Cognitive and Neural Mechanisms of Behavior Therapy for Tics: A Perception–Action Integration Approach
Abstract
1. Introduction
2. Behavioral Therapy Approaches in the Treatment of GTS
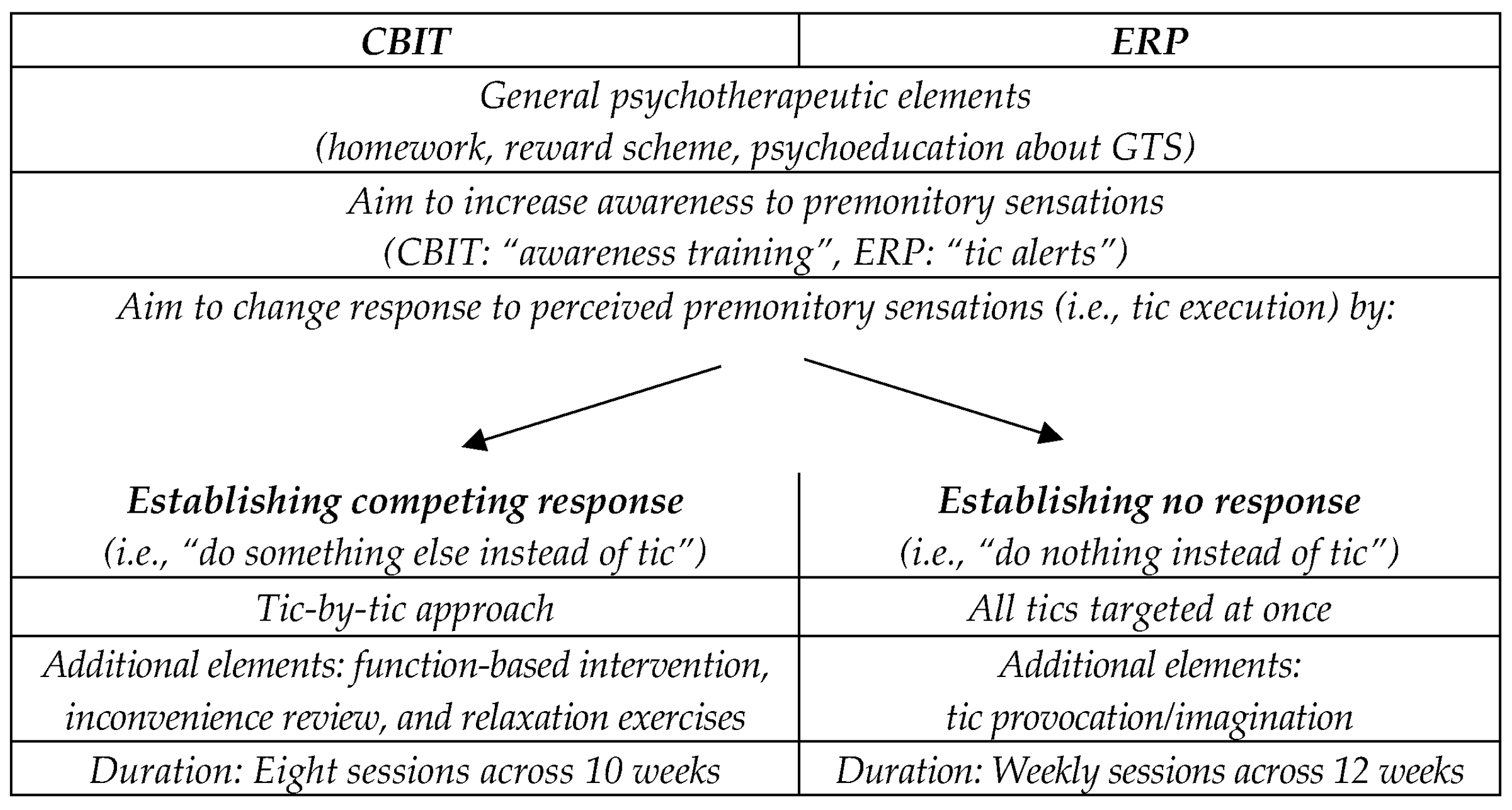
2.1. Comprehensive Behavioral Intervention for Tics (CBIT)/Habit Reversal Training (HRT)
2.2. Exposure and Response Prevention (ERP)
3. Current Evidence on the Effectiveness of CBIT/HRT and ERP for the Treatment of Tic Disorders
4. The Relevance of Neurophysiological Markers
5. Theory of Event Coding (TEC) and Binding and Retrieval in Action Control (BRAC)
6. GTS as a Disorder of Perception–Action Integration
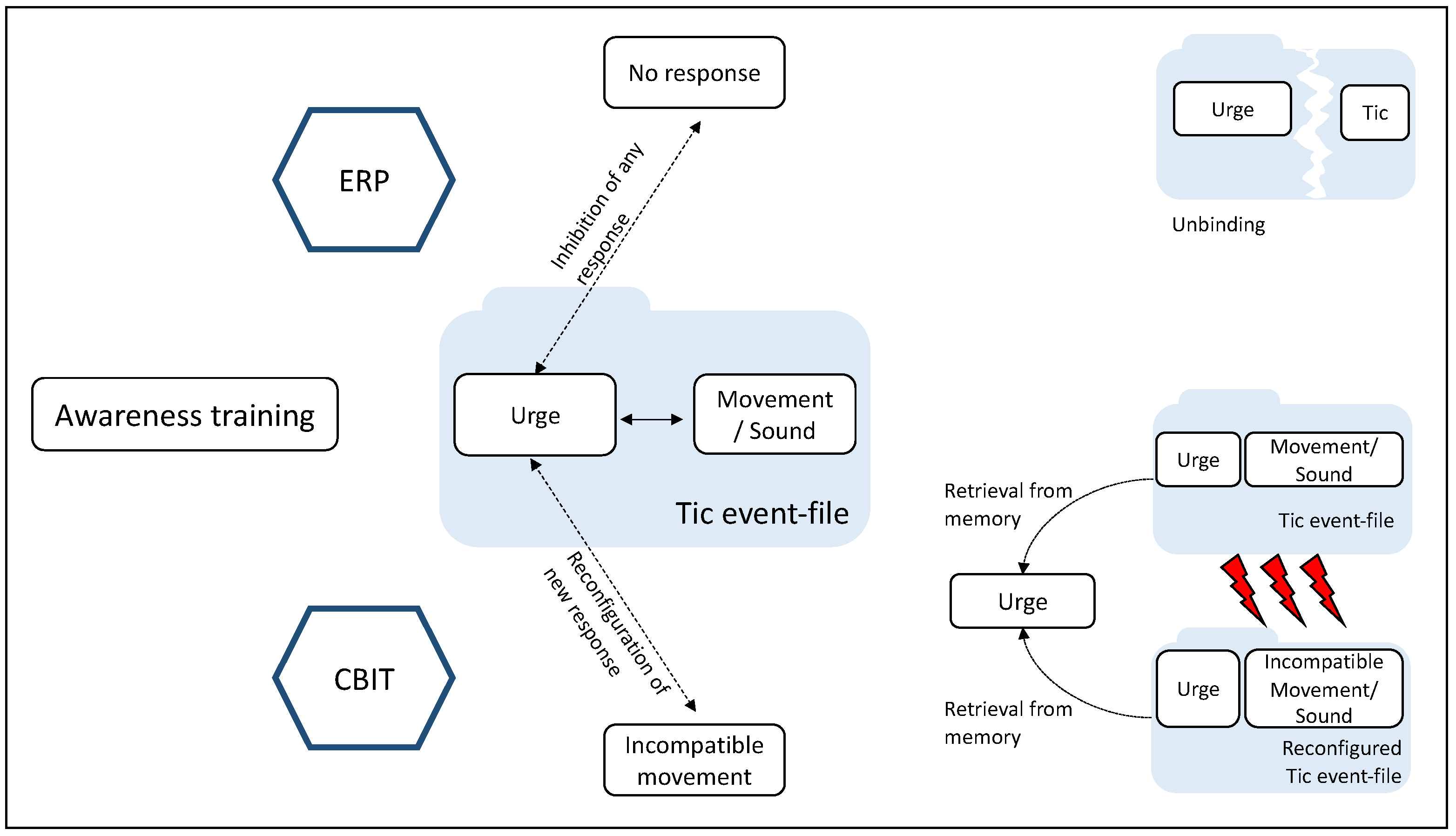
7. Cognitive and Neural Mechanisms Underlying CBIT/HRT and ERP in Light of TEC and BRAC
8. CBIT/HRT and ERP Mechanisms of Action Conceptualized by TEC/BRAC
9. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013; ISBN 978-0-89042-554-1. [Google Scholar]
- Bartha, S.; Bluschke, A.; Rawish, T.; Naumann, K.E.R.; Wendiggensen, P.; Bäumer, T.; Roessner, V.; Münchau, A.; Beste, C. Extra Movements in Healthy People: Challenging the Definition and Diagnostic Practice of Tic Disorders. Ann. Neurol. 2023, 93, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Paulus, T.; Schappert, R.; Bluschke, A.; Alvarez-Fischer, D.; Naumann, K.E.R.; Roessner, V.; Bäumer, T.; Beste, C.; Münchau, A. Questioning the Definition of Tourette Syndrome—Evidence from Machine Learning. Brain Commun. 2021, 3, fcab282. [Google Scholar] [CrossRef]
- Beste, C.; Münchau, A. Tics and Tourette Syndrome—Surplus of Actions Rather than Disorder? Mov. Disord. 2018, 33, 238–242. [Google Scholar] [CrossRef]
- Leckman, J.F.; Walker, D.E.; Cohen, D.J. Premonitory Urges in Tourette’s Syndrome. Am. J. Psychiatry 1993, 150, 98–102. [Google Scholar] [CrossRef]
- Cohen, A.J.; Leckman, J.F. Sensory Phenomena Associated with Gilles de La Tourette’s Syndrome. J. Clin. Psychiatry 1992, 53, 319–323. [Google Scholar] [PubMed]
- Nowak, D.A.; Rothwell, J.; Topka, H.; Robertson, M.M.; Orth, M. Grip Force Behavior in Gilles de La Tourette Syndrome. Mov. Disord. 2005, 20, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Delorme, C.; Salvador, A.; Valabrègue, R.; Roze, E.; Palminteri, S.; Vidailhet, M.; de Wit, S.; Robbins, T.; Hartmann, A.; Worbe, Y. Enhanced Habit Formation in Gilles de La Tourette Syndrome. Brain 2016, 139, 605–615. [Google Scholar] [CrossRef]
- Paulus, T.; Kleimaker, M.; Münchau, A. Das Tourette-Syndrom und dessen Abgrenzung zu wichtigen Differenzialdiagnosen. PSYCH Up2date 2021, 15, 321–335. [Google Scholar] [CrossRef]
- Pringsheim, T.; Holler-Managan, Y.; Okun, M.S.; Jankovic, J.; Piacentini, J.; Cavanna, A.E.; Martino, D.; Müller-Vahl, K.; Woods, D.W.; Robinson, M.; et al. Comprehensive Systematic Review Summary: Treatment of Tics in People with Tourette Syndrome and Chronic Tic Disorders. Neurology 2019, 92, 907–915. [Google Scholar] [CrossRef]
- Andrén, P.; Jakubovski, E.; Murphy, T.L.; Woitecki, K.; Tarnok, Z.; Zimmerman-Brenner, S.; van de Griendt, J.; Debes, N.M.; Viefhaus, P.; Robinson, S.; et al. European Clinical Guidelines for Tourette Syndrome and Other Tic Disorders-Version 2.0. Part II: Psychological Interventions. Eur. Child Adolesc. Psychiatry 2021, 31, 403–423. [Google Scholar] [CrossRef]
- Piacentini, J.; Woods, D.W.; Scahill, L.; Wilhelm, S.; Peterson, A.L.; Chang, S.; Ginsburg, G.S.; Deckersbach, T.; Dziura, J.; Levi-Pearl, S.; et al. Behavior Therapy for Children with Tourette Disorder: A Randomized Controlled Trial. JAMA 2010, 303, 1929–1937. [Google Scholar] [CrossRef]
- Rowe, J.; Yuen, H.K.; Dure, L.S. Comprehensive Behavioral Intervention to Improve Occupational Performance in Children with Tourette Disorder. Am. J. Occup. Ther. 2013, 67, 194–200. [Google Scholar] [CrossRef]
- Wilhelm, S.; Peterson, A.L.; Piacentini, J.; Woods, D.W.; Deckersbach, T.; Sukhodolsky, D.G.; Chang, S.; Liu, H.; Dziura, J.; Walkup, J.T.; et al. Randomized Trial of Behavior Therapy for Adults with Tourette Syndrome. Arch. Gen. Psychiatry 2012, 69, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Verdellen, C.; van de Griendt, J.; Hartmann, A.; Murphy, T. ESSTS Guidelines Group European Clinical Guidelines for Tourette Syndrome and Other Tic Disorders. Part III: Behavioural and Psychosocial Interventions. Eur. Child Adolesc. Psychiatry 2011, 20, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Steeves, T.; McKinlay, B.D.; Gorman, D.; Billinghurst, L.; Day, L.; Carroll, A.; Dion, Y.; Doja, A.; Luscombe, S.; Sandor, P.; et al. Canadian Guidelines for the Evidence-Based Treatment of Tic Disorders: Behavioural Therapy, Deep Brain Stimulation, and Transcranial Magnetic Stimulation. Can. J. Psychiatry 2012, 57, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Okun, M.S.; Müller-Vahl, K.; Martino, D.; Jankovic, J.; Cavanna, A.E.; Woods, D.W.; Robinson, M.; Jarvie, E.; Roessner, V.; et al. Practice Guideline Recommendations Summary: Treatment of Tics in People with Tourette Syndrome and Chronic Tic Disorders. Neurology 2019, 92, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Morand-Beaulieu, S.; O’Connor, K.P.; Blanchet, P.J.; Lavoie, M.E. Electrophysiological Predictors of Cognitive-Behavioral Therapy Outcome in Tic Disorders. J. Psychiatr. Res. 2018, 105, 113–122. [Google Scholar] [CrossRef]
- Petruo, V.; Bodmer, B.; Bluschke, A.; Münchau, A.; Roessner, V.; Beste, C. Comprehensive Behavioral Intervention for Tics Reduces Perception-Action Binding during Inhibitory Control in Gilles de La Tourette Syndrome. Sci. Rep. 2020, 10, 1174. [Google Scholar] [CrossRef]
- Woods, D.W.; Piacentini, J.C.; Chang, S.W.; Deckersbach, T.; Ginsburg, G.S.; Peterson, A.L.; Scahill, L.D.; Walkup, J.T.; Wilhelm, S. Managing Tourette Syndrome: A Behavioral Intervention for Children and Adults: Therapist Guide; Treatments that work; Oxford University Press: Oxford, UK; New York, NY, USA, 2008; ISBN 978-0-19-534128-7. [Google Scholar]
- Langelage, J.; Verrel, J.; Friedrich, J.; Siekmann, A.; Schappert, R.; Bluschke, A.; Roessner, V.; Paulus, T.; Bäumer, T.; Frings, C.; et al. Urge-Tic Associations in Children and Adolescents with Tourette Syndrome. Sci. Rep. 2022, 12, 16008. [Google Scholar] [CrossRef]
- Schubert, L.; Verrel, J.; Behm, A.; Bäumer, T.; Beste, C.; Münchau, A. Inter-Individual Differences in Urge-Tic Associations in Tourette Syndrome. Cortex 2021, 143, 80–91. [Google Scholar] [CrossRef]
- Müller-Vahl, K.; Münchau, A.; Brandt, V.; Jakubovski, E. Tourette-Syndrom und Andere Tic-Störungen: Mit einem Manual zur Habit Reversal Therapie; 1. Auflage; Batra, A., Hohagen, F., Eds.; Störungsspezifische Psychotherapie; Kohlhammer: Stuttgart, Germany, 2019; ISBN 978-3-17-032653-8. [Google Scholar]
- Kim, K.M.; Bae, E.; Lee, J.; Park, T.-W.; Lim, M.H. A Review of Cognitive and Behavioral Interventions for Tic Disorder. J. Korean Acad. Child Adolesc. Psychiatry 2021, 32, 51–62. [Google Scholar] [CrossRef]
- Bloch, M.H.; Leckman, J.F.; Zhu, H.; Peterson, B.S. Caudate Volumes in Childhood Predict Symptom Severity in Adults with Tourette Syndrome. Neurology 2005, 65, 1253–1258. [Google Scholar] [CrossRef]
- Peterson, B.; Riddle, M.A.; Cohen, D.J.; Katz, L.D.; Smith, J.C.; Hardin, M.T.; Leckman, J.F. Reduced Basal Ganglia Volumes in Tourette’s Syndrome Using Three-Dimensional Reconstruction Techniques from Magnetic Resonance Images. Neurology 1993, 43, 941. [Google Scholar] [CrossRef]
- Worbe, Y.; Marrakchi-Kacem, L.; Lecomte, S.; Valabregue, R.; Poupon, F.; Guevara, P.; Tucholka, A.; Mangin, J.-F.; Vidailhet, M.; Lehericy, S.; et al. Altered Structural Connectivity of Cortico-Striato-Pallido-Thalamic Networks in Gilles de La Tourette Syndrome. Brain 2015, 138, 472–482. [Google Scholar] [CrossRef]
- Brandt, V.C.; Beck, C.; Sajin, V.; Baaske, M.K.; Bäumer, T.; Beste, C.; Anders, S.; Münchau, A. Temporal Relationship between Premonitory Urges and Tics in Gilles de La Tourette Syndrome. Cortex 2016, 77, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Van de Griendt, J.M.T.M.; Verdellen, C.W.J.; van Dijk, M.K.; Verbraak, M.J.P.M. Behavioural Treatment of Tics: Habit Reversal and Exposure with Response Prevention. Neurosci. Biobehav. Rev. 2013, 37, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Verdellen, C.W.J.; Keijsers, G.P.J.; Cath, D.C.; Hoogduin, C.A.L. Exposure with Response Prevention versus Habit Reversal in Tourettes’s Syndrome: A Controlled Study. Behav. Res. 2004, 42, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Verdellen, C.; van de Griendt, J.; Kriens, S.; van Oostrum, I. Tics—Therapist Manual & Workbook for Children; BT-Tics Foundation: Heteren, The Netherlands, 2016. [Google Scholar]
- Verdellen, C.W.J.; Hoogduin, C.A.L.; Kato, B.S.; Keijsers, G.P.J.; Cath, D.C.; Hoijtink, H.B. Habituation of Premonitory Sensations during Exposure and Response Prevention Treatment in Tourette’s Syndrome. Behav. Modif. 2008, 32, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Hoogduin, K.; Verdellen, C.; Cath, D. Exposure and Response Prevention in the Treatment of Gilles de La Tourette’s Syndrome: Four Case Studies. Clin. Psychol. Psychother. 1997, 4, 125–137. [Google Scholar] [CrossRef]
- Houghton, D.C.; Capriotti, M.R.; Scahill, L.D.; Wilhelm, S.; Peterson, A.L.; Walkup, J.T.; Piacentini, J.; Woods, D.W. Investigating Habituation to Premonitory Urges in Behavior Therapy for Tic Disorders. Behav. Ther. 2017, 48, 834–846. [Google Scholar] [CrossRef]
- Leckman, J.F.; Riddle, M.A.; Hardin, M.T.; Ort, S.I.; Swartz, K.L.; Stevenson, J.; Cohen, D.J. The Yale Global Tic Severity Scale: Initial Testing of a Clinician-Rated Scale of Tic Severity. J. Am. Acad. Child Adolesc. Psychiatry 1989, 28, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Deckersbach, T.; Rauch, S.; Buhlmann, U.; Wilhelm, S. Habit Reversal versus Supportive Psychotherapy in Tourette’s Disorder: A Randomized Controlled Trial and Predictors of Treatment Response. Behav. Res. Ther. 2006, 44, 1079–1090. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Deckersbach, T.; Coffey, B.J.; Bohne, A.; Peterson, A.L.; Baer, L. Habit Reversal Versus Supportive Psychotherapy for Tourette’s Disorder: A Randomized Controlled Trial. Am. J. Psychiatry 2003, 160, 1175–1177. [Google Scholar] [CrossRef] [PubMed]
- McGuire, J.F.; Piacentini, J.; Brennan, E.A.; Lewin, A.B.; Murphy, T.K.; Small, B.J.; Storch, E.A. A Meta-Analysis of Behavior Therapy for Tourette Syndrome. J. Psychiatr. Res. 2014, 50, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.; Pellico, A.; Silvestri, P.R.; Chiarotti, F.; Cardona, F. A Randomized Controlled Trial Comparing Behavioral, Educational, and Pharmacological Treatments in Youths with Chronic Tic Disorder or Tourette Syndrome. Front. Psychiatry 2018, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, E.J.; Goetz, A.R.; Capriotti, M.R.; Bauer, C.C.; Brei, N.G.; Himle, M.B.; Espil, F.M.; Snorrason, Í.; Ran, D.; Woods, D.W. A Randomized Waitlist-Controlled Pilot Trial of Voice over Internet Protocol-Delivered Behavior Therapy for Youth with Chronic Tic Disorders. J. Telemed. Telecare 2016, 22, 153–162. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Zhang, J.; Yan, C.; Wen, F.; Yan, J.; Wang, F.; Liu, J.; Cui, Y. The Therapeutic Effect of Habit Reversal Training for Tourette Syndrome: A Meta-Analysis of Randomized Control Trials. Expert Rev. Neurother. 2020, 20, 1189–1196. [Google Scholar] [CrossRef]
- Dutta, N.; Cavanna, A.E. The Effectiveness of Habit Reversal Therapy in the Treatment of Tourette Syndrome and Other Chronic Tic Disorders: A Systematic Review. Funct. Neurol. 2013, 28, 7–12. [Google Scholar]
- Andrén, P.; Wachtmeister, V.; Franzé, J.; Speiner, C.; Fernández de la Cruz, L.; Andersson, E.; de Schipper, E.; Rautio, D.; Silverberg-Mörse, M.; Serlachius, E.; et al. Effectiveness of Behaviour Therapy for Children and Adolescents with Tourette Syndrome and Chronic Tic Disorder in a Naturalistic Setting. Child Psychiatry Hum. Dev. 2021, 52, 739–750. [Google Scholar] [CrossRef]
- Busner, J.; Targum, S.D. The Clinical Global Impressions Scale: Applying a Research Tool in Clinical Practice. Psychiatry 2007, 4, 28–37. [Google Scholar]
- Ueda, K.; Black, K.J. Recent Progress on Tourette Syndrome. Fac. Rev. 2021, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Himle, M.B.; Freitag, M.; Walther, M.; Franklin, S.A.; Ely, L.; Woods, D.W. A Randomized Pilot Trial Comparing Videoconference versus Face-to-Face Delivery of Behavior Therapy for Childhood Tic Disorders. Behav. Res. Ther. 2012, 50, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Rachamim, L.; Zimmerman-Brenner, S.; Rachamim, O.; Mualem, H.; Zingboim, N.; Rotstein, M. Internet-Based Guided Self-Help Comprehensive Behavioral Intervention for Tics (ICBIT) for Youth with Tic Disorders: A Feasibility and Effectiveness Study with 6 Month-Follow-Up. Eur. Child Adolesc. Psychiatry 2020, 31, 275–287. [Google Scholar] [CrossRef]
- Andrén, P.; Aspvall, K.; Fernández de la Cruz, L.; Wiktor, P.; Romano, S.; Andersson, E.; Murphy, T.; Isomura, K.; Serlachius, E.; Mataix-Cols, D. Therapist-Guided and Parent-Guided Internet-Delivered Behaviour Therapy for Paediatric Tourette’s Disorder: A Pilot Randomised Controlled Trial with Long-Term Follow-Up. BMJ Open 2019, 9, e024685. [Google Scholar] [CrossRef]
- Deckersbach, T.; Chou, T.; Britton, J.C.; Carlson, L.E.; Reese, H.E.; Siev, J.; Scahill, L.; Piacentini, J.C.; Woods, D.W.; Walkup, J.T.; et al. Neural Correlates of Behavior Therapy for Tourette’s Disorder. Psychiatry Res. 2014, 224, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.W.; McGuire, J.F.; Walkup, J.T.; Woods, D.W.; Scahill, L.; Wilhelm, S.; Peterson, A.L.; Dziura, J.; Piacentini, J. Neurocognitive Correlates of Treatment Response in Children with Tourette’s Disorder. Psychiatry Res. 2018, 261, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Morand-Beaulieu, S.; O’Connor, K.P.; Sauvé, G.; Blanchet, P.J.; Lavoie, M.E. Cognitive-Behavioral Therapy Induces Sensorimotor and Specific Electrocortical Changes in Chronic Tic and Tourette’s Disorder. Neuropsychologia 2015, 79, 310–321. [Google Scholar] [CrossRef]
- O’Connor, K.; Lavoie, M.; Blanchet, P.; St-Pierre-Delorme, M.-È. Evaluation of a Cognitive Psychophysiological Model for Management of Tic Disorders: An Open Trial. Br. J. Psychiatry 2016, 209, 76–83. [Google Scholar] [CrossRef]
- Morand-Beaulieu, S.; Crowley, M.J.; Grantz, H.; Leckman, J.F.; Scahill, L.; Sukhodolsky, D.G. Evaluation of EEG Biomarkers of Comprehensive Behavioral Intervention for Tics in Children with Tourette Syndrome. Clin. Neurophysiol. 2022, 142, 75–85. [Google Scholar] [CrossRef]
- Beste, C.; Mückschel, M.; Rauch, J.; Bluschke, A.; Takacs, A.; Dilcher, R.; Toth-Faber, E.; Bäumer, T.; Roessner, V.; Li, S.-C.; et al. Distinct Brain-Oscillatory Neuroanatomical Architecture of Perception-Action Integration in Adolescents With Tourette Syndrome. Biol. Psychiatry Glob. Open Sci. 2021, 1, 123–134. [Google Scholar] [CrossRef]
- Kleimaker, A.; Kleimaker, M.; Bäumer, T.; Beste, C.; Münchau, A. Gilles de La Tourette Syndrome—A Disorder of Action-Perception Integration. Front. Neurol. 2020, 11, 597898. [Google Scholar] [CrossRef] [PubMed]
- Mielke, E.; Takacs, A.; Kleimaker, M.; Schappert, R.; Conte, G.; Onken, R.; Künemund, T.; Verrel, J.; Bäumer, T.; Beste, C.; et al. Tourette Syndrome as a Motor Disorder Revisited—Evidence from Action Coding. Neuroimage Clin. 2021, 30, 102611. [Google Scholar] [CrossRef]
- Wendiggensen, P.; Paulus, T.; Bluschke, A.; Takacs, A.; Toth-Faber, E.; Weissbach, A.; Bäumer, T.; Frings, C.; Roessner, V.; Münchau, A.; et al. Theta Activity Dynamics during Embedded Response Plan Processing in Tourette Syndrome. Biomedicines 2023, 11, 393. [Google Scholar] [CrossRef]
- Hommel, B.; Müsseler, J.; Aschersleben, G.; Prinz, W. The Theory of Event Coding (TEC): A Framework for Perception and Action Planning. Behav. Brain Sci. 2001, 24, 849–878. [Google Scholar] [CrossRef] [PubMed]
- Treisman, A. The Binding Problem. Curr. Opin. Neurobiol. 1996, 6, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Hommel, B. Event Files: Evidence for Automatic Integration of Stimulus-Response Episodes. Vis. Cogn. 1998, 5, 183–216. [Google Scholar] [CrossRef]
- Hommel, B. Automatic Stimulus–Response Translation in Dual-Task Performance. J. Exp. Psychol. Hum. Percept. Perform. 1998, 24, 1368–1384. [Google Scholar] [CrossRef]
- Hommel, B. Event Files: Feature Binding in and across Perception and Action. Trends Cogn. Sci. 2004, 8, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Hommel, B. Action Control According to TEC (Theory of Event Coding). Psychol. Res. 2009, 73, 512–526. [Google Scholar] [CrossRef]
- Hommel, B. How Much Attention Does an Event File Need? J. Exp. Psychol. Hum. Percept. Perform. 2005, 31, 1067–1082. [Google Scholar] [CrossRef] [PubMed]
- Beste, C.; Münchau, A.; Frings, C. Towards a Systematization of Brain Oscillatory Activity in Actions. Commun. Biol. 2023, 6, 137. [Google Scholar] [CrossRef]
- Frings, C.; Hommel, B.; Koch, I.; Rothermund, K.; Dignath, D.; Giesen, C.; Kiesel, A.; Kunde, W.; Mayr, S.; Moeller, B.; et al. Binding and Retrieval in Action Control (BRAC). Trends Cogn. Sci. 2020, 24, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Schmalbrock, P.; Hommel, B.; Münchau, A.; Beste, C.; Frings, C. Predictability Reduces Event File Retrieval. Atten. Percept. Psychophys. 2022, 85, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Schmalbrock, P.; Frings, C. A Mighty Tool Not Only in Perception: Figure-Ground Mechanisms Control Binding and Retrieval Alike. Atten. Percept. Psychophys. 2022, 84, 2255–2270. [Google Scholar] [CrossRef] [PubMed]
- Hommel, B. Theory of Event Coding (TEC) V2.0: Representing and Controlling Perception and Action. Atten. Percept. Psychophys. 2019, 81, 2139–2154. [Google Scholar] [CrossRef]
- Kleimaker, M.; Takacs, A.; Conte, G.; Onken, R.; Verrel, J.; Bäumer, T.; Münchau, A.; Beste, C. Increased Perception-Action Binding in Tourette Syndrome. Brain 2020, 143, 1934–1945. [Google Scholar] [CrossRef] [PubMed]
- Petruo, V.A.; Bodmer, B.; Brandt, V.C.; Baumung, L.; Roessner, V.; Münchau, A.; Beste, C. Altered Perception-Action Binding Modulates Inhibitory Control in Gilles de La Tourette Syndrome. J. Child Psychol. Psychiatr. 2019, 60, 953–962. [Google Scholar] [CrossRef]
- Opitz, A.; Beste, C.; Stock, A.-K. Using Temporal EEG Signal Decomposition to Identify Specific Neurophysiological Correlates of Distractor-Response Bindings Proposed by the Theory of Event Coding. Neuroimage 2020, 209, 116524. [Google Scholar] [CrossRef]
- Takacs, A.; Zink, N.; Wolff, N.; Münchau, A.; Mückschel, M.; Beste, C. Connecting EEG Signal Decomposition and Response Selection Processes Using the Theory of Event Coding Framework. Hum. Brain Mapp. 2020, 41, 2862–2877. [Google Scholar] [CrossRef]
- Takacs, A.; Mückschel, M.; Roessner, V.; Beste, C. Decoding Stimulus–Response Representations and Their Stability Using EEG-Based Multivariate Pattern Analysis. Cereb. Cortex Commun. 2020, 1, tgaa016. [Google Scholar] [CrossRef]
- Ouyang, G.; Sommer, W.; Zhou, C. A Toolbox for Residue Iteration Decomposition (RIDE)—A Method for the Decomposition, Reconstruction, and Single Trial Analysis of Event Related Potentials. J. Neurosci. Methods 2015, 250, 7–21. [Google Scholar] [CrossRef]
- Ouyang, G.; Herzmann, G.; Zhou, C.; Sommer, W. Residue Iteration Decomposition (RIDE): A New Method to Separate ERP Components on the Basis of Latency Variability in Single Trials: RIDE: A New Method to Separate ERP Components. Psychophysiology 2011, 48, 1631–1647. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.; Hildebrandt, A.; Sommer, W.; Zhou, C. Exploiting the Intra-Subject Latency Variability from Single-Trial Event-Related Potentials in the P3 Time Range: A Review and Comparative Evaluation of Methods. Neurosci. Biobehav. Rev. 2017, 75, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wolff, N.; Mückschel, M.; Beste, C. Neural Mechanisms and Functional Neuroanatomical Networks during Memory and Cue-Based Task Switching as Revealed by Residue Iteration Decomposition (RIDE) Based Source Localization. Brain Struct. Funct. 2017, 222, 3819–3831. [Google Scholar] [CrossRef]
- Van de Griendt, J.M.T.M.; van Dijk, M.K.; Verdellen, C.W.J.; Verbraak, M.J.P.M. The Effect of Shorter Exposure versus Prolonged Exposure on Treatment Outcome in Tourette Syndrome and Chronic Tic Disorders—An Open Trial. Int. J. Psychiatry Clin. Pract. 2018, 22, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Moeller, B.; Frings, C. Designers Beware: Response Retrieval Effects Influence Drivers’ Response Times to Local Danger Warnings. Transp. Res. Part F Traffic Psychol. Behav. 2014, 24, 117–132. [Google Scholar] [CrossRef]
- Wells, A. Metacognitive Therapy. In Acceptance and Mindfulness in Cognitive Behavior Therapy; Herbert, J.D., Forman, E.M., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 83–108. ISBN 978-1-118-00185-1. [Google Scholar]
- Hayes, S.C.; Pierson, H. Acceptance and Commitment Therapy. In Encyclopedia of Cognitive Behavior Therapy; Freeman, A., Felgoise, S.H., Nezu, C.M., Nezu, A.M., Reinecke, M.A., Eds.; Springer: New York, NY, USA, 2005; pp. 1–4. ISBN 978-0-306-48580-0. [Google Scholar]
- Schaich, A.; Brandt, V.; Senft, A.; Schiemenz, C.; Klein, J.P.; Fassbinder, E.; Munchau, A.; Alvarez-Fischer, D. Treatment of Tourette Syndrome with Attention Training Technique—A Case Series. Front. Psychiatry 2020, 11, 519931. [Google Scholar] [CrossRef]
- Herrmann, K.; Sprenger, A.; Baumung, L.; Alvarez-Fischer, D.; Munchau, A.; Brandt, V. Help or Hurt? How Attention Modulates Tics under Different Conditions. Cortex 2019, 120, 471–482. [Google Scholar] [CrossRef]
- Misirlisoy, E.; Brandt, V.; Ganos, C.; Tübing, J.; Münchau, A.; Haggard, P. The Relation between Attention and Tic Generation in Tourette Syndrome. Neuropsychology 2015, 29, 658–665. [Google Scholar] [CrossRef]
- Franklin, M.E.; Best, S.H.; Wilson, M.A.; Loew, B.; Compton, S.N. Habit Reversal Training and Acceptance and Commitment Therapy for Tourette Syndrome: A Pilot Project. J. Dev. Phys. Disabil. 2011, 23, 49–60. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Friedrich, J.; Rawish, T.; Bluschke, A.; Frings, C.; Beste, C.; Münchau, A. Cognitive and Neural Mechanisms of Behavior Therapy for Tics: A Perception–Action Integration Approach. Biomedicines 2023, 11, 1550. https://doi.org/10.3390/biomedicines11061550
Friedrich J, Rawish T, Bluschke A, Frings C, Beste C, Münchau A. Cognitive and Neural Mechanisms of Behavior Therapy for Tics: A Perception–Action Integration Approach. Biomedicines. 2023; 11(6):1550. https://doi.org/10.3390/biomedicines11061550
Chicago/Turabian StyleFriedrich, Julia, Tina Rawish, Annet Bluschke, Christian Frings, Christian Beste, and Alexander Münchau. 2023. "Cognitive and Neural Mechanisms of Behavior Therapy for Tics: A Perception–Action Integration Approach" Biomedicines 11, no. 6: 1550. https://doi.org/10.3390/biomedicines11061550
APA StyleFriedrich, J., Rawish, T., Bluschke, A., Frings, C., Beste, C., & Münchau, A. (2023). Cognitive and Neural Mechanisms of Behavior Therapy for Tics: A Perception–Action Integration Approach. Biomedicines, 11(6), 1550. https://doi.org/10.3390/biomedicines11061550




