AMPK Activation as a Protective Mechanism to Restrain Oxidative Stress in the Insulin-Resistant State in Skeletal Muscle of Rat Model of PCOS Subjected to Postnatal Overfeeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Animals
2.2. Assessment of Systemic Insulin Sensitivity
2.3. Preparation of Whole Cell Extracts and Western Blot Analyses
2.4. Isolation of RNA and RT-qPCR
2.5. Measurement of Lipid Peroxidation
2.6. Statistical Analyses
3. Results
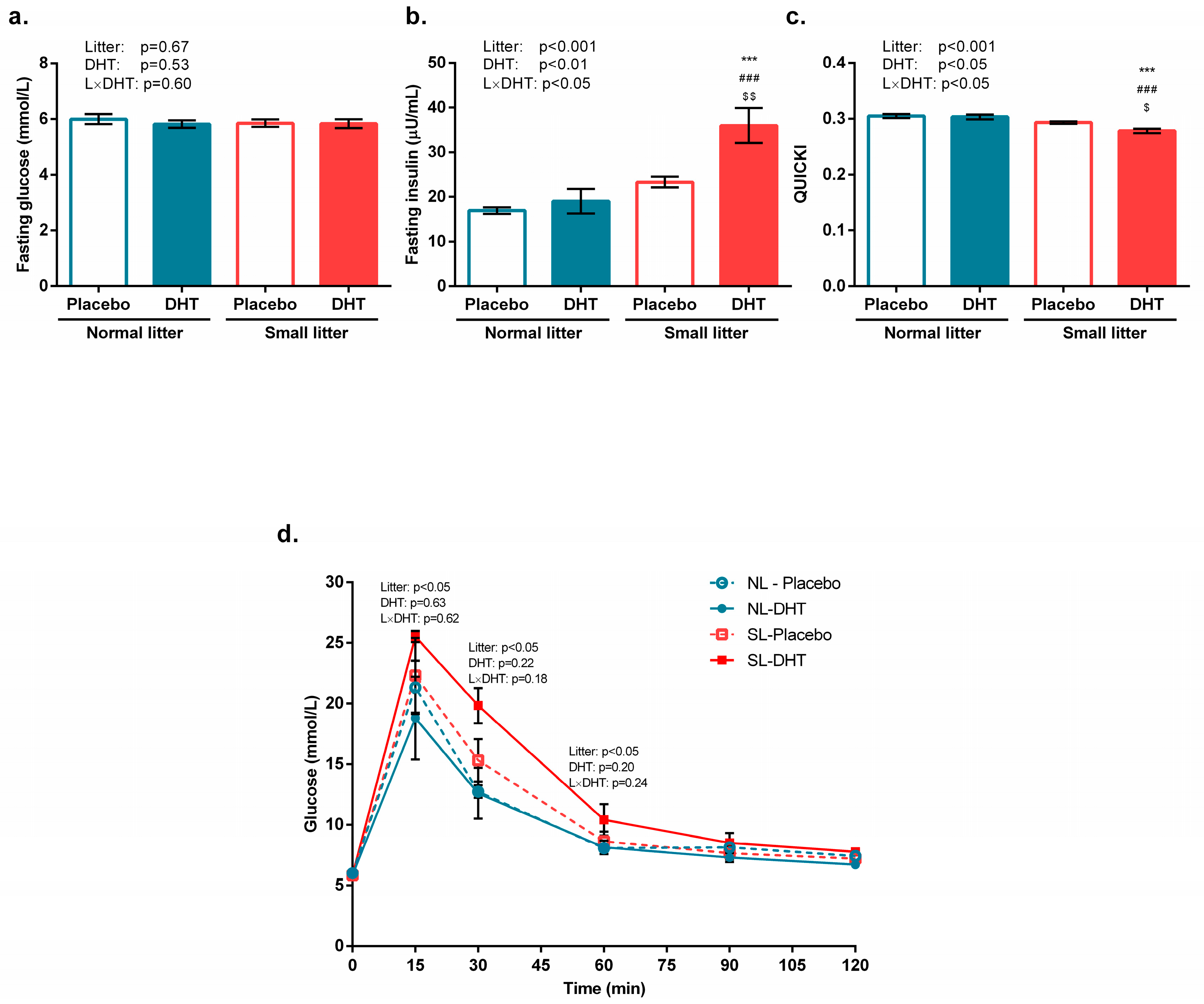
3.1. Parameters of Systemic Insulin Sensitivity
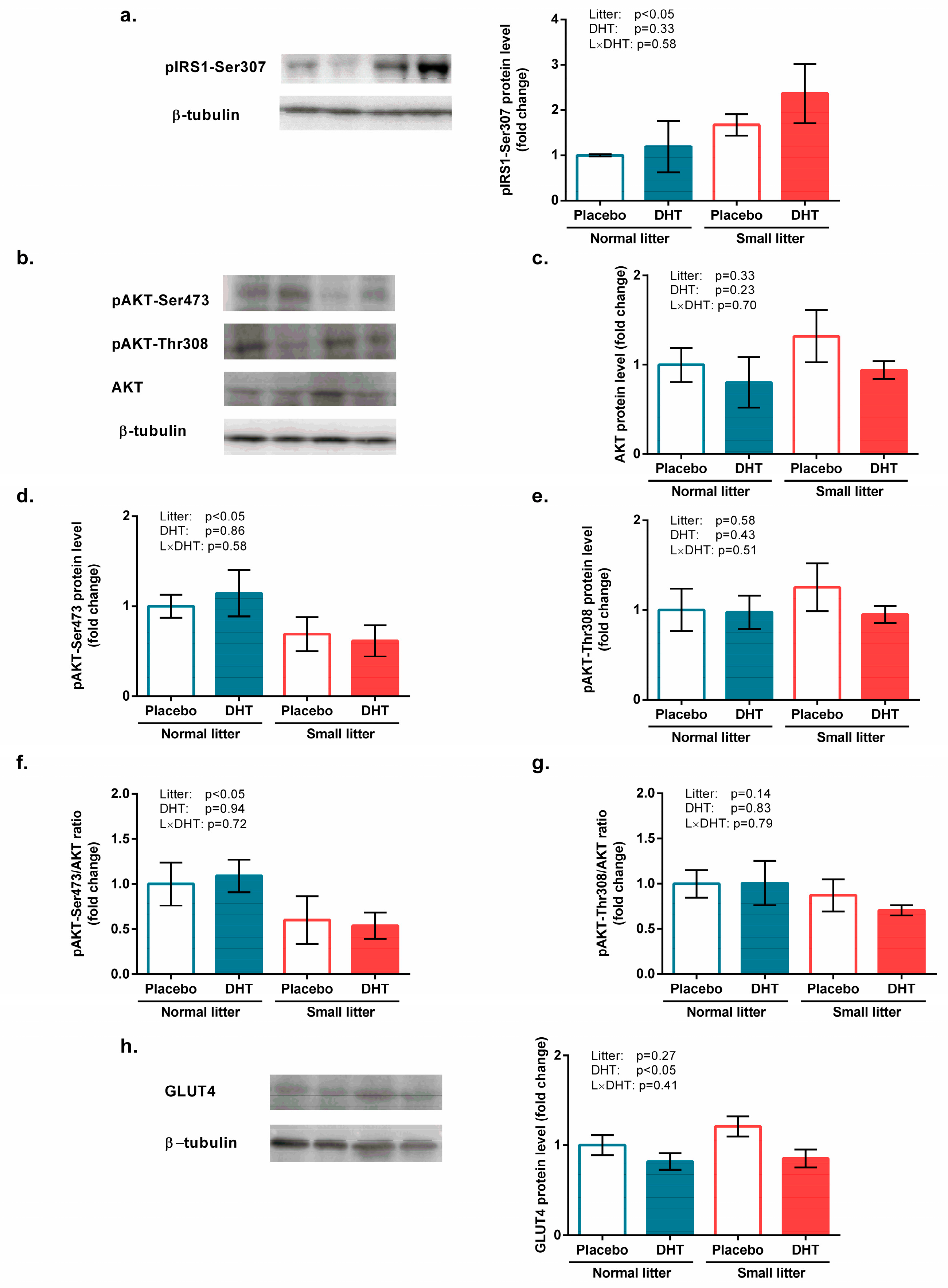
3.2. Insulin Signaling and Glucose Uptake
3.3. Energy Sensing and Lipid Metabolism
3.4. Markers of Oxidative Stress and Inflammation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [CrossRef]
- Stepto, N.K.; Moreno-Asso, A.; Mcilvenna, L.C.; Walters, K.A.; Rodgers, R.J. Molecular Mechanisms of Insulin Resistance in Polycystic Ovary Syndrome: Unraveling the Conundrum in Skeletal Muscle? J. Clin. Endocrinol. Metab. 2019, 104, 5372–5381. [Google Scholar] [CrossRef]
- Conway, G.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Franks, S.; Gambineri, A.; Kelestimur, F.; Macut, D.; Micic, D.; Pasquali, R.; et al. The Polycystic Ovary Syndrome: A Position Statement from the European Society of Endocrinology. Eur. J. Endocrinol. 2014, 171, P1–P29. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Piltonen, T.T.; Giudice, L.C. Endometrial Function in Women with Polycystic Ovary Syndrome: A Comprehensive Review. Hum. Reprod. Update 2018, 27, 584–618. [Google Scholar] [CrossRef] [PubMed]
- Bahri Khomami, M.; Teede, H.J.; Joham, A.E.; Moran, L.J.; Piltonen, T.T.; Boyle, J.A. Clinical Management of Pregnancy in Women with Polycystic Ovary Syndrome: An Expert Opinion. Clin. Endocrinol. 2022, 97, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garrido, M.A.; Tena-Sempere, M. Metabolic Dysfunction in Polycystic Ovary Syndrome: Pathogenic Role of Androgen Excess and Potential Therapeutic Strategies. Mol. Metab. 2020, 35, 100937. [Google Scholar] [CrossRef]
- Franks, S. Polycystic Ovary Syndrome in Adolescents. Int. J. Obes. 2008, 32, 1035–1041. [Google Scholar] [CrossRef]
- McCartney, C.R.; Prendergast, K.A.; Chhabra, S.; Eagleson, C.A.; Yoo, R.; Chang, R.J.; Foster, C.M.; Marshall, J.C. The Association of Obesity and Hyperandrogenemia during the Pubertal Transition in Girls: Obesity as a Potential Factor in the Genesis of Postpubertal Hyperandrogenism. J. Clin. Endocrinol. Metab. 2006, 91, 1714–1722. [Google Scholar] [CrossRef]
- Habbout, A.; Li, N.; Rochette, L.; Vergely, C. Postnatal Overfeeding in Rodents by Litter Size Reduction Induces Major Short- and Long-Term Pathophysiological Consequences. J. Nutr. 2013, 143, 553–562. [Google Scholar] [CrossRef]
- Morrison, J.A.; Friedman, L.A.; Wang, P.; Glueck, C.J. Metabolic Syndrome in Childhood Predicts Adult Metabolic Syndrome and Type 2 Diabetes Mellitus 25 to 30 Years Later. J. Pediatr. 2008, 152, 201–206. [Google Scholar] [CrossRef]
- Voits, M.; Förster, S.; Rödel, S.; Voigt, J.P.; Plagemann, A.; Fink, H. Obesity Induced by Unspecific Early Postnatal Overfeeding in Male and Female Rats: Hypophagic Effect of CCK-8S. Naunyn. Schmiedebergs. Arch. Pharmacol. 1996, 354, 374–378. [Google Scholar] [CrossRef]
- Plagemann, A.; Harder, T.; Rake, A.; Melchior, K.; Rohde, W. Increased Number of Galanin-Neurons in the Paraventricular Hypothalamic Nucleus of Neonatally Overfed Weanling Rats. Brain Res. 1999, 818, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Habbout, A.; Delemasure, S.; Goirand, F.; Guilland, J.C.; Chabod, F.; Sediki, M.; Rochette, L.; Vergely, C. Postnatal Overfeeding in Rats Leads to Moderate Overweight and to Cardiometabolic and Oxidative Alterations in Adulthood. Biochimie 2012, 94, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Puttabyatappa, M.; Cardoso, R.C.; Padmanabhan, V. Effect of Maternal PCOS and PCOS-like Phenotype on the Offspring’s Health. Mol. Cell. Endocrinol. 2016, 435, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Filippou, P.; Homburg, R. Is Foetal Hyperexposure to Androgens a Cause of PCOS? Hum. Reprod. Update 2017, 23, 421–432. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Padmanabhan, V.; Walters, K.A.; Campbell, R.E.; Benrick, A.; Giacobini, P.; Dumesic, D.A.; Abbott, D.H. Animal Models to Understand the Etiology and Pathophysiology of Polycystic Ovary Syndrome. Endocr. Rev. 2020, 41, bnaa010. [Google Scholar] [CrossRef]
- Barber, T.M.; Franks, S. Obesity and Polycystic Ovary Syndrome. Clin. Endocrinol. 2021, 95, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, R.; Oriolo, C. Obesity and Androgens in Women. Front. Horm. Res. 2019, 53, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Anagnostis, P.; Paparodis, R.D.; Bosdou, J.K.; Bothou, C.; Macut, D.; Goulis, D.G.; Livadas, S. Risk of Type 2 Diabetes Mellitus in Polycystic Ovary Syndrome Is Associated with Obesity: A Meta-Analysis of Observational Studies. Endocrine 2021, 74, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Gambineri, A.; Pelusi, C. Sex Hormones, Obesity and Type 2 Diabetes: Is There a Link? Endocr. Connect. 2019, 8, R1–R9. [Google Scholar] [CrossRef]
- Macut, D.; Bjekić-Macut, J.; Rahelić, D.; Doknić, M. Insulin and the Polycystic Ovary Syndrome. Diabetes Res. Clin. Pract. 2017, 130, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Misso, M.L.; Hopkins, W.G.; Shaw, C.S.; Teede, H.J.; Stepto, N.K. Insulin Resistance in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Euglycaemic—Hyperinsulinaemic Clamp Studies. Hum. Reprod. 2016, 31, 2619–2631. [Google Scholar] [CrossRef] [PubMed]
- Stepto, N.K.; Cassar, S.; Joham, A.E.; Hutchison, S.K.; Harrison, C.L.; Goldstein, R.F.; Teede, H.J. Women with Polycystic Ovary Syndrome Have Intrinsic Insulin Resistance on Euglycaemic—Hyperinsulaemic Clamp. Hum. Reprod. 2013, 28, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.E.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic Ovary Syndrome. Nat. Rev. Dis. Prim. 2016, 2, 16057. [Google Scholar] [CrossRef]
- McDonnell, T.; Cussen, L.; McIlroy, M.; O’Reilly, M.W. Characterizing Skeletal Muscle Dysfunction in Women with Polycystic Ovary Syndrome. Ther. Adv. Endocrinol. Metab. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32 (Suppl. S2), S157–S163. [Google Scholar] [CrossRef]
- Silfen, M.E.; Denburg, M.R.; Manibo, A.M.; Lobo, R.A.; Jaffe, R.; Ferin, M.; Levine, L.S.; Oberfield, S.E. Early Endocrine, Metabolic, and Sonographic Characteristics of Polycystic Ovary Syndrome (PCOS): Comparison between Nonobese and Obese Adolescents. J. Clin. Endocrinol. Metab. 2003, 88, 4682–4688. [Google Scholar] [CrossRef]
- Mannerås, L.; Cajander, S.; Holmäng, A.; Seleskovic, Z.; Lystig, T.; Lönn, M.; Stener-Victorin, E. A New Rat Model Exhibiting Both Ovarian and Metabolic Characteristics of Polycystic Ovary Syndrome. Endocrinology 2007, 148, 3781–3791. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Pinola, P.; Lashen, H.; Bloigu, A.; Puukka, K.; Ulmanen, M.; Ruokonen, A.; Martikainen, H.; Pouta, A.; Franks, S.; Hartikainen, A.L.; et al. Menstrual Disorders in Adolescence: A Marker for Hyperandrogenaemia and Increased Metabolic Risks in Later Life? Finnish General Population-Based Birth Cohort Study. Hum. Reprod. 2012, 27, 3279–3286. [Google Scholar] [CrossRef]
- Nikolić, M.; Macut, D.; Djordjevic, A.; Veličković, N.; Nestorović, N.; Bursać, B.; Antić, I.B.; Macut, J.B.; Matić, G.; Vojnović Milutinović, D. Possible Involvement of Glucocorticoids in 5α-Dihydrotestosterone-Induced PCOS-like Metabolic Disturbances in the Rat Visceral Adipose Tissue. Mol. Cell. Endocrinol. 2015, 399, 22–31. [Google Scholar] [CrossRef]
- Walters, K.A. Androgens in Polycystic Ovary Syndrome: Lessons from Experimental Models. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.D.; Solorzano, C.M.B.; McCartney, C.R. Childhood Obesity and Its Impact on the Development of Adolescent PCOS. Semin. Reprod. Med. 2014, 32, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Šefčíková, Z.; Bujňáková, D.; Raček, L.; Kmet, V.; Mozeš, Š. Developmental Changes in Gut Microbiota and Enzyme Activity Predict Obesity Risk in Rats Arising From Reduced Nests. Physiol. Res. 2011, 8408, 337–346. [Google Scholar] [CrossRef]
- Mićić, B.; Teofilović, A.; Djordjevic, A.; Veličković, N.; Macut, D.; Vojnović Milutinović, D. AMPK Activation Is Important for the Preservation of Insulin Sensitivity in Visceral, but Not in Subcutaneous Adipose Tissue of Postnatally Overfed Rat Model of Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2022, 23, 8942. [Google Scholar] [CrossRef] [PubMed]
- Chun, S. Predictive Capability of Fasting-State Glucose and Insulin Measurements for Abnormal Glucose Tolerance in Women with Polycystic Ovary Syndrome. Clin. Exp. Reprod. Med. 2021, 48, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.M.; Emanuelli, B.; Kahn, C.R. Critical Nodes in Signalling Pathways: Insights into Insulin Action. Nat. Rev. 2006, 7, 85–96. [Google Scholar] [CrossRef]
- Shelley, P.; Martin-Gronert, M.S.; Rowlerson, A.; Poston, L.; Heales, S.J.R.; Hargreaves, I.P.; McConnell, J.M.; Ozanne, S.E.; Fernandez-Twinn, D.S. Altered Skeletal Muscle Insulin Signaling and Mitochondrial Complex II-III Linked Activity in Adult Offspring of Obese Mice. Am. J. Physiol.—Regul. Integr. Comp. Physiol. 2009, 297, R675–R681. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.A.; Defronzo, R.A. Pathogenesis of Insulin Resistance in Skeletal Muscle. J. Biomed. Biotechnol. 2010, 2010, 476279. [Google Scholar] [CrossRef]
- Sebastián, D.; Guitart, M.; García-Martínez, C.; Mauvezin, C.; Orellana-Gavaldà, J.M.; Serra, D.; Gómez-Foix, A.M.; Hegardt, F.G.; Asins, G. Novel Role of FATP1 in Mitochondrial Fatty Acid Oxidation in Skeletal Muscle Cells. J. Lipid Res. 2009, 50, 1789–1799. [Google Scholar] [CrossRef]
- Stamatikos, A.D.; Paton, C.M. Role of Stearoyl-CoA Desaturase-1 in Skeletal Muscle Function and Metabolism. Am. J. Physiol.—Endocrinol. Metab. 2013, 305, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyn, P.; Dobrzyn, A.; Miyazaki, M.; Cohen, P.; Asilmaz, E.; Hardie, D.G.; Friedman, J.M.; Ntambi, J.M. Stearoyl-CoA Desaturase 1 Deficiency Increases Fatty Acid Oxidation by Activating AMP-Activated Protein Kinase in Liver. Proc. Natl. Acad. Sci. USA 2004, 101, 6409–6414. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.Z.; Lund, J.; Li, Y.; Knabenes, I.K.; Bakke, S.S.; Kase, E.T.; Lee, Y.K.; Kimmel, A.R.; Thoresen, G.H.; Rustan, A.C.; et al. Loss of Perilipin 2 in Cultured Myotubes Enhances Lipolysis and Redirects the Metabolic Energy Balance from Glucose Oxidation towards Fatty Acid Oxidation. J. Lipid Res. 2017, 58, 2147–2161. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wang, L.; Li, Z.; Zhang, H.; Wu, J.; Rahman, N.; Guo, Y.; Li, D.; Li, N.; et al. Differential Effects of Estrogen/Androgen on the Prevention of Nonalcoholic Fatty Liver Disease in the Male Rat. J. Lipid Res. 2013, 54, 345–357. [Google Scholar] [CrossRef]
- Meex, R.C.R.; Hoy, A.J.; Mason, R.M.; Martin, S.D.; Mcgee, S.L.; Bruce, C.R.; Watt, M.J. ATGL-Mediated Triglyceride Turnover and the Regulation of Mitochondrial Capacity in Skeletal Muscle. Am. J. Endocrinol. Metab. 2015, 308, 960–970. [Google Scholar] [CrossRef]
- Yao, Q.; Zou, X.; Liu, S.; Wu, H.; Shen, Q.; Kang, J. Oxidative Stress as a Contributor to Insulin Resistance in the Skeletal Muscles of Mice with Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2022, 23, 11384. [Google Scholar] [CrossRef]
- Wu, S.B.; Wu, Y.T.; Wu, T.P.; Wei, Y.H. Role of AMPK-Mediated Adaptive Responses in Human Cells with Mitochondrial Dysfunction to Oxidative Stress. Biochim. Biophys. Acta 2014, 1840, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, L.; Li, B.; Jiang, H.; Duan, Y.; Xie, Z. AMP-Activated Protein Kinase (AMPK) Regulates Energy Metabolism through Modulating Thermogenesis in Adipose Tissue. Front. Physiol. 2018, 9, 122. [Google Scholar] [CrossRef]
- Jeon, S.M. Regulation and Function of AMPK in Physiology and Diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Liu, J.; Sun, Z.; Yin, Y.; Mao, Y.; Xu, D.; Liu, L.; Xu, Z.; Guo, Q.; Ding, C.; et al. AMPK-Dependent and -Independent Coordination of Mitochondrial Function and Muscle Fiber Type by FNIP1. PLoS Genet. 2021, 17, e1009488. [Google Scholar] [CrossRef]
- Lee, M.K.S.; Yvan-Charvet, L.; Masters, S.L.; Murphy, A.J. The Modern Interleukin-1 Superfamily: Divergent Roles in Obesity. Semin. Immunol. 2016, 28, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Kistner, T.M.; Pedersen, B.K.; Lieberman, D.E. Interleukin 6 as an Energy Allocator in Muscle Tissue. Nat. Metab. 2022, 4, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; St.-Pierre, J.; Jones, R.G. AMPK Maintains Cellular Metabolic Homeostasis through Regulation of Mitochondrial Reactive Oxygen Species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mićić, B.; Djordjevic, A.; Veličković, N.; Kovačević, S.; Martić, T.; Macut, D.; Vojnović Milutinović, D. AMPK Activation as a Protective Mechanism to Restrain Oxidative Stress in the Insulin-Resistant State in Skeletal Muscle of Rat Model of PCOS Subjected to Postnatal Overfeeding. Biomedicines 2023, 11, 1586. https://doi.org/10.3390/biomedicines11061586
Mićić B, Djordjevic A, Veličković N, Kovačević S, Martić T, Macut D, Vojnović Milutinović D. AMPK Activation as a Protective Mechanism to Restrain Oxidative Stress in the Insulin-Resistant State in Skeletal Muscle of Rat Model of PCOS Subjected to Postnatal Overfeeding. Biomedicines. 2023; 11(6):1586. https://doi.org/10.3390/biomedicines11061586
Chicago/Turabian StyleMićić, Bojana, Ana Djordjevic, Nataša Veličković, Sanja Kovačević, Teodora Martić, Djuro Macut, and Danijela Vojnović Milutinović. 2023. "AMPK Activation as a Protective Mechanism to Restrain Oxidative Stress in the Insulin-Resistant State in Skeletal Muscle of Rat Model of PCOS Subjected to Postnatal Overfeeding" Biomedicines 11, no. 6: 1586. https://doi.org/10.3390/biomedicines11061586
APA StyleMićić, B., Djordjevic, A., Veličković, N., Kovačević, S., Martić, T., Macut, D., & Vojnović Milutinović, D. (2023). AMPK Activation as a Protective Mechanism to Restrain Oxidative Stress in the Insulin-Resistant State in Skeletal Muscle of Rat Model of PCOS Subjected to Postnatal Overfeeding. Biomedicines, 11(6), 1586. https://doi.org/10.3390/biomedicines11061586







