Learning and Investigation of the Role of Angiotensin-Converting Enzyme in Radiotherapy for Nasopharyngeal Carcinoma
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis
2.2. Cell Culture and Transfection
2.3. Cell Viability Assay
2.4. Apoptosis Analysis
2.5. Immunofluorescence Staining
2.6. Alkaline Comet Assay
2.7. Western Blotting
2.8. Flow Cytometry Analysis of Intracellular ROS
2.9. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
2.10. Mitochondrial Membrane Potential Measurement
2.11. X-ray Irradiation
2.12. Statistical Analyses
3. Results
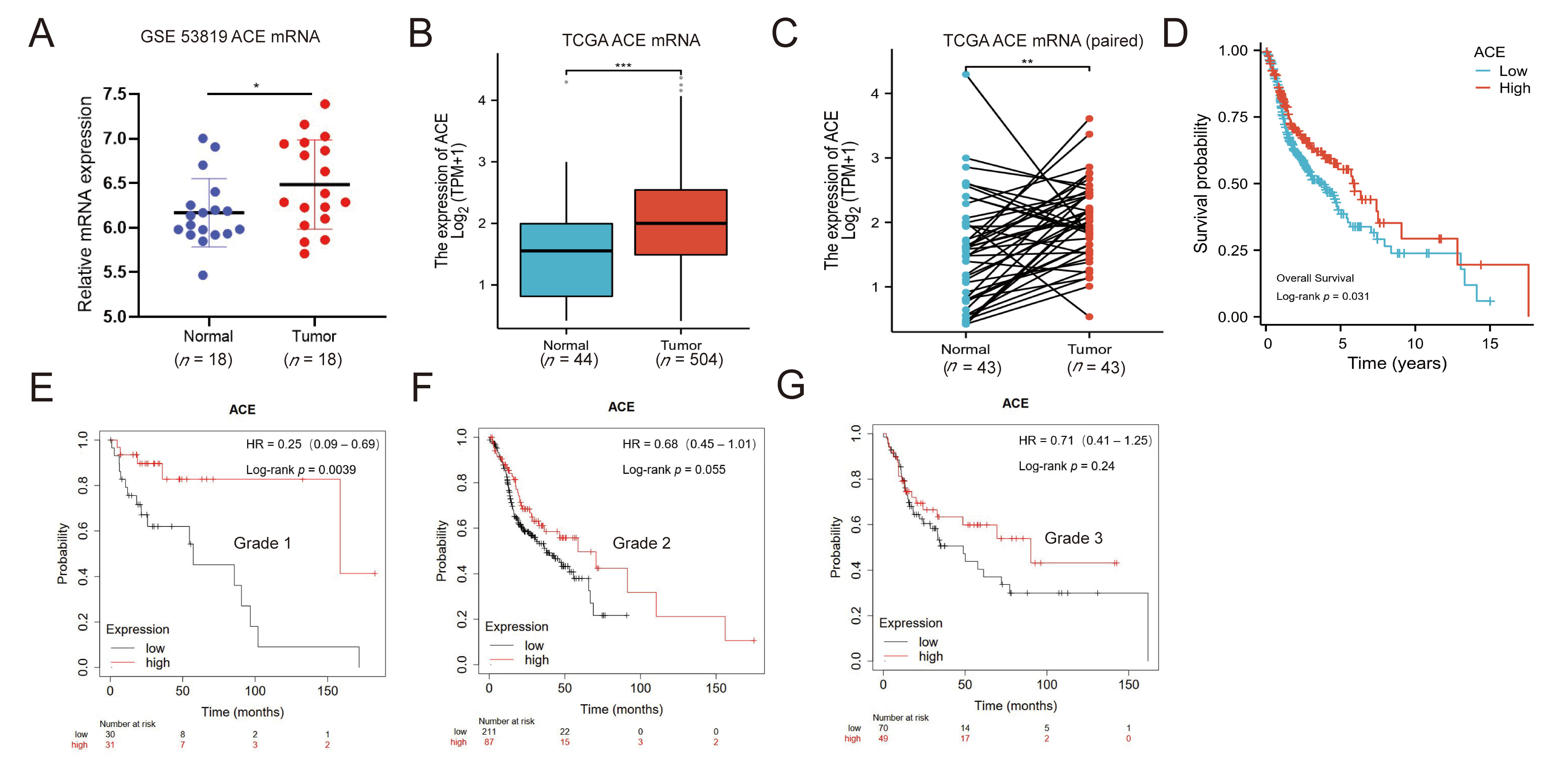
3.1. ACE Expression Is Upregulated in NPC Patients
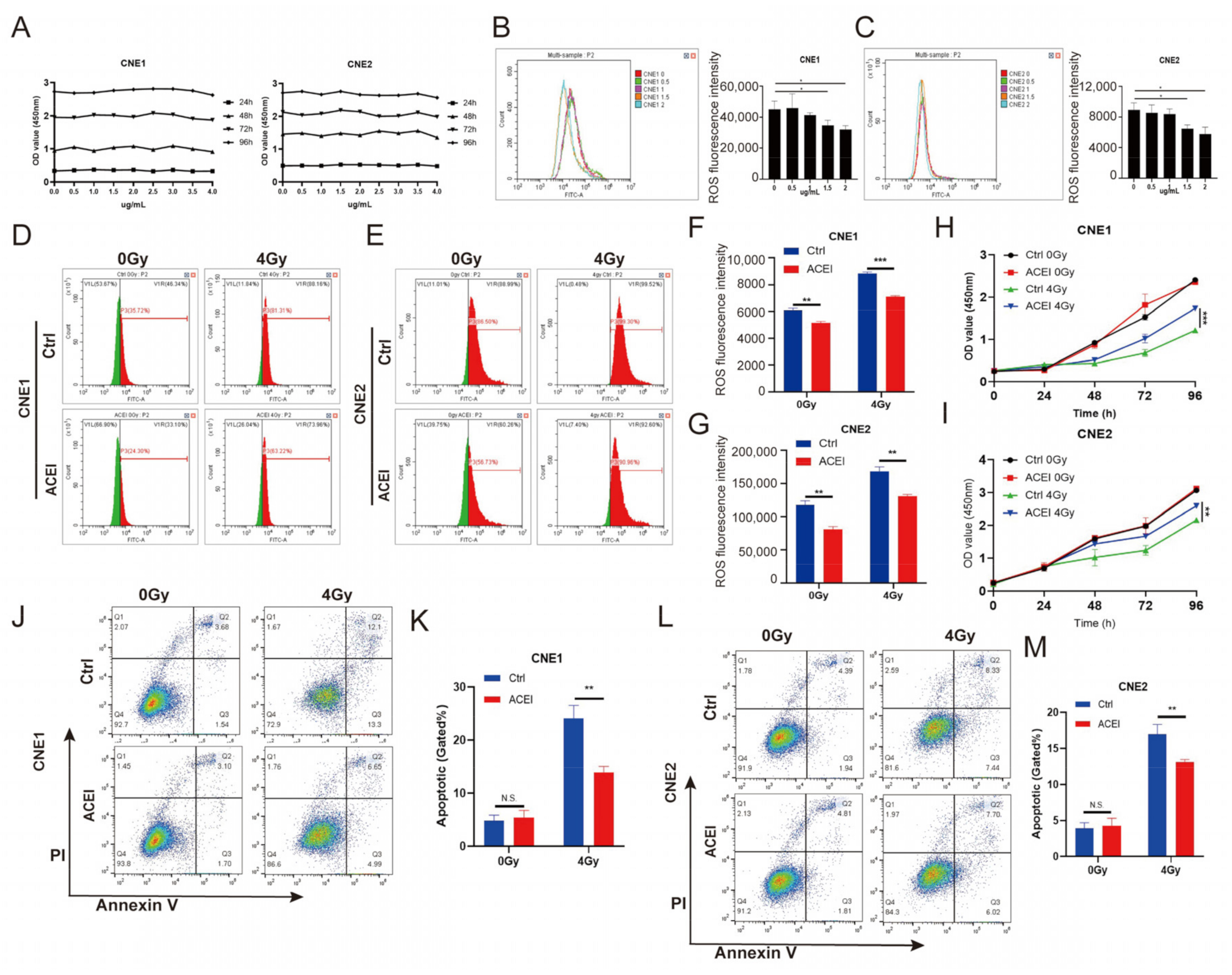
3.2. ACE Knockdown Reduces IR-Induced ROS Levels
3.3. ACE Knockdown Decreases Radiosensitivity in NPC Cells
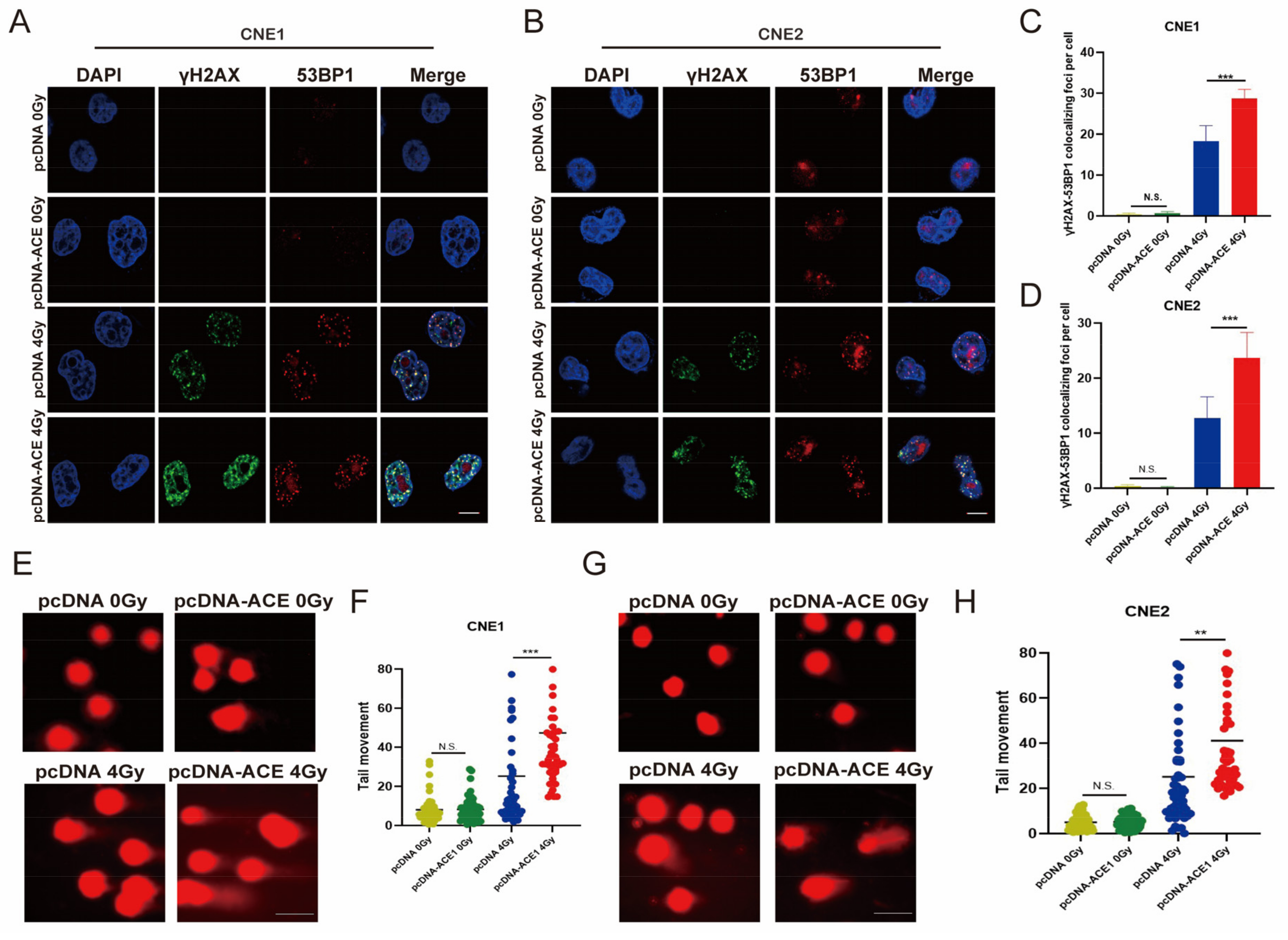
3.4. ACE Knockdown Reduces IR-Induced DNA Damage
3.5. Inhibition of ACE by Enalaprilat Reduces Levels of Endogenous ROS in NPC Cells
3.6. Inhibition of ACE by Enalaprilat Reduces Apoptosis in NPC Cells after IR
3.7. Inhibition of ACE by Enalaprilat Reduces IR-Induced DNA Damage
3.8. Increased Levels of ROS in NPC Cells Due to ACE Overexpression Lead to Enhanced Sensitivity to Radiotherapy
3.9. ACE Overexpression Enhances IR-Induced DNA Damage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bian, Z.; Shi, L.; Kidder, K.; Zen, K.; Garnett-Benson, C.; Liu, Y. Intratumoral SIRPα-deficient macrophages activate tumor antigen-specific cytotoxic T cells under radiotherapy. Nat. Commun. 2021, 12, 3229. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, H.; De Ridder, M. Targeting antioxidant enzymes as a radiosensitizing strategy. Cancer Lett. 2018, 438, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.L.; Dianova, I.I.; Dianov, G.L. APE1-dependent repair of DNA single-strand breaks containing 3′-end 8-oxoguanine. Nucleic Acids Res. 2005, 33, 2204–2209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheung, R.M.; Komaki, R.; Fang, B.; Chang, J.Y. Radiotherapy sensitization by tumor-specific TRAIL gene targeting improves survival of mice bearing human non-small cell lung cancer. Clin. Cancer Res. 2005, 11, 6657–6668. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wijesekera, O.; Salas, S.J.; Wang, J.Y.; Zhu, M.; Aprhys, C.; Chaichana, K.L.; Chesler, D.A.; Zhang, H.; Smith, C.L.; et al. Mesenchymal stem cells from human fat engineered to secrete BMP4 are nononcogenic, suppress brain cancer, and prolong survival. Clin. Cancer Res. 2014, 20, 2375–2387. [Google Scholar] [CrossRef]
- Lu, L.; Dong, J.; Wang, L.; Xia, Q.; Zhang, D.; Kim, H.; Yin, T.; Fan, S.; Shen, Q. Activation of STAT3 and Bcl-2 and reduction of reactive oxygen species (ROS) promote radioresistance in breast cancer and overcome of radioresistance with niclosamide. Oncogene 2018, 37, 5292–5304. [Google Scholar] [CrossRef]
- Diehn, M.; Cho, R.W.; Lobo, N.A.; Kalisky, T.; Dorie, M.J.; Kulp, A.N.; Qian, D.; Lam, J.S.; Ailles, L.E.; Wong, M.; et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature 2009, 458, 780–783. [Google Scholar] [CrossRef]
- Lin, M.Y.; Chang, Y.C.; Wang, S.Y.; Yang, M.H.; Chang, C.H.; Hsiao, M.; Kitsis, R.N.; Lee, Y.J. OncomiR miR-182-5p Enhances Radiosensitivity by Inhibiting the Radiation-Induced Antioxidant Effect through SESN2 in Head and Neck Cancer. Antioxidants 2021, 10, 1808. [Google Scholar] [CrossRef]
- Michaud, A.; Acharya, K.R.; Masuyer, G.; Quenech’du, N.; Gribouval, O.; Morinière, V.; Gubler, M.C.; Corvol, P. Absence of cell surface expression of human ACE leads to perinatal death. Hum. Mol. Genet. 2014, 23, 1479–1491. [Google Scholar] [CrossRef]
- Cao, D.Y.; Saito, S.; Veiras, L.C.; Okwan-Duodu, D.; Bernstein, E.A.; Giani, J.F.; Bernstein, K.E.; Khan, Z. Role of angiotensin-converting enzyme in myeloid cell immune responses. Cell. Mol. Biol. Lett. 2020, 25, 31. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Cai, Y.; Zhang, K.; Zhou, L.; Zhang, Y.; Zhang, X.; Li, Q.; Li, W.; Yang, S.; Zhao, X.; et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: A retrospective observational study. Eur. Heart. J. 2020, 41, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- De Cavanagh, E.M.; Inserra, F.; Ferder, L.; Fraga, C.G. Enalapril and captopril enhance glutathione-dependent antioxidant defenses in mouse tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R572–R577. [Google Scholar] [CrossRef] [PubMed]
- De Cavanagh, E.M.; Fraga, C.G.; Ferder, L.; Inserra, F. Enalapril and captopril enhance antioxidant defenses in mouse tissues. Am. J. Physiol. 1997, 272, R514–R518. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Chan, A.T.C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Chua, M.L.K.; Wee, J.T.S.; Hui, E.P.; Chan, A.T.C. Nasopharyngeal carcinoma. Lancet 2016, 387, 1012–1024. [Google Scholar] [CrossRef]
- Lee, A.W.M.; Ng, W.T.; Chan, J.Y.W.; Corry, J.; Mäkitie, A.; Mendenhall, W.M.; Rinaldo, A.; Rodrigo, J.P.; Saba, N.F.; Strojan, P.; et al. Management of locally recurrent nasopharyngeal carcinoma. Cancer Treat. Rev. 2019, 79, 101890. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, Y.H.; Xu, H.; Yuan, C.S.; Zhou, H.H.; Huang, W.H.; Wang, H.; Zhang, W. LncRNA linc00312 suppresses radiotherapy resistance by targeting DNA-PKcs and impairing DNA damage repair in nasopharyngeal carcinoma. Cell. Death Dis. 2021, 12, 69. [Google Scholar] [CrossRef]
- Wojewodzka-Zelezniakowicz, M.; Gromotowicz-Poplawska, A.; Kisiel, W.; Konarzewska, E.; Szemraj, J.; Ladny, J.R.; Chabielska, E. Angiotensin-converting enzyme inhibitors attenuate propofol-induced pro-oxidative and antifibrinolytic effect in human endothelial cells. J. Renin Angiotensin Aldosterone Syst. 2017, 18, 1470320316687197. [Google Scholar] [CrossRef]
- Mailloux, A.; Deslandes, B.; Vaubourdolle, M.; Baudin, B. Captopril and enalaprilat decrease antioxidant defences in human endothelial cells and are unable to protect against apoptosis. Cell Biol. Int. 2003, 27, 825–830. [Google Scholar] [CrossRef]
- Feng, X.; Weng, D.; Zhou, F.; Owen, Y.D.; Qin, H.; Zhao, J.; WenYu; Huang, Y.; Chen, J.; Fu, H.; et al. Activation of PPARγ by a Natural Flavonoid Modulator, Apigenin Ameliorates Obesity-Related Inflammation Via Regulation of Macrophage Polarization. EBioMedicine 2016, 9, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Jamsen, J.A.; Sassa, A.; Perera, L.; Shock, D.D.; Beard, W.A.; Wilson, S.H. Structural basis for proficient oxidized ribonucleotide insertion in double strand break repair. Nat. Commun. 2021, 12, 5055. [Google Scholar] [CrossRef]
- Ladstätter, S.; Tachibana-Konwalski, K. A Surveillance Mechanism Ensures Repair of DNA Lesions during Zygotic Reprogramming. Cell 2016, 167, 1774–1787.e1713. [Google Scholar] [CrossRef]
- Matsuno, Y.; Atsumi, Y.; Shimizu, A.; Katayama, K.; Fujimori, H.; Hyodo, M.; Minakawa, Y.; Nakatsu, Y.; Kaneko, S.; Hamamoto, R.; et al. Replication stress triggers microsatellite destabilization and hypermutation leading to clonal expansion in vitro. Nat. Commun. 2019, 10, 3925. [Google Scholar] [CrossRef]
- Szikriszt, B.; Póti, Á.; Pipek, O.; Krzystanek, M.; Kanu, N.; Molnár, J.; Ribli, D.; Szeltner, Z.; Tusnády, G.E.; Csabai, I.; et al. A comprehensive survey of the mutagenic impact of common cancer cytotoxics. Genome Biol. 2016, 17, 99. [Google Scholar] [CrossRef]
- Ngo, L.P.; Owiti, N.A.; Swartz, C.; Winters, J.; Su, Y.; Ge, J.; Xiong, A.; Han, J.; Recio, L.; Samson, L.D.; et al. Sensitive CometChip assay for screening potentially carcinogenic DNA adducts by trapping DNA repair intermediates. Nucleic Acids Res. 2020, 48, e13. [Google Scholar] [CrossRef] [PubMed]
- Perini, M.V.; Dmello, R.S.; Nero, T.L.; Chand, A.L. Evaluating the benefits of renin-angiotensin system inhibitors as cancer treatments. Pharmacol. Ther. 2020, 211, 107527. [Google Scholar] [CrossRef] [PubMed]
- Dineen, S.P.; Roland, C.L.; Greer, R.; Carbon, J.G.; Toombs, J.E.; Gupta, P.; Bardeesy, N.; Sun, H.; Williams, N.; Minna, J.D.; et al. Smac mimetic increases chemotherapy response and improves survival in mice with pancreatic cancer. Cancer Res. 2010, 70, 2852–2861. [Google Scholar] [CrossRef]
- Postow, M.A.; Knox, S.J.; Goldman, D.A.; Elhanati, Y.; Mavinkurve, V.; Wong, P.; Halpenny, D.; Reddy, S.K.; Vado, K.; McCabe, D.; et al. A Prospective, Phase 1 Trial of Nivolumab, Ipilimumab, and Radiotherapy in Patients with Advanced Melanoma. Clin. Cancer Res. 2020, 26, 3193–3201. [Google Scholar] [CrossRef]
- Jun, D.W.; Jeong, Y.S.; Kim, H.J.; Jeong, K.C.; Kim, S.; Lee, C.H. Characterization of DDRI-18 (3,3’-(1H,3’H-5,5’-bibenzo[d]imidazole-2,2’-diyl)dianiline), a novel small molecule inhibitor modulating the DNA damage response. Br. J. Pharmacol. 2012, 167, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, T.; Douglass, M.; Williamson, N.H.; Howard, D.; Bhardwaj, R.; Lawrence, M.; Paterson, D.J.; Bezak, E.; Thierry, B.; Kempson, I.M. Cross-Correlative Single-Cell Analysis Reveals Biological Mechanisms of Nanoparticle Radiosensitization. ACS Nano 2019, 13, 5077–5090. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.; Van De Gucht, M.; De Ridder, M. Hypoxic Radioresistance: Can ROS Be the Key to Overcome It? Cancers 2019, 11, 112. [Google Scholar] [CrossRef] [PubMed]
- Banskota, S.; Regmi, S.C.; Kim, J.A. NOX1 to NOX2 switch deactivates AMPK and induces invasive phenotype in colon cancer cells through overexpression of MMP-7. Mol. Cancer 2015, 14, 123. [Google Scholar] [CrossRef]
- van der Reest, J.; Lilla, S.; Zheng, L.; Zanivan, S.; Gottlieb, E. Proteome-wide analysis of cysteine oxidation reveals metabolic sensitivity to redox stress. Nat. Commun. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cao, R.; Wang, X.; Zhang, Y.; Wang, P.; Gao, H.; Li, C.; Yang, F.; Zeng, R.; Wei, P.; et al. Mitochondrial PKM2 regulates oxidative stress-induced apoptosis by stabilizing Bcl2. Cell Res. 2017, 27, 329–351. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Trachootham, D.; Liu, J.; Chen, G.; Pelicano, H.; Garcia-Prieto, C.; Lu, W.; Burger, J.A.; Croce, C.M.; Plunkett, W.; et al. Stromal control of cystine metabolism promotes cancer cell survival in chronic lymphocytic leukaemia. Nat. Cell Biol. 2012, 14, 276–286. [Google Scholar] [CrossRef]
- Tang, D.; Zhao, Y.C.; Liu, H.; Luo, S.; Clarke, J.M.; Glass, C.; Su, L.; Shen, S.; Christiani, D.C.; Gao, W.; et al. Potentially functional genetic variants in PLIN2, SULT2A1 and UGT1A9 genes of the ketone pathway and survival of nonsmall cell lung cancer. Int. J. Cancer 2020, 147, 1559–1570. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, S.J.; Cho, H.J.; Kim, J.T.; Yoon, H.R.; Lee, K.H.; Kim, B.Y.; Lee, Y.; Lee, H.G. Epsilon-Globin HBE1 Enhances Radiotherapy Resistance by Down-Regulating BCL11A in Colorectal Cancer Cells. Cancers 2019, 11, 498. [Google Scholar] [CrossRef]
- Thomas, J.; Smith, H.; Smith, C.A.; Coward, L.; Gorman, G.; De Luca, M.; Jumbo-Lucioni, P. The Angiotensin-Converting Enzyme Inhibitor Lisinopril Mitigates Memory and Motor Deficits in a Drosophila Model of Alzheimer’s Disease. Pathophysiology 2021, 28, 307–319. [Google Scholar] [CrossRef]
- Tanaka, M.; Umemoto, S.; Kawahara, S.; Kubo, M.; Itoh, S.; Umeji, K.; Matsuzaki, M. Angiotensin II type 1 receptor antagonist and angiotensin-converting enzyme inhibitor altered the activation of Cu/Zn-containing superoxide dismutase in the heart of stroke-prone spontaneously hypertensive rats. Hypertens. Res. 2005, 28, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Sahin, B.; Ergul, M. Captopril exhibits protective effects through anti-inflammatory and anti-apoptotic pathways against hydrogen peroxide-induced oxidative stress in C6 glioma cells. Metab. Brain Dis. 2022, 37, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Choi, E.B.; Kim, M.Y.; Choi, K.W. Angiotensin-converting enzyme Ance is cooperatively regulated by Mad and Pannier in Drosophila imaginal discs. Sci. Rep. 2017, 7, 13174. [Google Scholar] [CrossRef]
- Mackey, K.; King, V.J.; Gurley, S.; Kiefer, M.; Liederbauer, E.; Vela, K.; Sonnen, P.; Kansagara, D. Risks and Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin-Receptor Blockers on SARS-CoV-2 Infection in Adults: A Living Systematic Review. Ann. Intern. Med. 2020, 173, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.E.; Wo, J.Y.; Ryan, D.P.; Clark, J.W.; Jiang, W.; Yeap, B.Y.; Drapek, L.C.; Ly, L.; Baglini, C.V.; Blaszkowsky, L.S.; et al. Total Neoadjuvant Therapy with FOLFIRINOX in Combination with Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019, 5, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Z.; Li, P.; Weiss, D.; Fuchs, S.; Xiao, H.D.; Adams, J.A.; Williams, I.R.; Capecchi, M.R.; Taylor, W.R.; Bernstein, K.E. Mice with enhanced macrophage angiotensin-converting enzyme are resistant to melanoma. Am. J. Pathol. 2007, 170, 2122–2134. [Google Scholar] [CrossRef]
- Harashima, N.; Minami, T.; Uemura, H.; Harada, M. Transfection of poly(I:C) can induce reactive oxygen species-triggered apoptosis and interferon-β-mediated growth arrest in human renal cell carcinoma cells via innate adjuvant receptors and the 2-5A system. Mol. Cancer 2014, 13, 217. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Xiu, H.; Zhang, Y.; Ke, M.; Lin, L.; Yan, H.; Hu, P.; Xiao, M.; He, X.; Zhang, T. Learning and Investigation of the Role of Angiotensin-Converting Enzyme in Radiotherapy for Nasopharyngeal Carcinoma. Biomedicines 2023, 11, 1581. https://doi.org/10.3390/biomedicines11061581
Ding Y, Xiu H, Zhang Y, Ke M, Lin L, Yan H, Hu P, Xiao M, He X, Zhang T. Learning and Investigation of the Role of Angiotensin-Converting Enzyme in Radiotherapy for Nasopharyngeal Carcinoma. Biomedicines. 2023; 11(6):1581. https://doi.org/10.3390/biomedicines11061581
Chicago/Turabian StyleDing, Yanan, Huanhuan Xiu, Yanling Zhang, Miaola Ke, Letao Lin, Huzheng Yan, Pan Hu, Meigui Xiao, Xu He, and Tao Zhang. 2023. "Learning and Investigation of the Role of Angiotensin-Converting Enzyme in Radiotherapy for Nasopharyngeal Carcinoma" Biomedicines 11, no. 6: 1581. https://doi.org/10.3390/biomedicines11061581
APA StyleDing, Y., Xiu, H., Zhang, Y., Ke, M., Lin, L., Yan, H., Hu, P., Xiao, M., He, X., & Zhang, T. (2023). Learning and Investigation of the Role of Angiotensin-Converting Enzyme in Radiotherapy for Nasopharyngeal Carcinoma. Biomedicines, 11(6), 1581. https://doi.org/10.3390/biomedicines11061581





