The Role of Female Reproductive Hormones in the Association between Migraine and Breast Cancer: An Unanswered Question
Abstract
1. Introduction
2. Epidemiological Data: Conflicting Evidence?
3. An Epidemiological Focus on the Role of HR
4. Possible Causes for the Discrepancies among Studies Targeting the Associations between Mi and BC
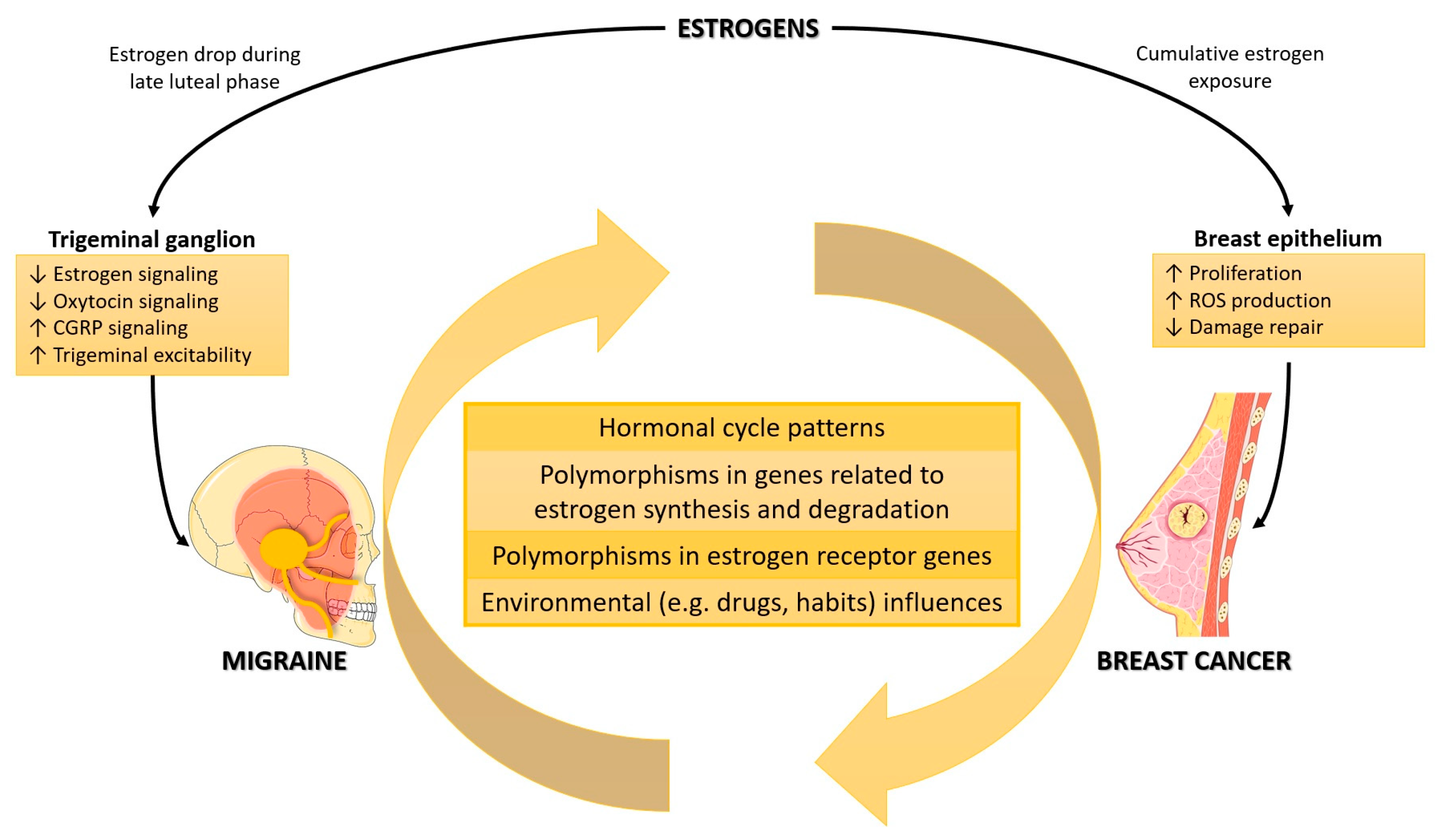
5. Female Reproductive Hormones
5.1. Hormonal Cycle Patterns
5.2. The Association between Polymorphisms in Estrogen Synthesis Pathways and Mi and BC
5.3. The Association between Polymorphisms in Estrogen Catabolism Pathways and Mi and BC
5.4. The Association between Polymorphisms in ER Genes and Mi and BC
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.A.; Schulz, K.F. Cohort studies: Marching towards outcomes. Lancet 2002, 359, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Schettini, F.; Buono, G.; Cardalesi, C.; Desideri, I.; De Placido, S.; Del Mastro, L. Hormone Receptor/Human Epidermal Growth Factor Receptor 2-positive breast cancer: Where we are now and where we are going. Cancer Treat. Rev. 2016, 46, 20–26. [Google Scholar] [CrossRef]
- ESHRE Capri Workshop Group. Hormones and breast cancer. Hum. Reprod. Update 2004, 10, 281–293. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- MacGregor, E.A. Oestrogen and attacks of migraine with and without aura. Lancet Neurol. 2004, 3, 354–361. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Victor, T.W.; Hu, X.; Xiang, Q.; Puenpatom, R.A.; Chen, W.; Campbell, J.C. Characteristics of menstrual vs nonmenstrual migraine: A post hoc, within-woman analysis of the usual-care phase of a nonrandomized menstrual migraine clinical trial. Headache 2010, 50, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Misakian, A.L.; Langer, R.D.; Bensenor, I.M.; Cook, N.R.; Manson, J.E.; Buring, J.E.; Rexrode, K.M. Postmenopausal hormone therapy and migraine headache. J. Womens Health 2003, 12, 1027–1036. [Google Scholar] [CrossRef]
- Mathes, R.W.; Malone, K.E.; Daling, J.R.; Davis, S.; Lucas, S.M.; Porter, P.L.; Li, C.I. Migraine in postmenopausal women and the risk of invasive breast cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3116–3322. [Google Scholar] [CrossRef]
- Li, C.I.; Mathes, R.W.; Malone, K.E.; Daling, J.R.; Bernstein, L.; Marchbanks, P.A.; Strom, B.L.; Simon, M.S.; Press, M.F.; Deapen, D.; et al. Relationship between migraine history and breast cancer risk among premenopausal and postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2030–2034. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A.; Moradi, A.; Gookizadeh, A.; Jokar, S.; Sonbolestan, S.A. Evaluation of relationship between breast cancer and migraine. Adv. Biomed. Res. 2015, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.C.; Rexrode, K.M.; Lee, I.M.; Bring, J.E.; Tamimi, R.M.; Kurth, T. Migraine and subsequent risk of breast cancer: A prospective cohort study. Cancer Causes Control 2013, 24, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.C.; Rice, M.S.; Fortner, R.T.; Eliassen, A.H.; Kurth, T.; Tamimi, R.M. Migraine and breast cancer risk: A prospective cohort study and meta-analysis. J. Natl. Cancer Inst. 2014, 107, 381. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.Y.; Lin, C.S.; Huang, W.Y.; Lin, K.T.; Chao, H.L.; Tsao, C.C.; Liu, M.Y.; Tsai, I.J.; Kao, C.H. Association between Migraine and Breast Cancer Risk: A Population-Based Cohort Study and Literature Review. J. Womens Health 2018, 27, 1499–1507. [Google Scholar] [CrossRef]
- Rezaeian, S.; Veisani, Y.; Ghorbani, M.; Delpisheh, A.; Abbastabar, H. Migraine History and Breast Cancer Risk: A Systematic Review and Meta-Analysis. Adv. Breast Cancer Res. 2015, 4, 63–70. [Google Scholar] [CrossRef]
- Wu, X.; Wang, M.; Li, S.; Zhang, Y. Migraine and breast cancer risk: A meta-analysis of observational studies based on MOOSE compliant. Medicine 2016, 95, e4031. [Google Scholar] [CrossRef]
- Hesari, E.; Ahmadinezhad, M.; Arshadi, M.; Azizi, H.; Khodamoradi, F. The association between migraine and breast cancer risk: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0263628. [Google Scholar] [CrossRef]
- Li, C.I.; Mathes, R.W.; Bluhm, E.C.; Caan, B.; Cavanagh, M.F.; Chlebowski, R.T.; Michael, Y.; O’Sullivan, M.J.; Stefanick, M.L.; Prentice, R. Migraine history and breast cancer risk among postmenopausal women. J. Clin. Oncol. 2010, 28, 1005–1010. [Google Scholar] [CrossRef]
- Lowry, S.J.; Malone, K.E.; Cushing-Haugen, K.L.; Li, C.I. The risk of breast cancer associated with specific patterns of migraine history. Cancer Causes Control 2014, 25, 1707–1715. [Google Scholar] [CrossRef]
- Shi, M.; DeRoo, L.A.; Sandler, D.P.; Weinberg, C.R. Migraine and possible etiologic heterogeneity for hormone-receptor-negative breast cancer. Sci. Rep. 2015, 5, 14943. [Google Scholar] [CrossRef] [PubMed]
- Cupini, L.M.; Corbelli, I.; Sarchelli, P. Menstrual migraine: What it is and does it matter? J. Neurol. 2021, 268, 2355–2363. [Google Scholar] [CrossRef] [PubMed]
- Serrano, D.; Lipton, R.B.; Scher, A.I.; Reed, M.L.; Stewart, W.B.F.; Adams, A.M.; Buse, D.C. Fluctuations in episodic and chronic migraine status over the course of 1 year: Implications for diagnosis, treatment and clinical trial design. J. Headache Pain 2017, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Lipton, R.B. The prognosis of migraine. Curr. Opin. Neurol. 2008, 21, 301–308. [Google Scholar] [CrossRef]
- De Sanctis, R.; Viganò, A.; Pindilli, S.; Torrisi, R.; Santoro, A. A pilot analysis of headache disorders in breast cancer patients. Neurol. Sci. 2022, 43, 3313–3320. [Google Scholar] [CrossRef]
- Ilieva, M.B.; Tiberio, P.; Torrisi, R.; Lanzone, J.; Di Piero, V.; Santoro, A.; Viganò, A.; De Sanctis, R. Profiling the Spectrum of Headache Disorders on 440 Breast Cancer Patients: Highlights on Clinical and Pathological Mechanisms. Biomedicines 2023, 11, 1059. [Google Scholar] [CrossRef]
- Krause, D.N.; Warfvinge, K.; Haanes, K.A.; Edvinsson, L. Hormonal influences in migraine—Interactions of oestrogen, oxytocin and CGRP. Nat. Rev. Neurol. 2021, 17, 621–633. [Google Scholar] [CrossRef]
- Travis, R.C.; Key, T.J. Oestrogen exposure and breast cancer risk. Breast Cancer Res. 2003, 5, 239–247. [Google Scholar] [CrossRef]
- MacGregor, E.A.; Hackshaw, A. Prevalence of migraine on each day of the natural menstrual cycle. Neurology 2004, 63, 351–353. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.T.; Lipton, R.B. Epidemiology and biology of menstrual migraine. Headache 2008, 48, S124–S130. [Google Scholar] [CrossRef]
- Somerville, B.W. The role of estradiol withdrawal in the etiology of menstrual migraine. Neurology 1972, 22, 355–365. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, J.M.; Allshouse, A.A.; Santoro, N.F.; Crawford, S.L.; Thurston, R.C.; Neal-Perry, G.S.; Lipton, R.B.; Derby, C.A. Sex hormones in women with and without migraine: Evidence of migraine-specific hormone profiles. Neurology 2016, 87, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; McCarson, K.E.; Welch, K.M.; Berman, N.E. Mechanisms of pain modulation by sex hormones in migraine. Headache 2011, 51, 905–922. [Google Scholar] [CrossRef] [PubMed]
- Warhurst, S.; Rofe, C.J.; Brew, B.J.; Bateson, D.; McGeechan, K.; Merki-Feld, G.S.; Garrick, R.; Tomlinson, S.E. Effectiveness of the progestin-only pill for migraine treatment in women: A systematic review and meta-analysis. Cephalalgia 2018, 38, 754–764. [Google Scholar] [CrossRef]
- Pogatzki-Zahn, E.M.; Drescher, C.; Englbrecht, J.S.; Klein, T.; Magerl, W.; Zahn, P.K. Progesterone relates to enhanced incisional acute pain and pinprick hyperalgesia in the luteal phase of female volunteers. Pain 2019, 160, 1781–1793. [Google Scholar] [CrossRef]
- Colciago, A.; Bonalume, V.; Melfi, V.; Magnaghi, V. Genomic and Non-genomic Action of Neurosteroids in the Peripheral Nervous System. Front. Neurosci. 2020, 14, 796. [Google Scholar] [CrossRef]
- Coronel, M.F.; Labombarda, F.; González, S.L. Neuroactive steroids, nociception and neuropathic pain: A flashback to go forward. Steroids 2016, 110, 77–87. [Google Scholar] [CrossRef]
- Mattsson, P. Serum levels of androgens and migraine in postmenopausal women. Clin. Sci. 2002, 103, 487–491. [Google Scholar] [CrossRef]
- Apostol, G.; Cady, R.K.; Laforet, G.A.; Robieson, W.Z.; Olson, E.; Abi-Saab, W.M.; Saltarelli, M. Divalproex extended-release in adolescent migraine prophylaxis: Results of a randomized, double-blind, placebo-controlled study. Headache 2008, 48, 1012–1025. [Google Scholar] [CrossRef]
- La Vecchia, C.; Decarli, A.; di Pietro, S.; Franceschi, S.; Negri, E.; Parazzini, F. Menstrual cycle patterns and the risk of breast disease. Eur. J. Cancer Clin. Oncol. 1985, 21, 417–422. [Google Scholar] [CrossRef]
- Parazzini, F.; La Vecchia, C.; Negri, E.; Franceschi, S.; Tozzi, L. Lifelong menstrual pattern and risk of breast cancer. Oncology 1993, 50, 222–225. [Google Scholar] [CrossRef]
- Hunter, D.J.; Spiegelman, D.; Adami, H.O.; van den Brandt, P.A.; Folsom, A.R.; Goldbohm, R.A.; Graham, S.; Howe, G.R.; Kushi, L.H.; Marshall, J.R.; et al. Non-dietary factors as risk factors for breast cancer, and as effect modifiers of the association of fat intake and risk of breast cancer. Cancer Causes Control 1997, 8, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormone replacement therapy: Collaborative reanalysis of data from 51 epidemiological studies of 52,705 women with breast cancer and 108,411 women without breast cancer. Lancet 1997, 350, 1047–1059. [Google Scholar] [CrossRef]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and breastfeeding: Collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50,302 women with breast cancer and 96,973 women without the disease. Lancet 2002, 360, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Collaborative Group on Hormonal Factors in Breast Cancer. Breast cancer and hormonal contraceptives: Collaborative reanalysis of individual data on 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet 1996, 347, 1713–1727. [Google Scholar] [CrossRef]
- Beral, V.; Banks, E.; Reeves, G. Evidence from randomised trials on the long-term effects of hormone replacement therapy. Lancet 2002, 360, 942–944. [Google Scholar] [CrossRef]
- Key, T.; Appleby, P.; Barnes, I.; Reeves, G.; Endogenous Hormones and Breast Cancer Collaborative Group. Endogenous sex hormones and breast cancer in postmenopausal women: Reanalysis of nine prospective studies. J. Natl. Cancer Inst. 2002, 94, 606–616. [Google Scholar] [CrossRef]
- Thomas, H.V.; Key, T.J.; Allen, D.S.; Moore, J.W.; Dowsett, M.; Fentiman, I.S.; Wang, D.Y. A prospective study of endogenous serum hormone concentrations and breast cancer risk in post-menopausal women on the island of Guernsey. Br. J. Cancer 1997, 76, 401–405. [Google Scholar] [CrossRef]
- Pike, M.C.; Spicer, D.V.; Dahmoush, L.; Press, M.F. Estrogens, progestogens, normal breast cell proliferation, and breast cancer risk. Epidemiol. Rev. 1993, 15, 17–35. [Google Scholar] [CrossRef]
- Clevenger, C.V.; Furth, P.A.; Hankinson, S.E.; Schuler, L.A. The role of prolactin in mammary carcinoma. Endocr. Rev. 2003, 24, 1–27. [Google Scholar] [CrossRef]
- Simpson, E.R.; Mahendroo, M.S.; Means, G.D.; Kilgore, M.W.; Hinshelwood, M.M.; Graham-Lorence, S.; Amarneh, B.; Ito, Y.; Fisher, C.R.; Michael, M.D. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994, 15, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.; Joshi, G.; Pradhan, S.; Mittal, B. Potential role of aromatase over estrogen receptor gene polymorphisms in migraine susceptibility: A case control study from North India. PLoS ONE 2012, 7, e34828. [Google Scholar] [CrossRef]
- Alanazi, M.; Alabdulkarim, H.A.; Shaik, J.P.; Al Naeem, A.; Elrobh, M.; Al Amri, A.; Al-Mukaynizi, F.B.; Semlali, A.; Warsy, A.; Parine, N.R. No associations between aromatase gene polymorphisms and breast cancer risk in Saudi patients. OncoTargets Ther. 2015, 8, 2453–2459. [Google Scholar] [CrossRef]
- Boone, S.D.; Baumgartner, K.B.; Baumgartner, R.N.; Connor, A.E.; Pinkston, C.M.; Rai, S.N.; Riley, E.C.; Hines, L.M.; Giuliano, A.R.; John, E.M.; et al. Associations between CYP19A1 polymorphisms, Native American ancestry, and breast cancer risk and mortality: The Breast Cancer Health Disparities Study. Cancer Causes Control 2014, 25, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Healey, C.S.; Dunning, A.M.; Durocher, F.; Teare, D.; Pharoah, P.D.; Luben, R.N.; Easton, D.F.; Ponder, B.A. Polymorphisms in the human aromatase cytochrome P450 gene (CYP19) and breast cancer risk. Carcinogenesis 2000, 21, 189–193. [Google Scholar] [CrossRef]
- Ma, X.; Qi, X.; Chen, C.; Lin, H.; Xiong, H.; Li, Y.; Jiang, J. Association between CYP19 polymorphisms and breast cancer risk: Results from 10,592 cases and 11,720 controls. Breast Cancer Res. Treat. 2010, 122, 495–501. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Dawling, S.; Roodi, N.; Mernaugh, R.L.; Wang, X.; Parl, F.F. Catechol-O-methyltransferase (COMT)-mediated metabolism of catechol estrogens: Comparison of wild-type and variant COMT isoforms. Cancer Res. 2001, 61, 6716–6722. [Google Scholar]
- Kocabaş, N.A.; Sardaş, S.; Cholerton, S.; Daly, A.K.; Karakaya, A.E. Cytochrome P450 CYP1B1 and catechol O-methyltransferase (COMT) genetic polymorphisms and breast cancer susceptibility in a Turkish population. Arch. Toxicol. 2002, 76, 643–649. [Google Scholar] [CrossRef]
- Wen, W.; Cai, Q.; Shu, X.O.; Cheng, J.R.; Parl, F.; Pierce, L.; Gao, Y.T.; Zheng, W. Cytochrome P450 1B1 and catechol-O-methyltransferase genetic polymorphisms and breast cancer risk in Chinese women: Results from the shanghai breast cancer study and a meta-analysis. Cancer Epidemiol. Biomark. Prev. 2005, 14, 329–335. [Google Scholar] [CrossRef]
- Hanna, I.H.; Dawling, S.; Roodi, N.; Guengerich, F.P.; Parl, F.F. Cytochrome P450 1B1 (CYP1B1) pharmacogenetics: Association of polymorphisms with functional differences in estrogen hydroxylation activity. Cancer Res. 2000, 60, 3440–3444. [Google Scholar] [PubMed]
- Qin, X.; Peng, Q.; Qin, A.; Chen, Z.; Lin, L.; Deng, Y.; Xie, L.; Xu, J.; Li, H.; Li, T.; et al. Association of COMT Val158Met polymorphism and breast cancer risk: An updated meta-analysis. Diagn. Pathol. 2012, 7, 136. [Google Scholar] [CrossRef] [PubMed]
- Tammimäki, A.; Männistö, P.T. Catechol-O-methyltransferase gene polymorphism and chronic human pain: A systematic review and meta-analysis. Pharmacogenet. Genom. 2012, 22, 673–691. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, H.G.; Champion, M.; Plays, A.; Stuart, S.; Haupt, L.M.; Frith, A.; MacGregor, E.A.; Griffiths, L.R. Investigation of polymorphisms in genes involved in estrogen metabolism in menstrual migraine. Gene 2017, 607, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Economopoulos, K.P.; Sergentanis, T.N. Three polymorphisms in cytochrome P450 1B1 (CYP1B1) gene and breast cancer risk: A meta-analysis. Breast Cancer Res. Treat. 2010, 122, 545–551. [Google Scholar] [CrossRef]
- Paracchini, V.; Raimondi, S.; Gram, I.T.; Kang, D.; Kocabas, N.A.; Kristensen, V.N.; Li, D.; Parl, F.F.; Rylander-Rudqvist, T.; Soucek, P.; et al. Meta- and pooled analyses of the cytochrome P-450 1B1 Val432Leu polymorphism and breast cancer: A HuGE-GSEC review. Am. J. Epidemiol. 2007, 165, 115–215. [Google Scholar] [CrossRef]
- Osterlund, M.K.; Grandien, K.; Keller, E.; Hurd, Y.L. The human brain has distinct regional expression patterns of estrogen receptor alpha mRNA isoforms derived from alternative promoters. J. Neurochem. 2000, 75, 1390–1397. [Google Scholar] [CrossRef]
- Hewitt, S.C.; Korach, K.S. Estrogen Receptors: New Directions in the New Millennium. Endocr. Rev. 2018, 39, 664–675. [Google Scholar] [CrossRef]
- Levin, E.R. Extranuclear steroid receptors are essential for steroid hormone actions. Annu. Rev. Med. 2015, 66, 271–280. [Google Scholar] [CrossRef]
- Prossnitz, E.R.; Hathaway, H.J. What have we learned about GPER function in physiology and disease from knockout mice? J. Steroid Biochem. Mol. Biol. 2015, 153, 114–126. [Google Scholar] [CrossRef]
- Vanderhorst, V.G.; Gustafsson, J.A.; Ulfhake, B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: Relationships to monoaminergic, cholinergic, and spinal projection systems. J. Comp. Neurol. 2005, 488, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Sandweiss, A.J.; Cottier, K.E.; McIntosh, M.I.; Dussor, G.; Davis, T.P.; Vanderah, T.W.; Largent-Milnes, T.M. 17-β-Estradiol induces spreading depression and pain behavior in alert female rats. Oncotarget 2017, 8, 114109–114122. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Burstein, R. Hypothalamic regulation of headache and migraine. Cephalalgia 2019, 39, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; McKenna, E.; Korach, K.S.; Pfaff, D.W.; Ogawa, S. Estrogen receptor-beta regulates transcript levels for oxytocin and arginine vasopressin in the hypothalamic paraventricular nucleus of male mice. Brain Res. Mol. Brain Res. 2002, 109, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Liu, R.; Dong, Z.; Wang, X.; Yu, S. Impact of ESR1 Gene Polymorphisms on Migraine Susceptibility: A Meta-Analysis. Medicine 2015, 94, e0976. [Google Scholar] [CrossRef]
- Schürks, M.; Rist, P.M.; Kurth, T. Sex hormone receptor gene polymorphisms and migraine: A systematic review and meta-analysis. Cephalalgia 2010, 30, 1306–1328. [Google Scholar] [CrossRef]
- González-Zuloeta Ladd, A.M.; Vásquez, A.A.; Rivadeneira, F.; Siemes, C.; Hofman, A.; Stricker, B.H.; Pols, H.A.; Uitterlinden, A.G.; van Duijn, C.M. Estrogen receptor alpha polymorphisms and postmenopausal breast cancer risk. Breast Cancer Res. Treat. 2008, 107, 415–419. [Google Scholar] [CrossRef][Green Version]
- Al-Amri, R.J.; Alotibi, M.K.H.; Al-Raddadi, R.I.; Shebli, W.T.Y.; Fallatah, E.I.Y.; Alhujaily, A.S.; Mohamed, H.S. Estrogen Receptor 1 Gene (ESR1) rs2234693 Polymorphism and Breast Cancer Risk in Saudi Women. Asian Pac. J. Cancer Prev. 2020, 21, 3235–3240. [Google Scholar] [CrossRef]
- Onland-Moret, N.C.; van Gils, C.H.; Roest, M.; Grobbee, D.E.; Peeters, P.H. The estrogen receptor alpha gene and breast cancer risk (The Netherlands). Cancer Causes Control 2005, 16, 1195–1202. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Yuan, X.; Zhang, Z.; Zhang, P.; Chao, H.; Jiang, L.; Jiang, J. Association between ESR1 PvuII, XbaI, and P325P Polymorphisms and Breast Cancer Susceptibility: A Meta-Analysis. Med. Sci. Monit. 2015, 21, 2986–2996. [Google Scholar] [CrossRef]
- Lukina, S.S.; Burdennyy, A.M.; Zavarykina, T.M.; Riabchikov, D.A.; Kazubskaya, T.P.; Kruglova, M.P.; Loginov, V.I. The Role of ESR1 Gene Polymorphic Markers in the Development of Breast Cancer and Resistance to Tamoxifen Therapy. Bull. Exp. Biol. Med. 2021, 170, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Kallel, I.; Rebai, M.; Rebai, A. Mutations and polymorphisms of estrogens receptors genes and diseases susceptibility. J. Recept. Signal Transduct. Res. 2012, 32, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.A.; Rahman, M.; Harun-Or-Rashid, M.; Hossain, S.; Kasuya, H.; Sakamoto, J.; Hamajima, N. Association of smoked and smokeless tobacco use with migraine: A hospital-based case-control study in Dhaka, Bangladesh. Tob. Induc. Dis. 2013, 11, 15. [Google Scholar] [CrossRef]
- Zlotnik, Y.; Plakht, Y.; Aven, A.; Engel, Y.; Am, N.B.; Ifergane, G. Alcohol consumption and hangover patterns among migraine sufferers. J. Neurosci. Rural Pract. 2014, 5, 128–134. [Google Scholar] [CrossRef]
- Chen, T.C.; Leviton, A.; Edelstein, S.; Ellenberg, J.H. Migraine and other diseases in women of reproductive age. The influence of smoking on observed associations. Arch. Neurol. 1987, 44, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- López-Mesonero, L.; Márquez, S.; Parra, P.; Gámez-Leyva, G.; Muñoz, P.; Pascual, J. Smoking as a precipitating factor for migraine: A survey in medical students. J. Headache Pain 2009, 10, 101–103. [Google Scholar] [CrossRef]
- Schramm, S.H.; Obermann, M.; Katsarava, Z.; Diener, H.C.; Moebus, S.; Yoon, M.S. Epidemiological profiles of patients with chronic migraine and chronic tension-type headache. J. Headache Pain 2013, 14, 40. [Google Scholar] [CrossRef]
- Li, T.; Zhao, J.; Yang, J.; Ma, X.; Dai, Q.; Huang, H.; Wang, L.; Liu, P. A Meta-Analysis of the Association between ESR1 Genetic Variants and the Risk of Breast Cancer. PLoS ONE 2016, 11, e0153314. [Google Scholar] [CrossRef]
| Authors | Title | Year | Type of Study | N. of Participants | Results | Protective Role of Mi on BC |
|---|---|---|---|---|---|---|
| Mathes, R.W., et al. [10] | Migraine in postmenopausal women and the risk of invasive breast cancer | 2008 | Combined data from two population-based case–control | 1199 ductal BC 739 lobular BC 1474 controls | Postmenopausal women presenting a history of Mi had a reduced BC risk, in particular for HR + ductal (OR = 0.67; 95% CI: 0.54–0.82; p < 0.05) and lobular (OR = 0.68; 95% CI: 0.52–0.90; p < 0.05) BC. | Yes |
| Li, C.I., et al. [11] | Relationship between migraine history and breast cancer risk among premenopausal and postmenopausal women | 2009 | Population-based case–control study | 4568 BC 4678 controls | Women with a history of Mi had a reduced risk of BC development (OR = 0.74; 95% CI: 0.66–0.82; p < 0.05), independent from menopausal status, age at diagnosis, use of Mi medications, and ability to avoid Mi triggers. | Yes |
| Ghorbani, A., et al. [12] | Evaluation of the relationship between breast cancer and migraine | 2015 | Case–control study | 400 BC 400 controls | The frequency of Mi and TTH was more prevalent in controls than in BC women (OR = 2.54; 95% CI: 1.78–3.60; p < 0.001; and OR = 2.18; 95% CI: 1.51–3.15; p < 0.001, respectively). | Yes |
| Winter, A.C., et al. [13] | Migraine and subsequent risk of breast cancer: a prospective cohort study | 2013 | Cohort study | 39,696 participants | Mi was not associated with BC risk (either total, in situ, or invasive BC). However, an increase in BC risk was shown in patients with an active diagnosis of MwoA at the time of the study (hazard ratio = 1.21; 95% CI: 1.03–1.43; p < 0.05). | No |
| Winter, A.C., et al. [14] | Migraine and breast cancer risk: a prospective cohort study and meta-analysis | 2014 | Cohort study + meta-analysis | 115,378 participants for cohort study + 11,207 BC 10,818 controls (three case–control studies) 164,190 participants (three cohort studies) for meta-analysis | In the cohort study, Mi was not associated with BC risk (either total, in situ, or invasive BC). In the meta-analysis, an inverse association between Mi and BC risk was shown (pool RR = 0.84; 95% CI: 0.73–0.98; p ≤ 0.001), but the association was present only in case–control studies. | Only in case–control studies |
| Fan, C.Y., et al. [15] | Association between Migraine and Breast Cancer Risk: A Population-Based Cohort Study and Literature Review | 2018 | Population-based cohort study | 25,606 Mi women 102,424 controls (frequency matched 1:4) | Mi was not associated with overall BC risk among Taiwanese women. However, the authors found a positive association between the risk of BC and the annual number of visits for Mi (hazard ratio = 2.00; 95% CI: 1.17–3.41; p = 0.0112) and between the presence of Mi and the number of BC-related examinations (p < 0.0001). | No |
| Rezaeian, S., et al. [16] | Migraine History and Breast Cancer Risk: A Systematic Review and Meta-Analysis | 2015 | Meta-analysis | 7568 BC 6828 controls (four case–control studies) + 130,812 participants (two cohort studies) | Having any Mi history was inversely associated with BC risk (OR = 0.77; 95% CI: 0.64–0.92; p = 0.005). The protective effect persisted even when the analyses were stratified for age at diagnosis, histological type, or HR status. | Yes |
| Wu, X., et al. [17] | Migraine and breast cancer risk: a meta-analysis of observational studies based on MOOSE compliant | 2016 | Meta-analysis | 7568 BC 6828 controls (four case–control studies) + 246,190 participants (three cohort studies) | A statistically significant inverse relationship between Mi and overall BC risk was found (RR = 0.78; 95%CI: 0.66–0.92; p < 0.05). However, the correlation seemed to be study-dependent, detected only in case–control studies and not in cohort ones. | Only in case–control studies |
| Hesari, E., et al. [18] | The association between migraine and breast cancer risk: A systematic review and meta-analysis | 2022 | Meta-analysis | 12,322 BC 11,594 controls (five case–control studies) 426,493 participants (five cohort studies) | The authors found a statistically significant negative correlation between Mi and BC only in case–control studies (RR = 0.68; 95% CI: 0.56–0.82; p = 0.000) and not in cohort ones. Mi also reduced the risk of ductal and lobular carcinomas. | Only in case–control studies |
| Li, C.I., et al. [19] | Migraine history and breast cancer risk among postmenopausal women | 2010 | Cohort study | 91,116 participants | Women with a Mi history showed a lower risk of BC development than those without Mi history (multivariate-adjusted hazard ratio = 0.89; 95% CI: 0.80–0.98; p < 0.05), independently from the use of non-steroidal anti-inflammatory drugs, and specifically in HR+ BC. | Yes |
| Lowry, S.J., et al. [20] | The risk of breast cancer is associated with specific patterns of migraine history; Cancer Causes and Control | 2014 | Population-based case–control study | 715 BC 376 controls | Women with a long history of Mi (RR = 0.4; 95% CI: 0.2–0.6; p < 0.05), those who experienced MwA (RR = 0.7; 95% CI: 0.5–0.98; p < 0.05), and those who had their first Mi before age 20 (RR = 0.5; 95% CI: 0.3–0.9; p < 0.05) had a lower risk of ER+ ductal BC. Women with a long history of Mi (RR = 0.5; 95% CI: 0.3–0.9; p < 0.05), those who experienced MwA (RR = 0.6; 95% CI: 0.4–0.9; p < 0.05), and those who had their first Mi before age 20 (RR = 0.5; 95% CI: 0.3–0.9; p < 0.05) showed a lower risk of ER + lobular BC, too. | Yes |
| Shi, M., et al. [21] | Migraine and possible etiologic heterogeneity for hormone-receptor-negative breast cancer | 2015 | Case-control study (using two different cohorts) | 50,884 women with a first-degree family history of BC 1418 case sisters who had been diagnosed recently with BC | Women with menstrual-related Mi showed a lower risk of developing an HR-BC than women with Mi independent from menses (hazard ratio = 0.63; 95% CI: 0.42–0.96; p = 0.005). The authors also found an inverse association between Mi and ductal carcinoma in situ. | Yes |
| Pathway | SNP | Mi Susceptibility | BC Risk | Ref |
|---|---|---|---|---|
| Estrogen synthesis | CYP19A1 rs4646 | ↓ | - | [52,53,54] |
| Estrogen catabolism | COMT rs4680 | - | - | [60,62,63,64] |
| CYP1B1 rs1056836 | n.d. | ↓ | [66] | |
| Estrogen receptor genes | ESR1 rs2234693 | ↑- | ↑↓- | [52,75,76,77,78,79,80,81] |
| ESR1 rs2228480 | ↑ | ↓ | [75,76,82,88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiberio, P.; Viganò, A.; Ilieva, M.B.; Pindilli, S.; Bianchi, A.; Zambelli, A.; Santoro, A.; De Sanctis, R. The Role of Female Reproductive Hormones in the Association between Migraine and Breast Cancer: An Unanswered Question. Biomedicines 2023, 11, 1613. https://doi.org/10.3390/biomedicines11061613
Tiberio P, Viganò A, Ilieva MB, Pindilli S, Bianchi A, Zambelli A, Santoro A, De Sanctis R. The Role of Female Reproductive Hormones in the Association between Migraine and Breast Cancer: An Unanswered Question. Biomedicines. 2023; 11(6):1613. https://doi.org/10.3390/biomedicines11061613
Chicago/Turabian StyleTiberio, Paola, Alessandro Viganò, Mariya Boyanova Ilieva, Sebastiano Pindilli, Anna Bianchi, Alberto Zambelli, Armando Santoro, and Rita De Sanctis. 2023. "The Role of Female Reproductive Hormones in the Association between Migraine and Breast Cancer: An Unanswered Question" Biomedicines 11, no. 6: 1613. https://doi.org/10.3390/biomedicines11061613
APA StyleTiberio, P., Viganò, A., Ilieva, M. B., Pindilli, S., Bianchi, A., Zambelli, A., Santoro, A., & De Sanctis, R. (2023). The Role of Female Reproductive Hormones in the Association between Migraine and Breast Cancer: An Unanswered Question. Biomedicines, 11(6), 1613. https://doi.org/10.3390/biomedicines11061613







