Abstract
Head and neck cancer (H&NC) is a diverse category of tumors related to malignancies in the common aerodigestive pathway, with high metabolic rate, poor nutritional and treatment outcomes, and elevated mortality despite the best standard treatment. Herein, we focus on determining how the phase angle (PA) differs across sex as a predictor of poor prognosis, low quality-of-life (QoL) scores, and mortality in patients with head and neck cancer. This follow-up study presents a sex-differential analysis in a prospective cohort of 139 head and neck cancer patients categorized by sex as male (n = 107) and female (n = 32). Patients were compared in terms of nutritional, biochemical, and quality-of-life indicators between low and normal PA in women (<3.9° (n = 14, 43.75%) and ≥3.9°) and men (<4.5° (n = 62, 57.9%) and ≥4.5°). Our results show that most patients were in locally advanced clinical stages (women: n = 21 (65.7%); men: n = 67 (62.6%)) and that patients with low PA had a lower punctuation in parameters such as handgrip strength, four-meter walking speed, albumin, C-reactive protein (CRP), and CRP/albumin ratio (CAR), as well as the worst QoL scores in functional and symptomatic scales in both the male and female groups. A comparison between sexes revealed significant disparities; malnourishment and tumor cachexia related to an inflammatory state was more evident in the women’s group.
1. Introduction
Head and neck cancer (H&NC) is a highly prevalent malignant disease in Asian countries, with around 900,000 total cases, 550,000 newly diagnosed cases per year, and 400,000 deaths annually [1]. Males are predominantly affected, with the male/female ratio ranging from 2:1 to 4:1 [2]. The geographic distribution of N&NC is determined by habits considered risk factors, such as tobacco, alcohol, and betel nut consumption. Other identified risk factors are opium use; viral infections (Epstein–Barr virus, human papillomavirus, herpes simplex virus, hepatitis C virus, and even human immunodeficiency virus); radiation; occupational exposure to perchloroethylene, pesticides, asbestos, and polycyclic aromatic hydrocarbons; dietary aspects, such as high levels of nitrites; mouthwashes with alcohol derivates; and poor oral hygiene and periodontal disease [3,4,5,6,7].
Head and neck cancer describes a group of malignancies, mainly squamous histologic varieties (over 90%), involving various anatomic sites and subsites, including the oral cavity, pharynx, larynx, nasal cavity, and paranasal sinuses or salivary glands. This condition affects the initial symptoms, progression, and malnourishment rate (Overview of the diagnosis and staging of head and neck cancer in up to date).
All these tumors share biological characteristics [8], with a high mortality rate in both sexes compared with other malignant neoplasms [9,10,11,12], even in the patients treated in our facility care center, where the rate reached 70% [13].
A 2020 report on the global burden of cancer by The Global Cancer Observatory (GLOBOCAN) [6] differentiated the disease prevalence according to anatomical site and subsite, with lip and oral cavity cancer accounting for 377,713 new cases in both sexes of all ages. The incidence in males accounted for 264,211 new cases versus 113,502 new cases in females. The number of male deaths was 125,022 (cumulated risk: 0.32), whereas the number of female deaths was 52,735 (cumulated risk: 0.12) [14]. The differential biological behavior between males and females is crucial to understanding the role of sex-specific biological characteristics in disease development associated with structural, hormonal, and immunological influences [15].
Sex differences in cancer vary considerably through mechanisms that impact incidence, prognosis, treatment, and survival [16], an outcome of differences in metabolism, sex-hormone-dependent systems, cell cycle control, and immune response specific to biological sex, which may be the reason for these repercussions [16,17].
In 2020, De Courcy et al. postulated about the radiotherapy response and side effects related to sex-differentiated biological behavior, which was radiogenomically investigated [18].
The radiotherapy basement refers to the use of ionizing radiation to ionize and oxidate biological molecules inside cells, resulting in the generation of free radicals and DNA damage due to radiation itself or through free oxygens radicals, causing membrane damage, senescence with alterations in cellular reproduction, apoptosis, or mitotic catastrophe [19].
All these mechanisms involve approximately 52% of malignant neoplastic cells causing tumor lysis. Another benefit is enhancement of innate immune system function associated with tumor regression during metastasis phenomena [20].
The adverse events associated with radiotherapy are acute and chronic with respect to the anatomic site and subsite. In H&NC, those events affect the vicinity of the aerodigestive tract, including superficial skin damage, mucositis in the oral cavity and esophagus, and, in severe cases, stenosis or perforation and fistulae, as well as osteoradionecrosis depending on the radiation dose, the volume of irradiated healthy tissue and irradiated tissue, and even the sex of the patient. According to Alsbeth et al. and Barnett et al., sex-related variations occur in the ability to recognize and repair DNA double-strand breaks as part of a differential treatment response [21,22,23].
In 2019, Benchetrit L et al. described the behavior of a population with squamous H&NC between 1985 and 2015. Women represented 26% of the total subjects, and a lower percentage of women were candidates to receive chemotherapy or chemoradiotherapy, considering their clinical condition. The clinical stages of H&NC were similar in proportion to the studied men [24].
H&NC is also one of the most aggressive types of cancer, causing patients to undergo life-changing interventions that impact their individual and social well-being and reduce function and quality of life (QoL) [25,26]. H&NC frequently leads to severe nutritional problems that result from the disease itself, even before treatment or treatment sequelae of surgery with extensive resection and reconstruction, including the aerodigestive tract, radiation, or acute or chronic effects of chemotherapy [27].
The clinical course of the disease also comprises many complications, such as dysphagia, swallowing difficulties, pain, inadequate dietary intake, hemoptysis, dyspnea, and oral mucositis, which impact the ability of patients to speak, breathe, and eat, with a high association with malnutrition [28,29].
Aerodigestive symptomatology interferes with food intake, which, in addition to systemic inflammation, leads to anorexia, loss of skeletal muscle mass and functionality, and adipose tissue depletion [16,17]. Patients with H&NC also exhibit poor overall survival rates [30].
The natural history of H&NC, as well as its treatments, impairs nutritional status; thus, anorexia and cachexia resulting from the therapeutic approach are associated with increased morbidity and mortality rates, QoL deterioration, and elevated medical treatment costs.
The high metabolic rate of H&NC, combined with the abovementioned factors, contributes to malnutrition-related weight loss [31], loss of function, and a high rate of adverse outcomes, even with the best available treatment, leading to increased morbidity and mortality rates [32,33].
The criteria related to malnutrition include biochemical and anthropometric markers [34]. Body composition can be used to identify a patient’s nutritional condition by dividing body weight into several compartments to obtain information about the patient’s health and functionality [34,35].
Nutritional phenotype diagnosis by bioelectrical impedance analysis (BIA) is a body composition assessment tool used in clinical settings to detect weight loss and muscle mass changes and determine the integrity of cell membranes using phase-angle (PA) analyses [36,37,38,39].
BIA is a simple, non-invasive technique that estimates body composition by measuring the opposition (impedance) to a current passing through the body. Impedance is the result of two components: resistance (R), which is the opposition to the flow of an alternating current through intra- and extracellular ionic solutions, and reactance (Xc), which is the delay in conduction because of capacitance by cell membranes and tissue interfaces. Capacitance conditions a phase shift or PA that is quantified as an arc tangent ((Xc/R) * 180°/π) [40]. PA is a biological marker of cellular health, reflecting membrane integrity, cell mass, and hydration status. PA has been proven to be a suitable predictor of morbidity and mortality in various patient groups [41,42].
PA data relate to functionality, QoL, and even the risk of complications and mortality [10]; their use is based on estimation of the integrity of cell membranes, serving as a critical parameter for prediction of clinical and functional outcomes [43]. Moreover, these measures predict patient functionality and mortality [44]. Therefore, it is possible to identify the role of skeletal muscle mass loss and lean mass loss in patients with H&NC, predisposing this population to sarcopenia and dynapenia.
PA ranges from 4 to 10 in healthy individuals [39]. Low PA values are related to damage to cell membrane integrity, leading to cell death. In contrast, moderate or high values are related to cell membrane integrity and an appropriate water ratio in the intracellular-to-extracellular spaces, which can predict body mass [32,45].
Therefore, oncologic care for H&NC patients should focus on the importance of prognosis and prediction in decision making and treatment approaches that contribute to the best response and minimal toxicity of treatment [46].
The high mortality and the poor nutritional response of patients undergoing treatment for this type of cancer are the factors determining the sex-specific variation in PA as a predictor of poor prognosis and mortality in patients with head and neck cancer.
In this report, we emphasize the sex-differential behavior of nutritional status, functionality, and mortality in H&NC patients.
2. Materials and Methods
2.1. Patients
The current study presents a sex-differential analysis in a prospective cohort of 139 patients with H&NC categorized by sex as male (n = 107) and female (n = 32), all of whom had a biopsy to confirm their diagnosis. They were recruited to participate in the study at an Instituto Mexicano del Seguro Social (IMSS) tertiary care hospital in Guadalajara, Mexico. All patients provided consent in writing, and the study was approved by our institutional review board (Comisión Nacional de Investigación en Salud del IMSS). The processes were carried out in accordance with the requirements of the Helsinki Declaration.
Patients with more than one malignant neoplasm, autoimmune diseases, chronic illnesses in the lung or kidney, or any contraindication to perform bioelectrical impedance analysis were excluded. We retrieved the patients’ clinical features, clinical stage, anatomical localization of the tumor, treatment, and lab results from medical records.
The following requirements had to be followed to complete the anthropometric analysis: the patients had to fast for eight hours and be free of any objects and conditions that would interfere with the BIA (metal prosthesis, shoes, socks, fixtures, electronic implants, severe edema, limb amputations, weight greater than 300 kg, etc.).
2.2. Anthropometric Analysis
An SECA 213 instrument (Seca, Hamburg, Germany) was used to measure weight (kg) and height (m), whereas a SECA 514 bioelectrical impedance device (Seca, Germany) was used to assess body composition and PA.
The body mass index (BMI) and skeletal muscle mass index (SMMI) were calculated by dividing the body weight or total skeletal muscle mass (kg) by the height squared (m2). The patients were then classified into three categories based on nutritional phenotype:
- Non-sarcopenic group (NSG):
- (a)
- Women with SMMI ≥ 6.42 kg/m2 and BMI < 25 kg/m2;
- (b)
- Men with SMMI ≥ 8.86 kg/m2 and BMI < 25 kg/m2.
- Sarcopenic group (SG):
- (a)
- Women with SMMI < 6.42 kg/m2 and BMI < 25 kg/m2;
- (b)
- Men with SMMI < 8.87 kg/m2 and BMI < 25 kg/m2.
- Sarcopenic obesity group (SOG):
- (a)
- Women with SMMI < 6.42 kg/m2 and BMI ≥ 25 kg/m2;
- (b)
- Men with SMMI < 8.87 kg/m2 and BMI ≥ 25 kg/m2.
A dietitian validated the body composition analysis and the nutritional phenotypic classification. An mBCA SECA 514 bioelectric impedance device (Seca, Germany) was used to determine patient weight, phase angle, total skeletal muscle mass, and whole-body fat percentage, with data used to calculate the body mass index (BMI), skeletal muscle mass index (SMMI), and fat mass index (FMI). All of these indicators were used to calculate patient outcomes.
BMI was calculated as described by the World Health Organization. We obtained the SMMI by dividing the total skeletal muscle mass (kg) by the height squared (m2). The SMM-to-patient weight ratio was used to calculate the proportion of total weight corresponding to muscle mass, which was normalized by dividing by the square of height. Handgrip strength was assessed using a Jamar Plus+ Digital hand dynamometer (Patterson Medical Supply, Cedarburg, WI, USA). According to the American Association of Hand Therapists, patients held the device and compressed it with maximum force to obtain a maximum contraction. The test was repeated three times for each hand, with one-minute rest intervals between measurements. The highest result of all tests was recorded [47].
The EORTC QLQ-C30 v.3 (validated for Mexican Spanish; Brussels, Belgium) questionnaire was conducted to assess HRQoL. The instrument consists of six multi-item scales (related to patient functioning) and nine single-item scales (describing the severity of cancer-related symptoms). The EORTC QLQ-H&N35 supplementary module for H&NC patients was also used, which consists of 35 questions, 7 multi-item symptom scales, and 11 single-item symptom scales described by the EORTC Scoring Manual [48].
2.3. Statistical Analysis
Statistical analysis was performed with the IBM® SPSS® Statistics version 29 software package (Armonk, NY, USA).
Results are presented as means ± standard deviation (SD) for variables with normal distributions. Non-parametric variables are described as medians (interquartile intervals (IQIs)).
Categorical variables are expressed as numbers and percentages relative to the total. Pearson’s chi-squared tests were performed to assess differences between the two groups (Fisher’s tests if the estimated values were <5), and one-way ANOVA and Kruskal–Wallis tests with Bonferroni correction were used to assess differences between the three groups. Pearson’s correlation or Spearman’s Rho was calculated to determine the relationship, depending on the variable type. Survival analysis was carried out using the Kaplan–Meier method. Analyses were two-sided, and a p value of <0.05 was considered significant. Cronbach’s alpha value was used to determine reliability in the multi-item scales of the EORTC questionnaires.
3. Results
This study included 139 H&NC patients (107 (76.98%) men and 32 (23.02%) women), with a two-year follow-up. Population analysis showed a perspective between sexes across clinical, anthropometric, and biochemical parameters (Table 1). Significant differences in age (p = 0.012), phase angle (p = 0.035), handgrip strength, SMMI, hemoglobin, and total body fat were observed (all with p < 0.001; Table 1). We found differences around the anatomical location of the tumor; in women, a higher percentage presented a tumor in the oral cavity (n = 16; 50%), and for men, the larynx represented the most affected anatomical site (n = 51; 47.7%; p = 0.04).

Table 1.
Comparison of clinical characteristics of women and men with H&NC.
The median age of the 32 women was 59 (44–69) years. The predominant anatomical location was the oral cavity (n = 16; 50%), with histological diagnosis of squamous or epi-dermoid carcinoma (n = 22; 68.8%) and clinical stage IV (n = 14; 43.8%).
The median age of the 107 men was 67 (60–73) years. The predominant anatomical location was the larynx (n = 51; 47.7%), with histological diagnosis of squamous or epidermoid carcinoma (n = 101; 94.4%) and clinical stage IV (n = 49; 45.8%).
3.1. Population Characteristics by Gender
3.1.1. Comparison between Low and Normal PA in Women
We divided the women’s group into low PA (<3.9°) and normal PA (≥3.9°). In both groups, most of the tumors were of squamous histologic variety. In more than 50% of both groups, the tumors were in clinical stages III and IV. Sarcopenia was more common in women with low PA. A proportion of 71.4% of women with low PA were not treated with surgery, and 50% were managed using chemoradiotherapy (Table 2). Tumor location predominance in the oral cavity was observed in women with normal PA (n = 10; 55.6%), without any specific predominance in low-PA women.

Table 2.
Characteristics of the cohort of women and men with H&NC.
3.1.2. Comparison between Low and Normal PA in Men
In men, the cutoff for PA was 4.5, and the most affected anatomical area was the larynx, without specific distribution related to PA values. In both groups, the predominant histologic type was squamous, and the dominant clinical stage was CS IV. Sarcopenia was present in 46.8% of patients with low PA, versus 26.7% for normal-PA men (p = 0.023). Men in the low-PA group were not treated with surgery in 69.4% of cases, compared to 51.1% in normal-PA men (p = 0.043). We did not identify other differences conditioned by PA values in the male group.
3.2. Anthropometrical and Biochemical Indicators
3.2.1. Comparison between Low and Normal PA in Women
In the group of H&NC women, we observed differences in terms of age (64 ± 16.5 versus 51.8 ± 14.8; p = 0.013), handgrip strength (17 ± 4.5 kg/cm2 versus 22.6 kg/cm2; p = 0.024, respectively), four-meter walking speed (0.7 ± 0.3 versus 0.9 ± 0.2 m/seg), phase angle (3.2 ± 0.6° versus 4.6 ± 0.5°; p < 0.001), and SMMI (5.5 ± 2.5 versus 7.1 ± 1.7 kg/m2 BS; p = 0.041) between low and normal PA angle (Table 3).

Table 3.
Anthropometric and biochemical parameters in the cohort of head and neck cancer women and men.
Concerning biochemical markers in low and normal PA H&NC women, C-reactive protein (27.1 (19–48.5) mg/dL versus 10.4 (3.6–27); p = 0.027) and albumin levels (3.8 ± 0.6 versus 4.3 ± 0.3 g/dL; p = 0.011) showed significant differences (Table 3).
3.2.2. Comparison between Low and Normal PA in Men
For H&NC men, the differences observed between low and normal PA relevant in terms of age (69 ± 11 versus 60 ± 10; p < 0.001), handgrip strength (25.3 ± 8 versus 33 ± 7.2; p < 0.001), gait speed (0.75 ± 0.2 versus 0.95 ± 0.2; p > 0.001), phase angle (3.7 ± 0.6 versus 5.2 versus 0.5; p < 0.001), hemoglobin (12.9 ± 2 versus 14.3 ± 1.7 g/dL; p < 0.001), absolute lymphocyte count (1406 (1120–2015) versus 1739 (1311–2515); p = 0.024), albumin (4.1 ± 0.5 versus 4.3 ± 0.3; p < 0.001), and C-reactive protein (16.3 (5–27) versus 12 (3–21); p = 0.022; Table 3).
3.3. Health-Related Quality-of-Life Indicators
3.3.1. Comparison between Low and Normal PA in Women
We found profound alterations in various scales for the EORTC QLQ-C30 and EORTC QLQ-CX24 questionnaires, with significant differences in the scores for global health status/QoL (58.3 versus 75; p = 0.049), physical functioning (50 versus 93.3; p < 0.001), fatigue (61.1 versus 22; p = 0.014), loss of appetite (50 versus 0; p = 0.014), swallowing (33.3 versus 4.2; p = 0.020), trouble with social contact (33.3 versus 0.0; p = 0.030), teeth (100 versus 10.3; p = 0.020), and sticky saliva (100 versus 0.0; p = 0.027) (see Table 4).

Table 4.
Comparison of EORTC QLQ-C30 and EORTC QLQ H&N35 scores between female and male patients with head and neck cancer with low PA and normal PA.
3.3.2. Comparison between Low and Normal PA in Men
In the H&NC men group, the findings of the EORTC QLQ-C30 questionnaire were significant on the scales of global health status/QoL (69.8 versus 83.3; p = 0.006), physical functioning (73.3 versus 93.3; p < 0.001), role functioning (83.3 versus 100; p = 0.032), fatigue (44.4 versus 11.1; p < 0.001), loss of appetite (0.0 (0.0–33.3) versus 0.0 (0.0–0.0); p = 0.013). On the EORTC QLQ-CX24 questionnaire, we observed significant differences in swallowing (25 versus 8.3; p = 0.010), sensory problems (16.7 versus 0.0; p = 0.023), trouble with social eating (19.4 versus 8.3; p = 0.031), mouth opening (23 versus 0.0; p = 0.043), and pain killers (100 versus 0.0; p = 0.022) (see Table 4).
3.4. Survival Status by Sex and by PA
3.4.1. Comparison between Low and Normal PA in Women
In the women’s groups considering low and normal PA, the survival rate showed statistical differences between groups. The women with a low phase angle had 71.4% two-year mortality, whereas for normal phase angle women, the mortality rate was 27.8% (p = 0.046). The mean survival time was shorter (14.25 (95% CI, 7.8–20.7) months) in the low-PA group than in the normal-PA group (30.9 (95% CI, 23.5–38.5) months; p = 0.007, Table 5; Figure 1).

Table 5.
Survival, death, and loss to follow-up.
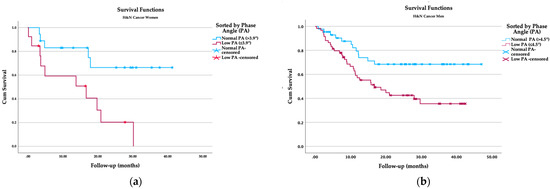
Figure 1.
Kaplan–Meier survival analysis: (a) survival function by phase angle in women; (b) survival function by phase angle in men.
3.4.2. Comparison between Low and Normal PA in Men
For the male group, the mortality rate in patients with PA lower than 4.5° was 53.2%, compared with the group of male patients with a PA over 4.5°, for which the mortality rate was 26.7% (p = 0.022). The survival time was also shorter for patients with low PA (22.5 (18–27) versus 35.2 (29.5–40.8) months; p = 0.008, Table 5; Figure 1).
The survival analysis by sex also reflected significant differences (23.7 (17.6–29.8) versus 28.7 (24.7–32.6) months, p = 0.001).
4. Discussion
The etiology, treatments, and clinical outcomes of oncologic illness are profoundly influenced by several biologic determinants, such as sex, age, physical function, and body composition [49,50]. A previous literature review discussed the evidence for sex dimorphism; men and women presented sex-specific regulation concerning several illness states [49].
In this instance, we discussed the involvement of biological issues and their relationship with development, treatment, and outcomes to comprehend sex-specific variations in cancer-induced gene regulation [49,50].
H&NC patients are affected by skeletal muscle loss. This situation might be caused by sex differences in metabolism, cellular function, immunological response, inflammatory phenomena, and stresses contributing to cachexia. Several mechanisms may be involved in the generation of cellular depletion that condition metabolic, inflammatory, and anthropometrical changes, including muscular atrophy and dynapenia, as demonstrated by handgrip strength (0.79 versus 0.82; p < 0.001) and the presence of sarcopenia (n = 52; 72.7%) in both sexes (Table 1), as well as four-meter walking speed between men with low and normal PA (0.75 versus 0.95; p < 0.001) [49,51]. The comparison of anthropometric and biochemical characteristics between sexes revealed significant disparities. The existence of muscle mass reduction because of SMMI is related not only to malnourishment but could also be the consequence of tumor cachexia; this phenomenon is supported by higher levels of C-reactive protein in men and women with low phase angle.
A scoping review considered data from 76 studies identified through a systematic literature search and published over six years; the prevalence of sarcopenia ranged from 3.8% to 78.7% [52]. Additionally, sarcopenia was found to have a substantial and unfavorable influence on functional, psychosocial, QoL, and survival outcomes in H&NC [52]. In contrast to our results, the prevalence of sarcopenia affects women (n = 11; 34.4%) and men (n = 41; 38.3%) and was significantly associated with handgrip strength (p < 0.001), four-meter walking speed (p < 0.001), and phase angle (p < 0.001) in men and grip strength (p = 0.024), four-meter walking speed (p = 0.004), and phase angle (p < 0.001) in women. Finally, our data revealed that women cancer patients have a higher prevalence of cachexia, muscle wasting, and poorer outcomes than men.
BIA assists in identifying changes in body composition through PA indicators, the contribution of fluids and cell components, and membrane cell integrity in the human body [53]. A decrease in PA indicates cell death or an alteration of cell membrane integrity [53]. Relevant publications demonstrate that PA assists in evaluating the nutritional and health state of cancer patients, supplying essential information that can be used as a prognostic factor [54].
Władysiuk et al. conducted a cohort study in which 75 H&NC presurgical patients were divided into PA < 4.73° and ≥ 4.73°. They observed that the odds of shorter survival were significantly higher in patients with PA < 4.73° compared to the rest of the patients (19.6 months vs. 45 months; p = 0.048) [55].
A study by Yamanaka et al. [56] in Asian patients suggested a 4° PA for women and 4.6° PA for men as a predictive reference value; the low-PA group had a higher risk of poor three-year survival (p = 0.005). Both demonstrated biological behavior similar to the pattern observed in our studied population; however, the cutoff for the Asian study was close to the characteristics that we found in our male and female patients, in which we used reference PA values of <3.9° and ≥3.9° in women and <4.5° and ≥4.5° in men. Women with low PA had a shorter survival time (14.25; (7.8–20.7) months) in comparison to the normal-PA group (30.9 (23.5–38.5)). Similar significant differences were observed in men, with reduced survival reported in the low-PA group (22.5 (18–27)) compared to the normal-PA group (35.2 (29.5–40.8)).
In our study, we found significant differences in low- and normal-PA H&NC women in terms of C-reactive protein (27.1 (19–48.5) mg/dL versus 10.4 (3.6–27); p = 0.027) and albumin levels (3.8 ± 0.6 versus 4.3 ± 0.3 g/dL; p = 0.011), which may be associated with the low PA observed in patients, a marker related to damage to cell membrane integrity, leading to cell death and, therefore, serving as a predictive marker of the functionality and mortality in association with poor outcome and prognosis.
In a cohort study, Harada et al. reviewed the records of 543 patients diagnosed with esophageal squamous cell carcinoma who underwent subtotal esophagectomy, collecting the results of CPR in blood tests performed on postoperative days. They found that CRP levels were highest on day 3. CRP levels after day 3 correlated with major complications, as well as day 7/8 high CRP levels (>3.52), combined with postoperative survival, which was significantly associated with poor prognosis (hazard ratio: 1.67; 95% CI: 1.14–2.43; p = 0.008), proving that CRP has potential prognostic value for patients with this diagnosis after esophagectomy [57].
Novel reports explain that specific biochemical markers emerge as an inflammation reflex. Some consider them valuable tools to evaluate prognosis in patients diagnosed with various malignancies associated with tumor cachexia [56].
Evidence shows that inflammatory response is highly associated with poor outcomes in patients diagnosed with different types of cancer, including H&NC [58], which appears to be a suitable marker for predicting survival in patients diagnosed with oral squamous cell carcinoma [59].
The CAR in Asian patients with oral squamous cell carcinoma was demonstrated to be an excellent prognosis marker. According to our results, we estimated the CAR in men and women according to PA classification. We found significant differences between females and males between low and normal PA groups (0.23 versus 0.66; p = 0.020, and 0.36 versus 0.28; p = 0.015, Table 3) [56].
Kruse et al. postulated that the C-reactive protein might be a relevant sign of chronic inflammation, serving as a prognostic marker for patients with cancer, reinforcing the findings mentioned above [60].
Finally, evaluating QoL allowed us to determine patients’ perception of their conditions as the disease progressed and was treated, as well their functional status affectation [61]. To this end, we used the questionnaires (1) the EORTC QLQ-C30 (30 questions; 6 multi-item scales related to functioning and nine single-item scales describe the severity of symptoms), (2) the EORTC QLQ-H&N35 module for H&NC patients (35 questions; 7 multi-item symptom scales, and 11 single-item symptom scales) questionnaires (validated in Mexican Spanish). The EORTC scoring system leads us to understand the functional symptoms of differential behavior according to sex [62].
The results emphasize that low PA affects QoL and global health. In women, physical function, fatigue, and loss of appetite are coincidental with a low PA and loss of muscle mass, in addition to impacting swallowing, social contact capacity, tooth state, and sticky saliva, all of which are related to functionality in women.
In the group of males, patient perception was a profound functional affectation on global health status, physical, and role functioning, and, similarly to the female group, fatigue, loss of appetite, and swallowing were also affected. According to men’s perception of low PA and loss of muscle mass, other relevant aspects include sensory issues, trouble with social eating, mouth opening, and pain management, reaching higher scores than women.
The role of sex in the perception of QoL is evident; however, there is currently a lack of differentiated information according to sex.
5. Conclusions
The comparison of anthropometric and biochemical characteristics between sexes reveals significant disparities. The existence of muscle mass reduction because of SMMI is more evident in women, but in terms of HRQoL, men appear to be more profoundly affected, especially in terms of the symptom scales.
The results observed in our population are associated with the incidence of malnourishment and tumor cachexia related to an inflammatory state triggered by malignant neoplasms in H&NC patients. PA was used as a significant prognostic, HRQoL, and survival indicator. PA is also linked the CAR, a novel outcome indicator for various diseases based on the systemic inflammatory response of patients with this condition.
Author Contributions
Conceptualization, L.-M.-A.B.-P., L.-M.C.-G., D.S.-M. and B.-E.M.-H.; methodology, L.-M.-A.B.-P., B.-E.M.-H., L.-X.G.-R. and D.S.-M.; software, L.-M.C.-G., L.-L.J.O., M.-G.M.-G. and J.-A.G.-R.; validation, B.T.-H., A.S.-M. and M.-C.V.-F.; formal analysis, L.-X.G.-R., L.-M.C.-G., B.-E.M.-H. and M.-C.V.-F.; investigation, L.-M.C.-G., E.G.-S., M.S.-P. and G.-G.C.-N.; resources, A.-H.N.-Z., M.-C.V.-F. and M.-A.M.-R.; data curation, A.-H.N.-Z., L.-L.J.O., C.-M.N.-G. and M.-A.M.-R.; writing—original draft preparation, L.-M.-A.B.-P., B.-E.M.-H. and D.S.-M.; writing—review and editing, L.-M.-A.B.-P., M.S.-P., G.-G.C.-N., B.-E.M.-H., C.-M.N.-G. and D.S.-M.; visualization, A.-H.N.-Z., M.-G.M.-G., M.-A.M.-R. and M.S.-P.; supervision, L.-M.-A.B.-P., D.S.-M. and B.-E.M.-H.; project administration, L.-M.-A.B.-P., B.-E.M.-H. and D.S.-M.; funding acquisition, L.-M.-A.B.-P., B.-E.M.-H. and D.S.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Universidad de Guadalajara (“Convocatoria Apoyo a la Incorporación de Nuevos Profesores de Tiempo Completo PRODEP SEP 2018”, assigned to Professor number UDG PTC 1388 Luz-Ma.-Adriana Balderas-Peña in document 511-6/18-9169; date: 31 July 2018). All authors supported the APC.
Institutional Review Board Statement
This study was carried out according to the Declaration of Helsinki guidelines and was approved by the Institutional Review Board of the Comité Local de Investigación en Salud 1301, Instituto Mexicano del Seguro Social, México with the project: “Comportamiento de las escalas funcionales de calidad de vida, indicadores de caquexia, leptina sérica y frecuencia del polimorfismo rs12409877 del gen LEPR en pacientes con carcinoma de células escamosas de cabeza y cuello sin sarcopenia versus sarcopenia u obesidad sarcopénica No. R-2019-1301-164”.
Informed Consent Statement
This study was carried out according to the Declaration of Helsinki guidelines and was approved by the Institutional Review Board of the Comite Local de Investigación en Salud 1301, Instituto Mexicano del Seguro Social, Mexico.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available because they are the property of the Instituto Mexicano del Seguro Social. Institutional and federal dispositions restrict unlimited access to personal data, but they are available from the corresponding authors upon reasonable request with prior authorization from the institution.
Conflicts of Interest
The authors declare no conflict of interest with respect to this manuscript, including financial, consultant, institutional, or other relationships that might lead to bias or a conflict of interest, and that the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- World Health Organization. Global Cancer Observatory; International Agency for Research on Cancer: Lyon, France, 2023. [Google Scholar]
- Bray, F.; Ren, J.-S.; Masuyer, E.; Ferlay, J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int. J. Cancer 2013, 132, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Wyss, A.B.; Hashibe, M.; Lee, Y.-C.A.; Chuang, S.-C.; Muscat, J.; Chen, C.; Schwartz, S.M.; Smith, E.; Zhang, Z.-F.; Morgenstern, H.; et al. Smokeless Tobacco Use and the Risk of Head and Neck Cancer: Pooled Analysis of US Studies in the INHANCE Consortium. Am. J. Epidemiol. 2016, 184, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Hashibe, M.; Brennan, P.; Benhamou, S.; Castellsague, X.; Chen, C.; Curado, M.P.; Maso, L.D.; Daudt, A.W.; Fabianova, E.; Wunsch-Filho, V.; et al. Alcohol Drinking in Never Users of Tobacco, Cigarette Smoking in Never Drinkers, and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. J. Natl. Cancer Inst. 2007, 99, 777–789. [Google Scholar] [CrossRef]
- Mohebbi, E.; Hadji, M.; Rashidian, H.; Rezaianzadeh, A.; Marzban, M.; Haghdoost, A.A.; Naghibzadeh Tahami, A.; Moradi, A.; Gholipour, M.; Najafi, F.; et al. Opium use and the risk of head and neck squamous cell carcinoma. Int. J. Cancer 2021, 148, 1066–1076. [Google Scholar] [CrossRef] [PubMed]
- Mahale, P.; Sturgis, E.M.; Tweardy, D.J.; Ariza-Heredia, E.J.; Torres, H.A. Association Between Hepatitis C Virus and Head and Neck Cancers. J. Natl. Cancer Inst. 2016, 108, djw035. [Google Scholar] [CrossRef]
- Dos Santos, E.S.; Pérez-de-Oliveira, M.E.; Normando, A.G.C.; Gueiros, L.A.M.; Rogatto, S.R.; Vargas, P.A.; Lopes, M.A.; Da Silva Guerra, E.N.; Leme, A.F.P.; Santos-Silva, A.R. Systemic conditions associated with increased risk to develop oral squamous cell carcinoma: Systematic review and meta-analysis. Head Neck 2022, 44, 2925–2937. [Google Scholar] [CrossRef]
- Mirza, A.H.; Thomas, G.; Ottensmeier, C.H.; King, E.V. Importance of the immune system in head and neck cancer. Head Neck 2019, 41, 2789–2800. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Head and Neck Carcinoma Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018, 24, 6–13. [Google Scholar] [CrossRef]
- Gomes, E.P.A.d.A.; Aranha, A.M.F.; Borges, A.H.; Volpato, L.E.R. Head and neck cancer patients’ quality of life: Analysis of three instruments. J. Dent. 2019, 21, 31–41. [Google Scholar] [CrossRef]
- Windon, M.J.; D’Souza, G.; Rettig, E.M.; Westra, W.H.; Van Zante, A.; Wang, S.J.; Ryan, W.R.; Mydlarz, W.K.; Ha, P.K.; Miles, B.A.; et al. Increasing prevalence of human papillomavirus-positive oropharyngeal cancers among older adults: HPV-OPSCC Increasing Among Older Adults. Cancer 2018, 124, 2993–2999. [Google Scholar] [CrossRef]
- Fakhry, C.; Westra, W.H.; Wang, S.J.; Van Zante, A.; Zhang, Y.; Rettig, E.; Yin, L.X.; Ryan, W.R.; Ha, P.K.; Wentz, A.; et al. The prognostic role of sex, race, and human papillomavirus in oropharyngeal and nonoropharyngeal head and neck squamous cell cancer: Role of Sex, Race, and HPV in HNSCC Prognosis. Cancer 2017, 123, 1566–1575. [Google Scholar] [CrossRef] [PubMed]
- Sat-Muñoz, D.; Martínez-Herrera, B.-E.; González-Rodríguez, J.-A.; Gutiérrez-Rodríguez, L.-X.; Trujillo-Hernández, B.; Quiroga-Morales, L.-A.; Alcaráz-Wong, A.-A.; Dávalos-Cobián, C.; Solórzano-Meléndez, A.; Flores-Carlos, J.-D.; et al. Phase Angle, a Cornerstone of Outcome in Head and Neck Cancer. Nutrients 2022, 14, 3030. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Agency for Research on Cancer. All Cancers; Cancer Today, Lip, Oral, Cavity and Pharynx; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- De Felice, F.; Locati, L.D.; Ronchi, S.; Thariat, J.; Orlandi, E. Quality of life and financial toxicity after (chemo)radiation therapy in head and neck cancer: Are there any sex- or gender-related differences? Tumori 2022, 108, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Rubin, J.B. The spectrum of sex differences in cancer. Trends Cancer 2022, 8, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Cioffi, G.; Wang, J.; Waite, K.A.; Ostrom, Q.T.; Kruchko, C.; Lathia, J.D.; Rubin, J.B.; Berens, M.E.; Connor, J.; et al. Sex Differences in Cancer Incidence and Survival: A Pan-Cancer Analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1389–1397. [Google Scholar] [CrossRef]
- De Courcy, L.; Bezak, E.; Marcu, L.G. Gender-dependent radiotherapy: The next step in personalised medicine? Crit. Rev. Oncol. Hematol. 2020, 147, 102881. [Google Scholar] [CrossRef]
- Delaney, G.; Jacob, S.; Featherstone, C.; Barton, M. The role of radiotherapy in cancer treatment: Estimating optimal utilization from a review of evidence-based clinical guidelines. Cancer 2005, 104, 1129–1137. [Google Scholar] [CrossRef]
- Golden, E.B.; Chhabra, A.; Chachoua, A.; Adams, S.; Donach, M.; Fenton-Kerimian, M.; Friedman, K.; Ponzo, F.; Babb, J.S.; Goldberg, J.; et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof-of-principle trial. Lancet Oncol. 2015, 16, 795–803. [Google Scholar] [CrossRef]
- Bavrina, A.P.; Monich, V.A.; Malinovskaya, S.L.; Yakovleva, E.I.; Bugrova, M.L.; Lazukin, V.F. Method for Correction of Consequences of Radiation-Induced Heart Disease using Low-Intensity Electromagnetic Emission under Experimental Conditions. Bull. Exp. Biol. Med. 2015, 159, 103–106. [Google Scholar] [CrossRef]
- Singh, A.; Kitpanit, S.; Neal, B.; Yorke, E.; White, C.; Yom, S.K.; Randazzo, J.D.; Wong, R.J.; Huryn, J.M.; Tsai, C.J.; et al. Osteoradionecrosis of the Jaw Following Proton Radiation Therapy for Patients With Head and Neck Cancer. JAMA Otolaryngol. Head Neck Surg. 2023, 149, 151. [Google Scholar] [CrossRef]
- Barnett, G.C.; Coles, C.E.; Elliott, R.M.; Baynes, C.; Luccarini, C.; Conroy, D.; Wilkinson, J.S.; Tyrer, J.; Misra, V.; Platte, R.; et al. Independent validation of genes and polymorphisms reported to be associated with radiation toxicity: A prospective analysis study. Lancet Oncol. 2012, 13, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Benchetrit, L.; Torabi, S.J.; Tate, J.P.; Mehra, S.; Osborn, H.A.; Young, M.R.; Burtness, B.; Judson, B.L. Gender disparities in head and neck cancer chemotherapy clinical trials participation and treatment. Oral Oncol. 2019, 94, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Bodd, M.H.; McCammon, S.D. Laryngeal Cancer and the End of Life (As We Know It). Otolaryngol. Clin. North Am. 2023, 56, 403–412. [Google Scholar] [CrossRef]
- Samuel, S.R.; Maiya, A.G.; Fernandes, D.J.; Guddattu, V.; Saxena, P.P.; Kurian, J.R.; Lin, P.-J.; Mustian, K.M. Effectiveness of exercise-based rehabilitation on functional capacity and quality of life in head and neck cancer patients receiving chemo-radiotherapy. Support Care Cancer 2019, 27, 3913–3920. [Google Scholar] [CrossRef] [PubMed]
- Müller-Richter, U.; Betz, C.; Hartmann, S.; Brands, R.C. Nutrition management for head and neck cancer patients improves clinical outcome and survival. Nutr. Res. 2017, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Navarro Expósito, F.; López González, J.L.; Álvarez-Mon Soto, M. Cáncer de cabeza y cuello. Med. Programa Form. Méd. Contin. Acreditado 2017, 12, 1833–1848. [Google Scholar] [CrossRef]
- León, X.; Pardo, L.; Sansa, A.; Puig, R.; Serrano, C.; López, M.; Quer, M.; Valero, C. Significado pronóstico de los niveles de albúmina previos al tratamiento en los pacientes con carcinoma escamoso de cabeza y cuello. Acta Otorrinolaringológica Española 2020, 71, 204–211. [Google Scholar] [CrossRef]
- Bhat, G.R.; Hyole, R.G.; Li, J. Head and neck cancer: Current challenges and future perspectives. In Advances in Cancer Research; Elsevier: Amsterdam, The Netherlands, 2021; Volume 152, pp. 67–102. ISBN 978-0-12-824125-7. [Google Scholar]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Gupta, D.; Lammersfeld, C.A.; Vashi, P.G.; King, J.; Dahlk, S.L.; Grutsch, J.F.; Lis, C.G. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer 2008, 8, 249. [Google Scholar] [CrossRef]
- Yu, B.; Park, K.B.; Park, J.Y.; Lee, S.S.; Kwon, O.K.; Chung, H.Y. Bioelectrical Impedance Analysis for Prediction of Early Complications after Gastrectomy in Elderly Patients with Gastric Cancer: The Phase Angle Measured Using Bioelectrical Impedance Analysis. J. Gastric Cancer 2019, 19, 278. [Google Scholar] [CrossRef]
- Findlay, M.; White, K.; Brown, C.; Bauer, J.D. Nutritional status and skeletal muscle status in patients with head and neck cancer: Impact on outcomes. J. Cachexia Sarcopenia Muscle 2021, 12, 2187–2198. [Google Scholar] [CrossRef]
- Mazzoccoli, G. Body composition: Where and when. Eur. J. Radiol. 2016, 85, 1456–1460. [Google Scholar] [CrossRef]
- Chasen, M.R.; Bhargava, R. A descriptive review of the factors contributing to nutritional compromise in patients with head and neck cancer. Support Care Cancer 2009, 17, 1345–1351. [Google Scholar] [CrossRef]
- Colín-Ramírez, E.; Castillo-Martínez, L.; Orea-Tejeda, A.; Vázquez-Durán, M.; Rodríguez, A.E.; Keirns-Davis, C. Bioelectrical impedance phase angle as a prognostic marker in chronic heart failure. Nutrition 2012, 28, 901–905. [Google Scholar] [CrossRef]
- Yavuzsen, T.; Davis, M.P.; Ranganathan, V.K.; Walsh, D.; Siemionow, V.; Kirkova, J.; Khoshknabi, D.; Lagman, R.; LeGrand, S.; Yue, G.H. Cancer-Related Fatigue: Central or Peripheral? J. Pain Symptom Manag. 2009, 38, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Silva, M.C.G.; Barros, A.J.; Wang, J.; Heymsfield, S.B.; Pierson, R.N. Bioelectrical impedance analysis: Population reference values for phase angle by age and sex. Am. J. Clin. Nutr. 2005, 82, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C. Evolution of bioimpedance: A circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. Eur. J. Clin. Nutr. 2013, 67, S2–S9. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gómez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.-C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef] [PubMed]
- Lukaski, H.C.; Kyle, U.G.; Kondrup, J. Assessment of adult malnutrition and prognosis with bioelectrical impedance analysis: Phase angle and impedance ratio. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Amano, K.; Bruera, E.; Hui, D. Diagnostic and prognostic utility of phase angle in patients with cancer. Rev. Endocr. Metab. Disord. 2022, 24, 479–489. [Google Scholar] [CrossRef]
- Ward, L.C. Bioelectrical impedance analysis for body composition assessment: Reflections on accuracy, clinical utility, and standardisation. Eur. J. Clin. Nutr. 2019, 73, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Selberg, O.; Selberg, D. Norms and correlates of bioimpedance phase angle in healthy human subjects, hospitalized patients, and patients with liver cirrhosis. Eur. J. Appl. Physiol. 2002, 86, 509–516. [Google Scholar] [CrossRef]
- Rocha, H.; Khouri, L.; Lopes, M.C.; Dias, J.; Ferreira, B. Treatment failure prediction for head-and-neck cancer radiation therapy. Cancer/Radiothérapie 2016, 20, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Fayers, P.; Aaronson, N.K.; Bjordal, K.; Sullivan, M. EORTC QLQ–C30 Scoring Manual; European Organisation for Research and Treatment of Cancer: Brussels, Belgium, 1995. [Google Scholar]
- Montalvo, R.N.; Counts, B.R.; Carson, J.A. Understanding sex differences in the regulation of cancer-induced muscle wasting. Curr. Opin. Support. Palliat. Care 2018, 12, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.R.; Marks, C.; Becker, J.B.; Hurn, P.D.; Chen, W.; Woodruff, T.; McCarthy, M.M.; Sohrabji, F.; Schiebinger, L.; Wetherington, C.L.; et al. Considering sex as a biological variable in preclinical research. FASEB J. 2017, 31, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Zimmers, T.A. Sex Differences in Cancer Cachexia. Curr. Osteoporos. Rep. 2020, 18, 646–654. [Google Scholar] [CrossRef]
- Jovanovic, N.; Chinnery, T.; Mattonen, S.A.; Palma, D.A.; Doyle, P.C.; Theurer, J.A. Sarcopenia in head and neck cancer: A scoping review. PLoS ONE 2022, 17, e0278135. [Google Scholar] [CrossRef]
- Santarpia, L.; Marra, M.; Montagnese, C.; Alfonsi, L.; Pasanisi, F.; Contaldo, F. Prognostic significance of bioelectrical impedance phase angle in advanced cancer: Preliminary observations. Nutrition 2009, 25, 930–931. [Google Scholar] [CrossRef]
- Grundmann, O.; Yoon, S.L.; Williams, J.J. The value of bioelectrical impedance analysis and phase angle in the evaluation of malnutrition and quality of life in cancer patients—A comprehensive review. Eur. J. Clin. Nutr. 2015, 69, 1290–1297. [Google Scholar] [CrossRef]
- Władysiuk, M.S.; Mlak, R.; Morshed, K.; Surtel, W.; Brzozowska, A.; Małecka-Massalska, T. Bioelectrical Impedance Phase Angle as a Prognostic Indicator of Survival in Head-and-Neck Cancer. Curr. Oncol. 2016, 23, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Fukuzawa, S.; Ishibashi-Kanno, N.; Uchida, F.; Bukawa, H. Association between the C-reactive protein/albumin ratio and prognosis in patients with oral squamous cell carcinoma. Sci. Rep. 2021, 11, 5446. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Matsumoto, C.; Toihata, T.; Kosumi, K.; Iwatsuki, M.; Baba, Y.; Ohuchi, M.; Eto, K.; Ogawa, K.; Sawayama, H.; et al. C-Reactive Protein Levels After Esophagectomy are Associated with Increased Surgical Complications and Poor Prognosis in Esophageal Squamous Cell Carcinoma Patients. Ann. Surg. Oncol. 2023, 30, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, F.; Zhang, D.; Qiu, M.; Ren, C.; Jin, Y.; Zhou, Y.; Wang, D.; He, M.; Bai, L.; et al. A novel inflammation-based prognostic score in esophageal squamous cell carcinoma: The C-reactive protein/albumin ratio. BMC Cancer 2015, 15, 350. [Google Scholar] [CrossRef] [PubMed]
- Keinänen, A.; Uittamo, J.; Marinescu-Gava, M.; Kainulainen, S.; Snäll, J. Preoperative C-reactive protein to albumin ratio and oral health in oral squamous cell carcinoma patients. BMC Oral Health 2021, 21, 132. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.L.; Luebbers, H.T.; Grätz, K.W. C-reactive protein levels: A prognostic marker for patients with head and neck cancer? Head Neck Oncol. 2010, 2, 21. [Google Scholar] [CrossRef]
- Carcamo, M.; Campo, V.; Behrmann, D.; Celedón, C.; Alvear, Á.; Vásquez, P.; Araya, C. Cáncer de cabeza y cuello: Validación de cuestionario QLQ-H&N35. Rev. Méd. Chile 2018, 146, 578–584. [Google Scholar] [CrossRef]
- Beck, A.-J.C.C.; Kieffer, J.M.; Retèl, V.P.; van Overveld, L.F.J.; Takes, R.P.; van den Brekel, M.W.M.; van Harten, W.H.; Stuiver, M.M. Mapping the EORTC QLQ-C30 and QLQ-H&N35 to the EQ-5D for head and neck cancer: Can disease-specific utilities be obtained? PLoS ONE 2019, 14, e0226077. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).