Anti-hypercholesterolemic influence of the spice cardamom (Elettaria cardamomum) in experimental rats. J. Sci. Food Agric. 2017, 97, 3204–3210. [Google Scholar] [CrossRef]
- Arbeláez, L.F.G.; Pardo, A.C.; Fantinelli, J.C.; Schinella, G.R.; Mosca, S.M.; Ríos, J.L. Cardioprotection and natural polyphenols: An update of clinical and experimental studies. Food Funct. 2018, 9, 6129–6145. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.W.; Tomlinson, B.; Chan, P.; Lam, C.W.K. The beneficial effects of Ganoderma lucidum on cardiovascular and metabolic disease risk. Pharm. Biol. 2021, 59, 1161–1171. [Google Scholar] [CrossRef]
- Aviram, M.; Dornfeld, L. Pomegranate juice consumption inhibits serum angiotensin converting enzyme activity and reduces systolic blood pressure. Atherosclerosis 2001, 158, 195–198. [Google Scholar] [CrossRef]
- Asgary, S.; Haghjooyjavanmard, S.; Setorki, M.; Rafieian, M.; Haghighi, S.; Eidi, A.; Rohani, A.H. The postprandial effect of apple juice intake on some of the biochemical risk factors of atherosclerosis in male rabbit. J. Med. Plants Res. 2009, 3, 785–790. [Google Scholar]
- Asgary, S.; Sahebkar, A.; Afshani, M.R.; Keshvari, M.; Haghjooyjavanmard, S.; Rafieian-Kopaei, M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother. Res. 2014, 28, 193–199. [Google Scholar] [CrossRef]
- Setorki, M.; Nazari, B.; Asgary, S.; Eidi, A.; Rohani, A.H. Acute effects of apple cider vinegar intake on some biochemical risk factors of atherosclerosis in rabbits fed with a high cholesterol diet. Qom Univ. Med. Sci. J. 2010, 3, 10. [Google Scholar]
- Setorki, M.; Asgary, S.; Eidi, A.; Rohani, A.H.; Esmaeil, N. Effects of apple juice on risk factors of lipid profile, inflammation and coagulation, endothelial markers and atherosclerotic lesions in high cholesterolemic rabbits. Lipids Health Dis. 2009, 8, 39. [Google Scholar] [CrossRef] [PubMed]
- Omoni, A.O.; Aluko, R.E. The anti-carcinogenic and anti-atherogenic effects of lycopene: A review. Trends Food Sci. Technol. 2005, 16, 344–350. [Google Scholar] [CrossRef]
- Naz, A.; Butt, M.S.; Sultan, M.T.; Qayyum, M.M.N.; Niaz, R.S. Watermelon lycopene and allied health claims. EXCLI J. 2014, 13, 650. [Google Scholar] [PubMed]
- Chiva-Blanch, G.; Visioli, F. Polyphenols and health: Moving beyond antioxidants. J. Berry Res. 2012, 2, 63–71. [Google Scholar] [CrossRef]
- Niki, E. Antioxidant capacity: Which capacity and how to assess it? J. Berry Res. 2011, 1, 169–176. [Google Scholar] [CrossRef]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef]
- Jang, M.; Cai, L.; Udeani, G.O.; Slowing, K.V.; Thomas, C.F.; Beecher, C.W.; Fong, H.H.; Farnsworth, N.R.; Kinghorn, A.D.; Mehta, R.G.; et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 1997, 275, 218–220. [Google Scholar] [CrossRef] [PubMed]
- Seymour, E.M.; Singer, A.A.; Bennink, M.R.; Parikh, R.V.; Kirakosyan, A.; Kaufman, P.B.; Bolling, S.F. Chronic intake of a phytochemical-enriched diet reduces cardiac fibrosis and diastolic dysfunction caused by prolonged salt-sensitive hypertension. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Varshney, R.; Budoff, M.J. Garlic and heart disease. J. Nutr. 2016, 146, 416S–421S. [Google Scholar] [CrossRef]
- Ko, F.N.; Yu, S.M.; Kang, Y.F.; Teng, C.M. Characterization of the thromboxane (TP-) receptor subtype involved in proliferation in cultured vascular smooth muscle cells of rat. Br. J. Pharmacol. 1995, 116, 1801–1808. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and biochemistry of dietary polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef]
- Vitrac, X.; Monti, J.P.; Vercauteren, J.; Deffieux, G.; Mérillon, J.M. Direct liquid chromatographic analysis of resveratrol derivatives and flavanonols in wines with absorbance and fluorescence detection. Anal. Chim. Acta 2002, 458, 103–110. [Google Scholar] [CrossRef]
- Prasad, R.; Kawaguchi, S.; Ng, D.T. Biosynthetic mode can determine the mechanism of protein quality control. Biochem. Biophys. Res. Commun. 2012, 425, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Korkina, L.; Kostyuk, V.; De Luca, C.; Pastore, S. Plant phenylpropanoids as emerging anti-inflammatory agents. Mini Rev. Med. Chem. 2011, 11, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Kim, H.J.; Ali, M.S.; Lee, Y.S. An update on bioactive plant lignans. Nat. Prod. Rep. 2005, 22, 696–716. [Google Scholar] [CrossRef]
- Ly, C.; Yockell-Lelievre, J.; Ferraro, Z.M.; Arnason, J.T.; Ferrier, J.; Gruslin, A. The effects of dietary polyphenols on reproductive health and early development. Hum. Reprod. Update 2015, 21, 228–248. [Google Scholar] [CrossRef]
- Smeds, A.I.; Eklund, P.C.; Willför, S.M. Content, composition, and stereochemical characterisation of lignans in berries and seeds. Food Chem. 2012, 134, 1991–1998. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Arts, I.C.W. Flavonols, flavones and flavanols–nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1081–1093. [Google Scholar] [CrossRef]
- Cassidy, A.; Hanley, B.; Lamuela-Raventos, R.M. Isoflavones, lignans and stilbenes–origins, metabolism and potential importance to human health. J. Sci. Food Agric. 2000, 80, 1044–1062. [Google Scholar] [CrossRef]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Martín, M.L.; Fuguet, E.; Quero, C.; Pérez-Jiménez, J.; Torres, J.L. New identification of proanthocyanidins in cinnamon (Cinnamomum zeylanicum L.) using MALDI-TOF/TOF mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 1327–1336. [Google Scholar] [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [Google Scholar] [CrossRef]
- Raj, P.; Louis, X.L.; Thandapilly, S.J.; Movahed, A.; Zieroth, S.; Netticadan, T. Potential of resveratrol in the treatment of heart failure. Life Sci. 2014, 95, 63–71. [Google Scholar] [CrossRef]
- Niu, L.; He, X.H.; Wang, Q.W.; Fu, M.Y.; Xu, F.; Xue, Y.; Wang, Z.Z.; An, X.J. Polyphenols in regulation of redox signaling and inflammation during cardiovascular diseases. Cell Biochem. Biophys. 2015, 72, 485–494. [Google Scholar] [CrossRef]
- Li, M.; Jiang, Y.; Jing, W.; Sun, B.; Miao, C.; Ren, L. Quercetin provides greater cardioprotective effect than its glycoside derivative rutin on isoproterenol-induced cardiac fibrosis in the rat. Can. J. Physiol. Pharmacol. 2013, 91, 951–959. [Google Scholar] [CrossRef]
- Panchal, S.K.; Poudyal, H.; Brown, L. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutr. 2012, 142, 1026–1032. [Google Scholar] [CrossRef]
- Mak, J.C. Potential role of green tea catechins in various disease therapies: Progress and promise. Clin. Exp. Pharmacol. Physiol. 2012, 39, 265–273. [Google Scholar] [CrossRef]
- Sheng, R.; Gu, Z.L.; Xie, M.L.; Zhou, W.X.; Guo, C.Y. EGCG inhibits cardiomyocyte apoptosis in pressure overload-induced cardiac hypertrophy and protects cardiomyocytes from oxidative stress in rats 1. Acta Pharmacol. Sin. 2007, 28, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Sheng, R.; Gu, Z.L.; Xie, M.L.; Zhou, W.X.; Guo, C.Y. EGCG inhibits proliferation of cardiac fibroblasts in rats with cardiac hypertrophy. Planta Med. 2009, 75, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Lan, L.; Chu, J.; Kang, W.Q.; Ge, Z.M. Epigallocatechin gallate attenuated the activation of rat cardiac fibroblasts induced by angiotensin II via regulating β-arrestin1. Cell. Physiol. Biochem. 2013, 31, 338–346. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, S.S.; Chen, T.T.; Gao, S.; Geng, B.; Yu, Y.; Ye, J.T.; Liu, P.Q. EGCG inhibits CTGF expression via blocking NF-κB activation in cardiac fibroblast. Phytomedicine 2013, 20, 106–113. [Google Scholar] [CrossRef]
- Cai, Y.; He, S.Q.; Hong, H.Q.; Cai, Y.P.; Zhao, L.; Zhang, M. High doses of (−)-epigallocatechin-3-gallate from green tea induces cardiac fibrosis in mice. Biotechnol. Lett. 2015, 37, 2371–2377. [Google Scholar] [CrossRef] [PubMed]
- Heymans, S.; González, A.; Pizard, A.; Papageorgiou, A.P.; López-Andrés, N.; Jaisser, F.; Thum, T.; Zannad, F.; Díez, J. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur. J. Heart Fail. 2015, 17, 764–771. [Google Scholar] [CrossRef]
- Wang, T.; Pan, D.; Zhang, Y.; Li, D.; Zhang, Y.; Xu, T.; Luo, Y.; Ma, Y. Luteolin antagonizes angiotensin II-dependent proliferation and collagen synthesis of cultured rat cardiac fibroblasts. Curr. Pharm. Biotechnol. 2015, 16, 430–439. [Google Scholar] [CrossRef]
- Nakayama, A.; Morita, H.; Nakao, T.; Yamaguchi, T.; Sumida, T.; Ikeda, Y.; Kumagai, H.; Motozawa, Y.; Takahashi, T.; Imaizumi, A.; et al. A food-derived flavonoid luteolin protects against angiotensin II-induced cardiac remodeling. PLoS ONE 2015, 10, e0137106. [Google Scholar] [CrossRef]
- Sun, C.D.; Zhang, B.; Zhang, J.K.; Xu, C.J.; Wu, Y.L.; Li, X.; Chen, K.S. Cyanidin-3-glucoside-rich extract from Chinese bayberry fruit protects pancreatic β cells and ameliorates hyperglycemia in streptozotocin-induced diabetic mice. J. Med. Food 2012, 15, 288–298. [Google Scholar] [CrossRef]
- Skemiene, K.; Liobikas, J.; Borutaite, V. Anthocyanins as substrates for mitochondrial complex I–protective effect against heart ischemic injury. FEBS J. 2015, 282, 963–971. [Google Scholar] [CrossRef]
- Škėmienė, K.; Jablonskienė, G.; Liobikas, J.; Borutaitė, V. Protecting the heart against ischemia/reperfusion-induced necrosis and apoptosis: The effect of anthocyanins. Medicina 2013, 49, 15. [Google Scholar] [CrossRef]
- Chen, Y.F.; Shibu, M.A.; Fan, M.J.; Chen, M.C.; Viswanadha, V.P.; Lin, Y.L.; Lai, C.H.; Lin, K.H.; Ho, T.J.; Kuo, W.W.; et al. Purple rice anthocyanin extract protects cardiac function in STZ-induced diabetes rat hearts by inhibiting cardiac hypertrophy and fibrosis. J. Nutr. Biochem. 2016, 31, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Sunagawa, Y.; Morimoto, T.; Wada, H.; Takaya, T.; Katanasaka, Y.; Kawamura, T.; Yanagi, S.; Marui, A.; Sakata, R.; Shimatsu, A.; et al. A natural p300-specific histone acetyltransferase inhibitor, curcumin, in addition to angiotensin-converting enzyme inhibitor, exerts beneficial effects on left ventricular systolic function after myocardial infarction in rats. Circ. J. 2011, 75, 2151–2159. [Google Scholar] [CrossRef]
- Sunagawa, Y.; Sono, S.; Katanasaka, Y.; Funamoto, M.; Hirano, S.; Miyazaki, Y.; Hojo, Y.; Suzuki, H.; Morimoto, E.; Marui, A.; et al. Optimal dose-setting study of curcumin for improvement of left ventricular systolic function after myocardial infarction in rats. J. Pharmacol. Sci. 2014, 126, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.P.; Wang, Z.F.; Tootle, S.; Philip, T.; Zhao, Z.Q. Curcumin promotes cardiac repair and ameliorates cardiac dysfunction following myocardial infarction. Br. J. Pharmacol. 2012, 167, 1550–1562. [Google Scholar] [CrossRef]
- Xiao, J.; Sheng, X.; Zhang, X.; Guo, M.; Ji, X. Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug Des. Dev. Ther. 2016, 10, 1267. [Google Scholar] [CrossRef]
- Zeng, C.; Zhong, P.; Zhao, Y.; Kanchana, K.; Zhang, Y.; Khan, Z.A.; Chakrabarti, S.; Wu, L.; Wang, J.; Liang, G. Curcumin protects hearts from FFA-induced injury by activating Nrf2 and inactivating NF-κB both in vitro and in vivo. J. Mol. Cell. Cardiol. 2015, 79, 1–12. [Google Scholar] [CrossRef]
- Sung, M.M.; Dyck, J.R. Therapeutic potential of resveratrol in heart failure. Ann. N. Y. Acad. Sci. 2015, 1348, 32–45. [Google Scholar] [CrossRef]
- Song, H.; Wang, Q.; He, A.; Li, S.; Guan, X.; Hu, Y.; Feng, S. Antioxidant activity, storage stability and in vitro release of epigallocatechin-3-gallate (EGCG) encapsulated in hordein nanoparticles. Food Chem. 2022, 388, 132903. [Google Scholar] [CrossRef]
- Qiu, C.; McClements, D.J.; Jin, Z.; Qin, Y.; Hu, Y.; Xu, X.; Wang, J. Resveratrol-loaded core-shell nanostructured delivery systems: Cyclodextrin-based metal-organic nanocapsules prepared by ionic gelation. Food Chem. 2020, 317, 126328. [Google Scholar] [CrossRef]
- Ding, H.W.; Huang, A.L.; Zhang, Y.L.; Li, B.; Huang, C.; Ma, T.T.; Meng, X.M.; Li, J. Design, synthesis and biological evaluation of hesperetin derivatives as potent anti-inflammatory agent. Fitoterapia 2017, 121, 212–222. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.J. Synergistic anticancer activity of resveratrol in combination with docetaxel in prostate carcinoma cells. Nutr. Res. Pract. 2021, 15, 12–25. [Google Scholar] [CrossRef]
- Quiñones, M.; Guerrero, L.; Suarez, M.; Pons, Z.; Aleixandre, A.; Arola, L.; Muguerza, B. Low-molecular procyanidin rich grape seed extract exerts antihypertensive effect in males spontaneously hypertensive rats. Food Res. Int. 2013, 51, 587–595. [Google Scholar] [CrossRef]
- Rains, T.M.; Agarwal, S.; Maki, K.C. Antiobesity effects of green tea catechins: A mechanistic review. J. Nutr. Biochem. 2011, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Hoad, C.L.; Jedamzik, P.; Madla, C.M.; Maliepaard, M.; et al. The mechanisms of pharmacokinetic food-drug interactions—A perspective from the UNGAP group. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2019, 134, 31–59. [Google Scholar] [CrossRef]
- O’Shea, J.P.; Holm, R.; O’Driscoll, C.M.; Griffin, B.T. Food for thought: Formulating away the food effect–a PEARRL review. J. Pharm. Pharmacol. 2019, 71, 510–535. [Google Scholar] [CrossRef] [PubMed]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olabiyi, A.A.; de Castro Brás, L.E. Cardiovascular Remodeling Post-Ischemia: Herbs, Diet, and Drug Interventions. Biomedicines 2023, 11, 1697. https://doi.org/10.3390/biomedicines11061697
Olabiyi AA, de Castro Brás LE. Cardiovascular Remodeling Post-Ischemia: Herbs, Diet, and Drug Interventions. Biomedicines. 2023; 11(6):1697. https://doi.org/10.3390/biomedicines11061697
Chicago/Turabian StyleOlabiyi, Ayodeji A., and Lisandra E. de Castro Brás. 2023. "Cardiovascular Remodeling Post-Ischemia: Herbs, Diet, and Drug Interventions" Biomedicines 11, no. 6: 1697. https://doi.org/10.3390/biomedicines11061697
APA StyleOlabiyi, A. A., & de Castro Brás, L. E. (2023). Cardiovascular Remodeling Post-Ischemia: Herbs, Diet, and Drug Interventions. Biomedicines, 11(6), 1697. https://doi.org/10.3390/biomedicines11061697






