Medical Prospect of Melatonin in the Intervertebral Disc Degeneration through Inhibiting M1-Type Macrophage Polarization via SIRT1/Notch Signaling Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Primary Culture of Mouse NP Cells
2.2. Culture of Mouse RAW 264.7 Mφs
2.3. Preparation of CM
2.4. Rat Model of IDD
2.5. Reagents and Antibodies
2.6. MTT Assay
2.7. Flow Cytometry Assay
2.8. Reactive Oxygen Species (ROS), Malondialdehyde (MDA), and Glutathione (GSH) Assays
2.9. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.10. Western Blot
2.11. Immunoprecipitation (IP) Assay
2.12. X-ray Radiograph and Magnetic Resonance Imaging (MRI)
2.13. Histopathologic Analysis
2.14. Immunofluorescence
2.15. Statistical Analysis
3. Results
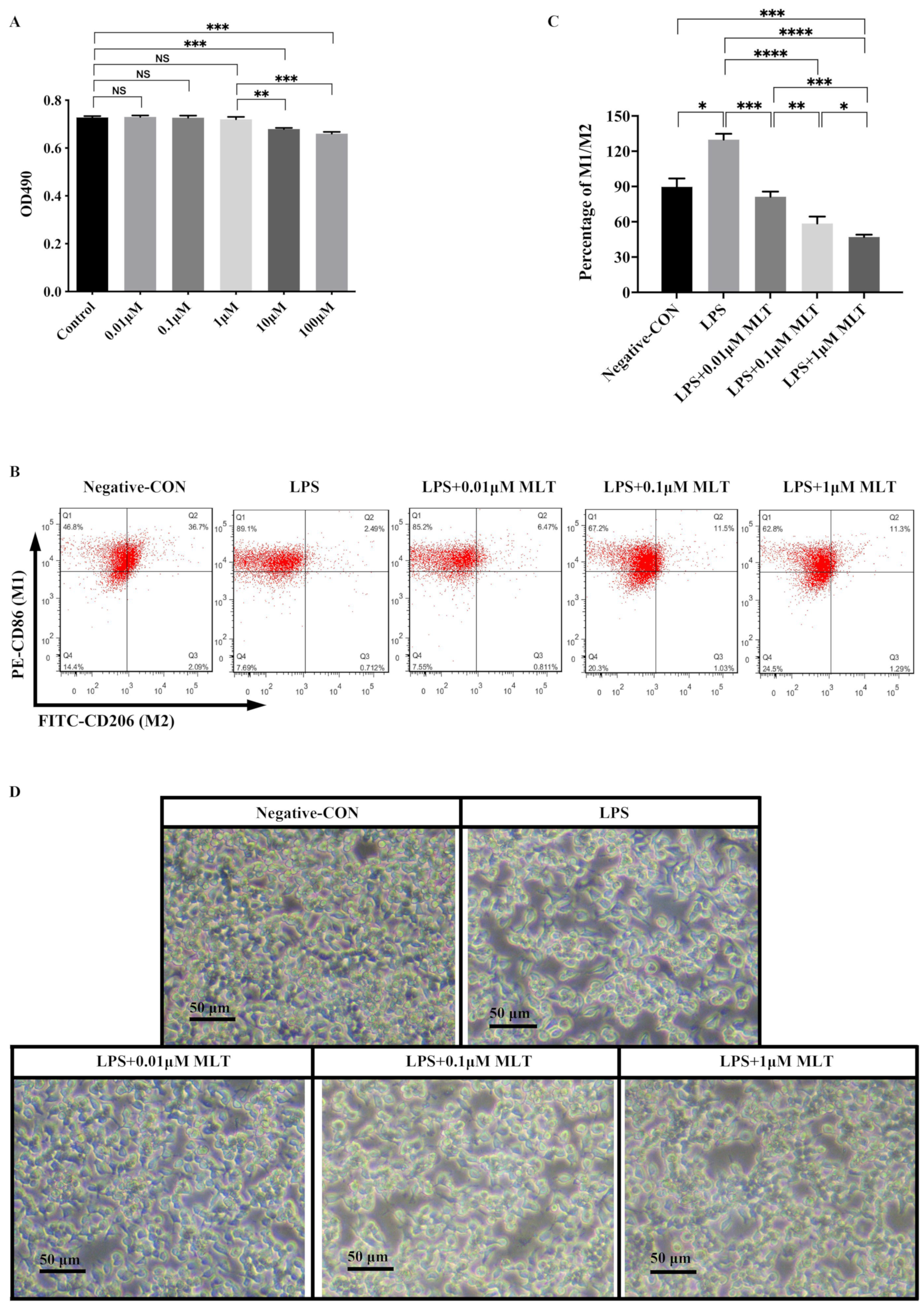
3.1. Low Concentration MLT Displays No Cytotoxicity on RAW 264.7 Mφs
3.2. Inhibition of M1-Type Polarization by MLT in Mφs
3.3. Induction of Cell Injury in NP Cells by M1-Type Mφ Polarization
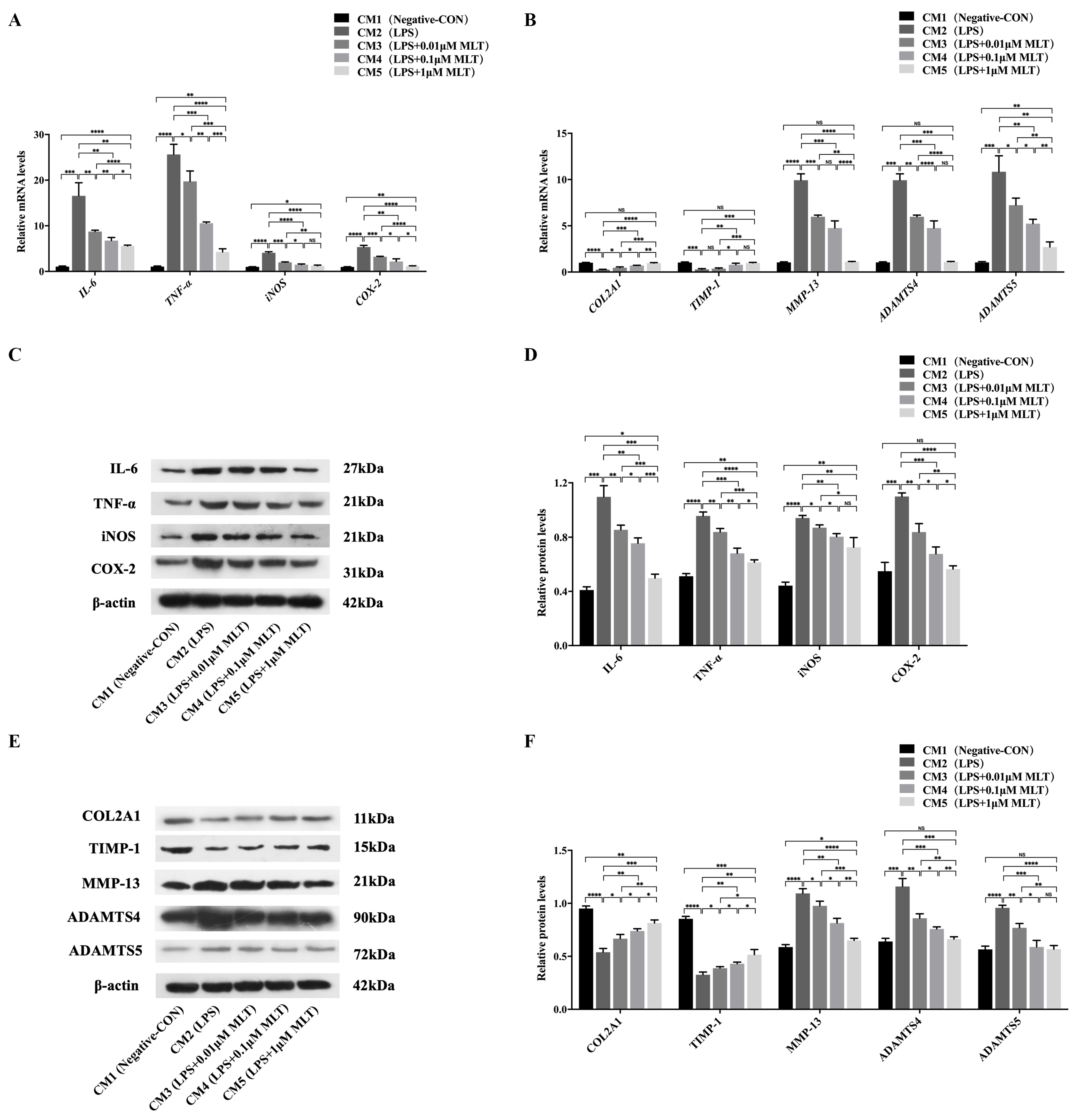
3.4. Protection on NP Cells against Cell Injury by the Treatment with MLT via Inhibiting M1-Type Mφ Polarization
3.5. Regulation of SIRT1/Notch Signal Pathway Contributes to the Effect of MLT on Inhibiting M1-Type Polarization of Mφs
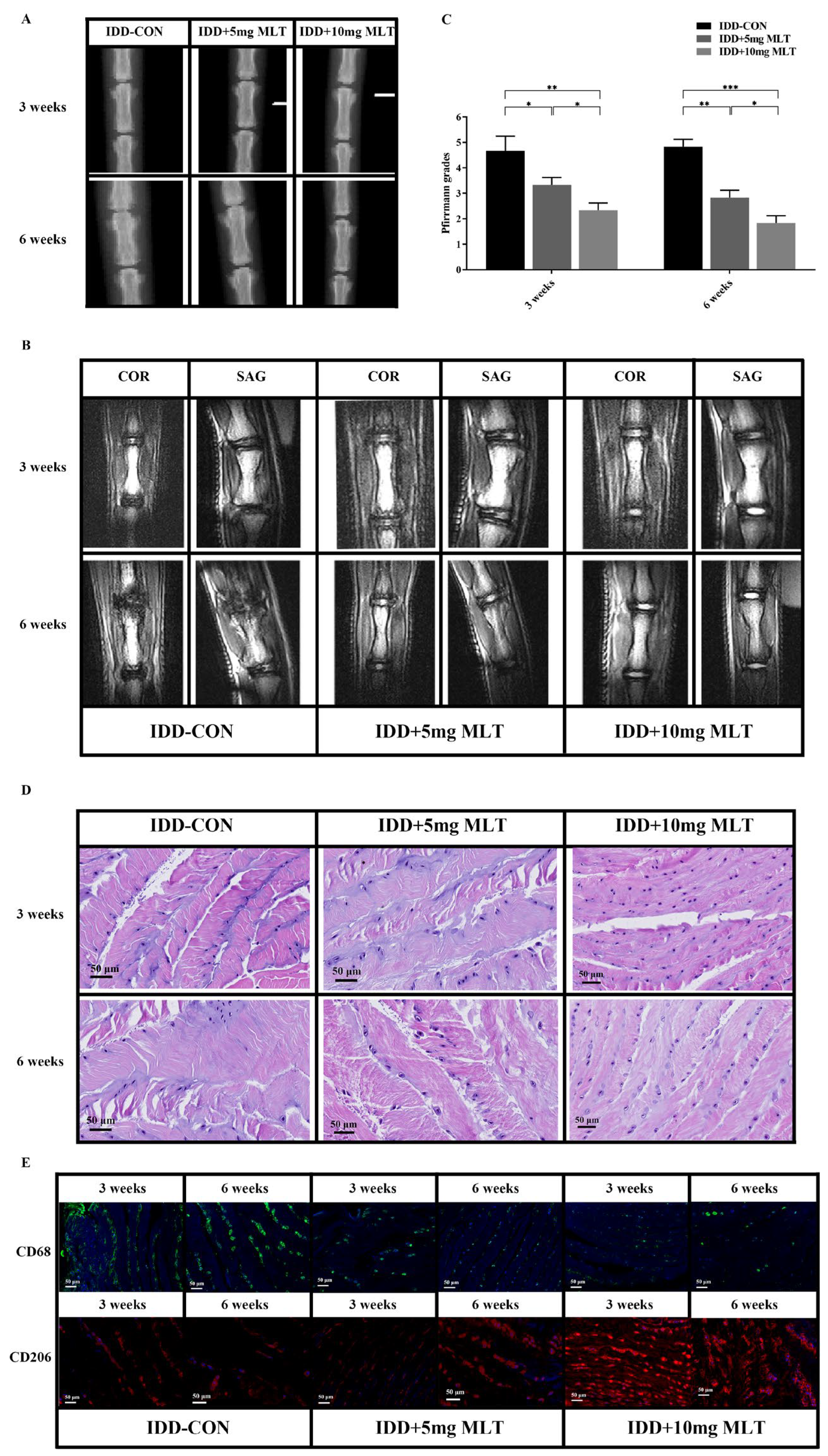
3.6. Evaluation of Therapeutic Effect of MLT on Rat Model of IDD
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sakai, D. Future perspectives of cell-based therapy for intervertebral disc disease. Eur. Spine J. 2008, 17 (Suppl. S4), 452–458. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Liu, H.; Yang, M.; Zhang, Y.; Huang, B.; Zhou, Y. Disc cell senescence in intervertebral disc degeneration: Causes and molecular pathways. Cell Cycle 2016, 15, 1674–1684. [Google Scholar] [CrossRef] [PubMed]
- Oichi, T.; Taniguchi, Y.; Oshima, Y.; Tanaka, S.; Saito, T. Pathomechanism of intervertebral disc degeneration. JOR Spine 2020, 3, e1076. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Hancock, M.J.; Kongsted, A.; Louw, Q.; Ferreira, M.L.; Genevay, S.; Hoy, D.; Karppinen, J.; Pransky, G.; Sieper, J.; et al. What low back pain is and why we need to pay attention. Lancet 2018, 391, 2356–2367. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, S.; Zeng, S.; Zhao, Y.; Zhu, C.; Deng, B.; Zhu, G.; Yin, Y.; Wang, W.; Hardeland, R.; et al. Melatonin in macrophage biology: Current understanding and future perspectives. J. Pineal Res. 2019, 66, e12547. [Google Scholar] [CrossRef]
- Nakazawa, K.R.; Walter, B.A.; Laudier, D.M.; Krishnamoorthy, D.; Mosley, G.E.; Spiller, K.L.; Iatridis, J.C. Accumulation and localization of macrophage phenotypes with human intervertebral disc degeneration. Spine J. 2018, 18, 343–356. [Google Scholar] [CrossRef]
- Yang, H.; Liu, B.; Liu, Y.; He, D.; Xing, Y.; An, Y.; Tian, W. Secreted Factors From Intervertebral Disc Cells and Infiltrating Macrophages Promote Degenerated Intervertebral Disc Catabolism. Spine 2019, 44, E520–E529. [Google Scholar] [CrossRef]
- Takada, T.; Nishida, K.; Doita, M.; Miyamoto, H.; Kurosaka, M. Interleukin-6 production is upregulated by interaction between disc tissue and macrophages. Spine 2004, 29, 1089–1092; discussion 1093. [Google Scholar] [CrossRef]
- Tarocco, A.; Caroccia, N.; Morciano, G.; Wieckowski, M.R.; Ancora, G.; Garani, G.; Pinton, P. Melatonin as a master regulator of cell death and inflammation: Molecular mechanisms and clinical implications for newborn care. Cell Death Dis. 2019, 10, 317. [Google Scholar] [CrossRef]
- Hsu, C.N.; Huang, L.T.; Tain, Y.L. Perinatal Use of Melatonin for Offspring Health: Focus on Cardiovascular and Neurological Diseases. Int. J. Mol. Sci. 2019, 20, 5681. [Google Scholar] [CrossRef]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; Gonzalez-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef]
- Korkmaz, A.; Reiter, R.J.; Topal, T.; Manchester, L.C.; Oter, S.; Tan, D.X. Melatonin: An established antioxidant worthy of use in clinical trials. Mol. Med. 2009, 15, 43–50. [Google Scholar] [CrossRef]
- El-Sokkary, G.H. Melatonin and vitamin C administration ameliorate diazepam-induced oxidative stress and cell proliferation in the liver of rats. Cell Prolif. 2008, 41, 168–176. [Google Scholar] [CrossRef]
- Turgut, M.; Basaloglu, H.K.; Yenisey, C.; Ozsunar, Y. Surgical pinealectomy accelerates intervertebral disc degeneration process in chicken. Eur. Spine J. 2006, 15, 605–612. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.; Shi, H.; Wang, J.; Zheng, Z.; Chen, J.; Chen, X.; Zhang, Z.; Xu, D.; Wang, X.; et al. Melatonin ameliorates intervertebral disc degeneration via the potential mechanisms of mitophagy induction and apoptosis inhibition. J. Cell Mol. Med. 2019, 23, 2136–2148. [Google Scholar] [CrossRef]
- He, R.; Cui, M.; Lin, H.; Zhao, L.; Wang, J.; Chen, S.; Shao, Z. Melatonin resists oxidative stress-induced apoptosis in nucleus pulposus cells. Life Sci. 2018, 199, 122–130. [Google Scholar] [CrossRef]
- Shin, N.R.; Ko, J.W.; Kim, J.C.; Park, G.; Kim, S.H.; Kim, M.S.; Kim, J.S.; Shin, I.S. Role of melatonin as an SIRT1 enhancer in chronic obstructive pulmonary disease induced by cigarette smoke. J. Cell Mol. Med. 2020, 24, 1151–1156. [Google Scholar] [CrossRef]
- Yi, W.J.; Kim, T.S. Melatonin protects mice against stress-induced inflammation through enhancement of M2 macrophage polarization. Int. Immunopharmacol. 2017, 48, 146–158. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and the pathologies of weakened or dysregulated circadian oscillators. J. Pineal Res. 2017, 62. [Google Scholar] [CrossRef]
- Ding, M.; Feng, N.; Tang, D.; Feng, J.; Li, Z.; Jia, M.; Liu, Z.; Gu, X.; Wang, Y.; Fu, F.; et al. Melatonin prevents Drp1-mediated mitochondrial fission in diabetic hearts through SIRT1-PGC1alpha pathway. J. Pineal Res. 2018, 65, e12491. [Google Scholar] [CrossRef]
- Yang, W.; Kang, X.; Qin, N.; Li, F.; Jin, X.; Ma, Z.; Qian, Z.; Wu, S. Melatonin protects chondrocytes from impairment induced by glucocorticoids via NAD(+)-dependent SIRT1. Steroids 2017, 126, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, S.; Dong, Y.; Fan, C.; Zhao, L.; Yang, X.; Li, J.; Di, S.; Yue, L.; Liang, G.; et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015, 58, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Cristofol, R.; Porquet, D.; Corpas, R.; Coto-Montes, A.; Serret, J.; Camins, A.; Pallas, M.; Sanfeliu, C. Neurons from senescence-accelerated SAMP8 mice are protected against frailty by the sirtuin 1 promoting agents melatonin and resveratrol. J. Pineal Res. 2012, 52, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zang, C.; Liu, X.S.; Aster, J.C. The role of Notch receptors in transcriptional regulation. J. Cell Physiol. 2015, 230, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; He, T.; Liu, Y.; Zhang, J.; Li, X.; Shi, J.; Wang, K.; Han, F.; Zhang, W.; Zhang, Y.; et al. Acetylation-Dependent Regulation of Notch Signaling in Macrophages by SIRT1 Affects Sepsis Development. Front. Immunol. 2018, 9, 762. [Google Scholar] [CrossRef]
- Yang, J.J.; Tao, H.; Liu, L.P.; Hu, W.; Deng, Z.Y.; Li, J. miR-200a controls hepatic stellate cell activation and fibrosis via SIRT1/Notch1 signal pathway. Inflamm. Res. 2017, 66, 341–352. [Google Scholar] [CrossRef]
- Shang, Y.; Smith, S.; Hu, X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell 2016, 7, 159–174. [Google Scholar] [CrossRef]
- Noebauer-Huhmann, I.M.; Juras, V.; Pfirrmann, C.W.; Szomolanyi, P.; Zbyn, S.; Messner, A.; Wimmer, J.; Weber, M.; Friedrich, K.M.; Stelzeneder, D.; et al. Sodium MR imaging of the lumbar intervertebral disk at 7 T: Correlation with T2 mapping and modified Pfirrmann score at 3 T—Preliminary results. Radiology 2012, 265, 555–564. [Google Scholar] [CrossRef]
- Ni, L.; Zheng, Y.; Gong, T.; Xiu, C.; Li, K.; Saijilafu; Li, B.; Yang, H.; Chen, J. Proinflammatory macrophages promote degenerative phenotypes in rat nucleus pulpous cells partly through ERK and JNK signaling. J. Cell Physiol. 2019, 234, 5362–5371. [Google Scholar] [CrossRef]
- Risbud, M.V.; Shapiro, I.M. Role of cytokines in intervertebral disc degeneration: Pain and disc content. Nat. Rev. Rheumatol. 2014, 10, 44–56. [Google Scholar] [CrossRef]
- Yamamoto, J.; Maeno, K.; Takada, T.; Kakutani, K.; Yurube, T.; Zhang, Z.; Hirata, H.; Kurakawa, T.; Sakai, D.; Mochida, J.; et al. Fas ligand plays an important role for the production of pro-inflammatory cytokines in intervertebral disc nucleus pulposus cells. J. Orthop Res. 2013, 31, 608–615. [Google Scholar] [CrossRef]
- Markus, R.P.; Fernandes, P.A.; Kinker, G.S.; da Silveira Cruz-Machado, S.; Marcola, M. Immune-pineal axis—acute inflammatory responses coordinate melatonin synthesis by pinealocytes and phagocytes. Br. J. Pharmacol. 2018, 175, 3239–3250. [Google Scholar] [CrossRef]
- Altaf, M.A.; Hao, Y.; Shu, H.; Mumtaz, M.A.; Cheng, S.; Alyemeni, M.N.; Ahmad, P.; Wang, Z. Melatonin enhanced the heavy metal-stress tolerance of pepper by mitigating the oxidative damage and reducing the heavy metal accumulation. J. Hazard. Mater. 2023, 454, 131468. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Y.; Wu, Z.; Wang, H.; Chen, Z.; Wang, J.; Bao, W. Protective effects of melatonin on deoxynivalenol-induced oxidative stress and autophagy in IPEC-J2 cells. Food Chem. Toxicol. 2023, 177, 113803. [Google Scholar] [CrossRef]
- Atacak, A.; Baltaci, S.B.; Akgun-Unal, N.; Mogulkoc, R.; Baltaci, A.K. Melatonin protects retinal tissue damage in streptozotocin-induced aged rats. Arch. Gerontol. Geriatr. 2023, 112, 105035. [Google Scholar] [CrossRef]
- Markowska, M.; Niemczyk, S.; Romejko, K. Melatonin Treatment in Kidney Diseases. Cells 2023, 12, 838. [Google Scholar] [CrossRef]
- Di, W.; Jin, Z.; Lei, W.; Liu, Q.; Yang, W.; Zhang, S.; Lu, C.; Xu, X.; Yang, Y.; Zhao, H. Protection of melatonin treatment and combination with traditional antibiotics against septic myocardial injury. Cell Mol. Biol. Lett. 2023, 28, 35. [Google Scholar] [CrossRef]
- Thangwong, P.; Jearjaroen, P.; Tocharus, C.; Govitrapong, P.; Tocharus, J. Melatonin suppresses inflammation and blood–brain barrier disruption in rats with vascular dementia possibly by activating the SIRT1/PGC-1alpha/PPARgamma signaling pathway. Inflammopharmacology 2023, 31, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Dehpour, A.R.; Shirooie, S.; Silva, A.S.; Baldi, A.; Khan, H.; Daglia, M. Anti-inflammatory effects of Melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 2019, 59, S4–S16. [Google Scholar] [CrossRef]
- Zou, J.; Yang, J.; Chen, B.; Jiang, J.; Liu, J.; Wang, C.; Yu, J.; Peng, Q.; Zeng, J.; Zhang, L.; et al. Melatonin protects against NMDA-induced retinal ganglion cell injury by regulating the microglia-TNFalpha-RGC p38 MAPK pathway. Int. Immunopharmacol. 2023, 118, 109976. [Google Scholar] [CrossRef]
- Dong, L.; Sun, Q.; Qiu, H.; Yang, K.; Xiao, B.; Xia, T.; Wang, A.; Gao, H.; Zhang, S. Melatonin protects against developmental PBDE-47 neurotoxicity by targeting the AMPK/mitophagy axis. J. Pineal Res. 2023, e12871. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Yuan, X.; Cui, Y.Y.; Liu, J.; Shen, J.; Jin, B.Y.; Feng, B.C.; Zhai, Y.J.; Zheng, M.Q.; Kou, G.J.; et al. Melatonin Mitigates Oxazolone-Induced Colitis in Microbiota-Dependent Manner. Front. Immunol. 2021, 12, 783806. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.N.; Wang, P.; Mao, Y.M.; Dan, Y.L.; Wu, Q.; Li, X.M.; Wang, D.G.; Davis, C.; Hu, W.; Pan, H.F. Potential role of melatonin in autoimmune diseases. Cytokine Growth Factor Rev. 2019, 48, 1–10. [Google Scholar] [CrossRef]
- Plaimee, P.; Khamphio, M.; Weerapreeyakul, N.; Barusrux, S.; Johns, N.P. Immunomodulatory effect of melatonin in SK-LU-1 human lung adenocarcinoma cells co-cultured with peripheral blood mononuclear cells. Cell Prolif. 2014, 47, 406–415. [Google Scholar] [CrossRef]
- Cheng, Z.; Xiang, Q.; Wang, J.; Zhang, Y. The potential role of melatonin in retarding intervertebral disc ageing and degeneration: A systematic review. Ageing Res. Rev. 2021, 70, 101394. [Google Scholar] [CrossRef]
- Chen, F.; Jiang, G.; Liu, H.; Li, Z.; Pei, Y.; Wang, H.; Pan, H.; Cui, H.; Long, J.; Wang, J.; et al. Melatonin alleviates intervertebral disc degeneration by disrupting the IL-1beta/NF-kappaB-NLRP3 inflammasome positive feedback loop. Bone Res. 2020, 8, 10. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Chen, C.; Chan, M.T.V.; Wu, W.K.K.; Shen, J. Melatonin inhibits nucleus pulposus (NP) cell proliferation and extracellular matrix (ECM) remodeling via the melatonin membrane receptors mediated PI3K-Akt pathway. J. Pineal Res. 2017, 63, e12435. [Google Scholar] [CrossRef]
- Ding, S.; Lin, N.; Sheng, X.; Zhao, Y.; Su, Y.; Xu, L.; Tong, R.; Yan, Y.; Fu, Y.; He, J.; et al. Melatonin stabilizes rupture-prone vulnerable plaques via regulating macrophage polarization in a nuclear circadian receptor RORalpha-dependent manner. J. Pineal Res. 2019, 67, e12581. [Google Scholar] [CrossRef]
- Minich, D.M.; Henning, M.; Darley, C.; Fahoum, M.; Schuler, C.B.; Frame, J. Is Melatonin the “Next Vitamin D”?: A Review of Emerging Science, Clinical Uses, Safety, and Dietary Supplements. Nutrients 2022, 14, 3934. [Google Scholar] [CrossRef]



| Gene | Forward Primer (5′-→-3′) | Reverse Primer (5′-→-3′) |
|---|---|---|
| IL-4 | CTCGAATGTACCAGGAGCCA | AGTCTCTGCAGCTCCATGAG |
| IL-6 | ATACCACTCCCAACAGACCT | GTTCTTCATGTACTCCAGGT |
| IL-10 | TAACTGCACCCACTTCCCAG | GTCTTCAGCTTCTCACCCAG |
| TNF-a | CCACGCTCTTCTGTCTACTG | CCTTGAAGAGAACCTGGGAG |
| iNOS | CTGGACAAGCTGCATGTGAC | TGGGTCCTCTGGTCAAACTC |
| COX-2 | CATAAGCGAGGACCTGGGTT | TGGCATACATCATCAGACCA |
| Notch1 | TAAGGACCTCAAGGCACGGA | CATCTGACAAGTAGCCATG |
| HES1 | GATCAACGCCATGACCTACC | GTTGGGGATGAGAAAGGCAA |
| HEY2 | GAGCATTGGATTCCGAGAGT | GGGTTGACTCTGATGTGTGG |
| NRARP | CAGCACTACACCAGTCAGTC | ACTTGGCCTTGGTGATGAGA |
| COL2A1 | TGAAGGGTGAGAGTGGTTCC | ACGAGAACCTTGAGCACCTT |
| TIMP-1 | AGACCACCTTATACCAGCGT | GAGAACCTTGAGCACCTTCA |
| MMP-13 | TCTATGATGGCACTGCTGAC | GTTGTAGCCTTTGGAACTG |
| ADAMTS4 | CATCACTGACTTCCTGGACA | CGAAGGTCAGTTGGCATTG |
| ADAMTS5 | GGCAGACGTTGGGACCATAT | TCTGTGATGGTGGCTGACGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, X.; Luo, Q.; Xie, L.; Zhou, X.; Song, C.; Liu, M.; Liu, X.; Ma, Y.; Liu, X. Medical Prospect of Melatonin in the Intervertebral Disc Degeneration through Inhibiting M1-Type Macrophage Polarization via SIRT1/Notch Signaling Pathway. Biomedicines 2023, 11, 1615. https://doi.org/10.3390/biomedicines11061615
Dou X, Luo Q, Xie L, Zhou X, Song C, Liu M, Liu X, Ma Y, Liu X. Medical Prospect of Melatonin in the Intervertebral Disc Degeneration through Inhibiting M1-Type Macrophage Polarization via SIRT1/Notch Signaling Pathway. Biomedicines. 2023; 11(6):1615. https://doi.org/10.3390/biomedicines11061615
Chicago/Turabian StyleDou, Xinyu, Qipeng Luo, Linzhen Xie, Xuchang Zhou, Chunyu Song, Meijuan Liu, Xiao Liu, Yunlong Ma, and Xiaoguang Liu. 2023. "Medical Prospect of Melatonin in the Intervertebral Disc Degeneration through Inhibiting M1-Type Macrophage Polarization via SIRT1/Notch Signaling Pathway" Biomedicines 11, no. 6: 1615. https://doi.org/10.3390/biomedicines11061615
APA StyleDou, X., Luo, Q., Xie, L., Zhou, X., Song, C., Liu, M., Liu, X., Ma, Y., & Liu, X. (2023). Medical Prospect of Melatonin in the Intervertebral Disc Degeneration through Inhibiting M1-Type Macrophage Polarization via SIRT1/Notch Signaling Pathway. Biomedicines, 11(6), 1615. https://doi.org/10.3390/biomedicines11061615







