Exploring Sex Differences of Beta-Blockers in the Treatment of Hypertension: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Series of Meta-Analysis
2.2. Literature Search
- -
- To determine the representation of females in studies on the effect of antihypertensive drugs on CVD for the past century.
- -
- To study differences and similarities between males and females on the effect of antihypertensive medication on cardiac function and structure.
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Data Extraction
2.6. Quality Assessment
2.7. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Quality Assessment
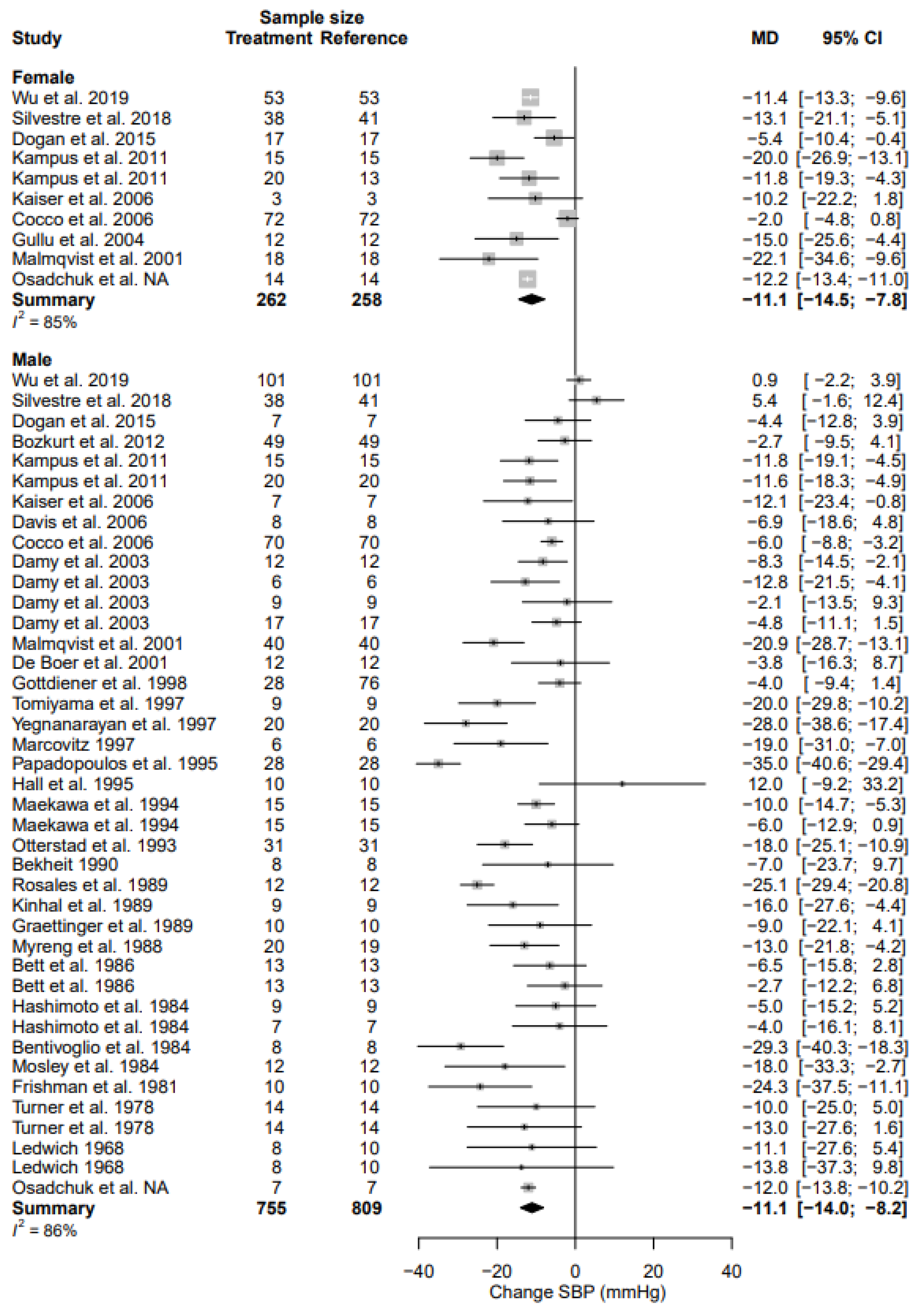
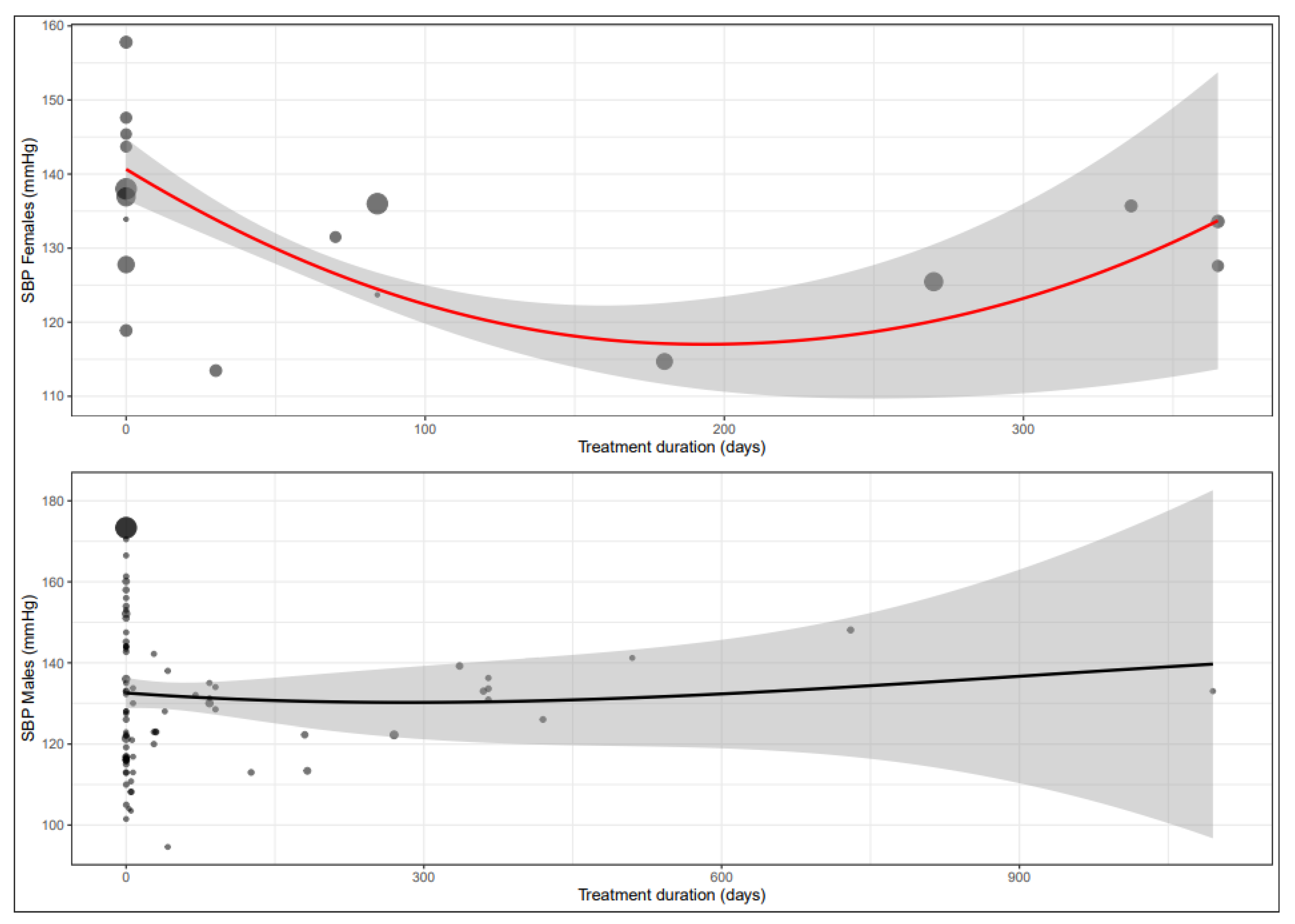
3.4. Systolic Blood Pressure
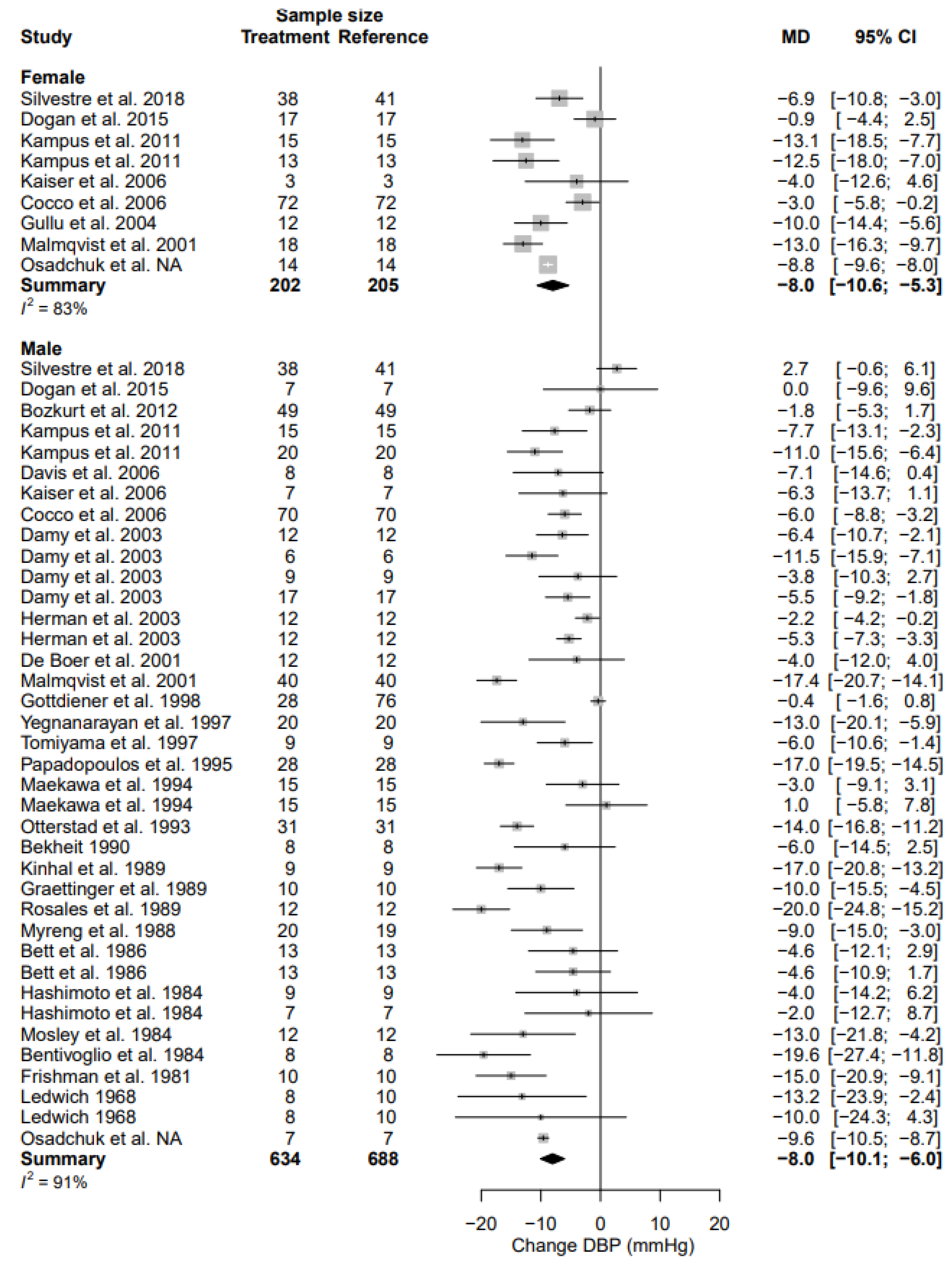
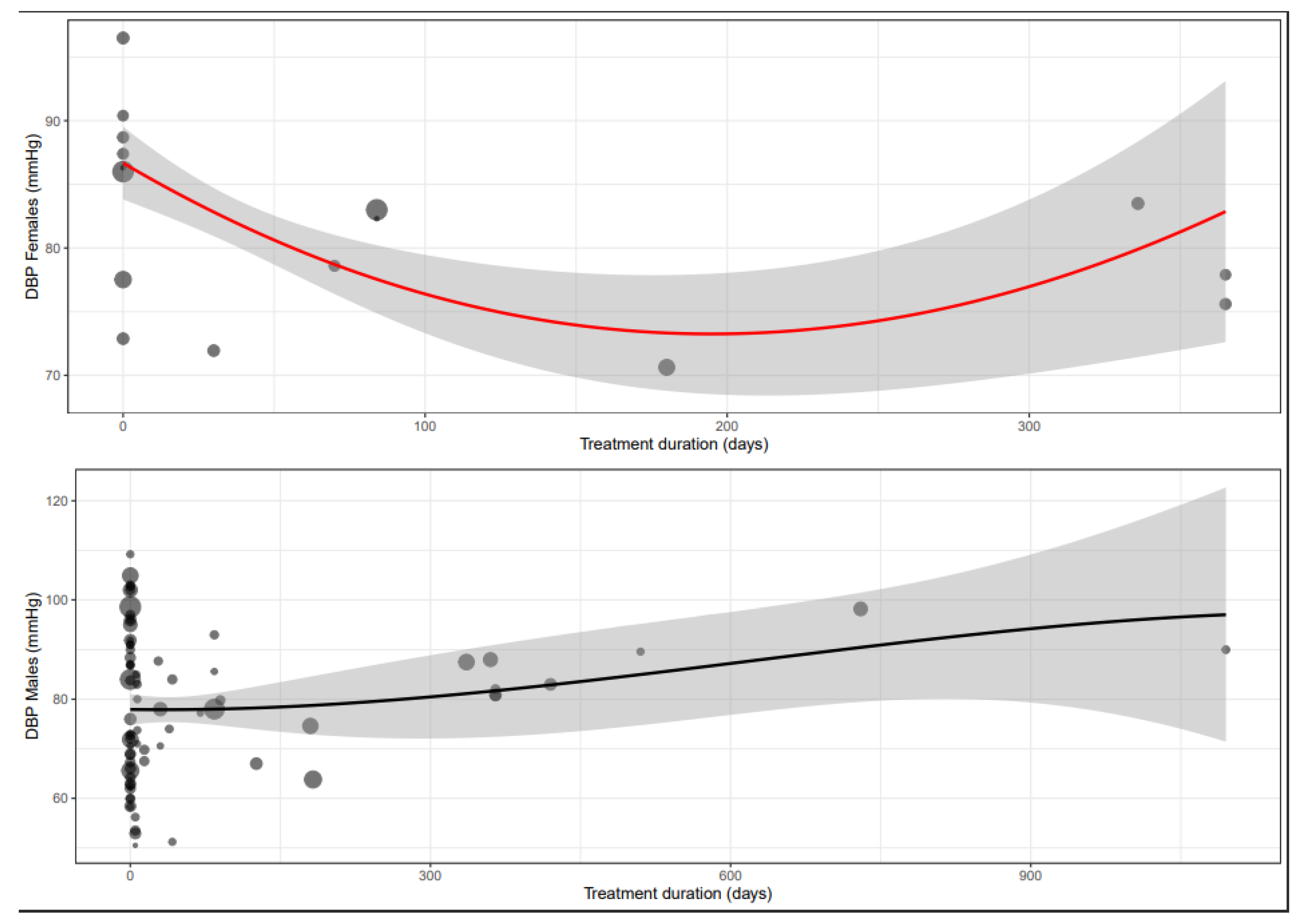
3.5. Diastolic Blood Pressure
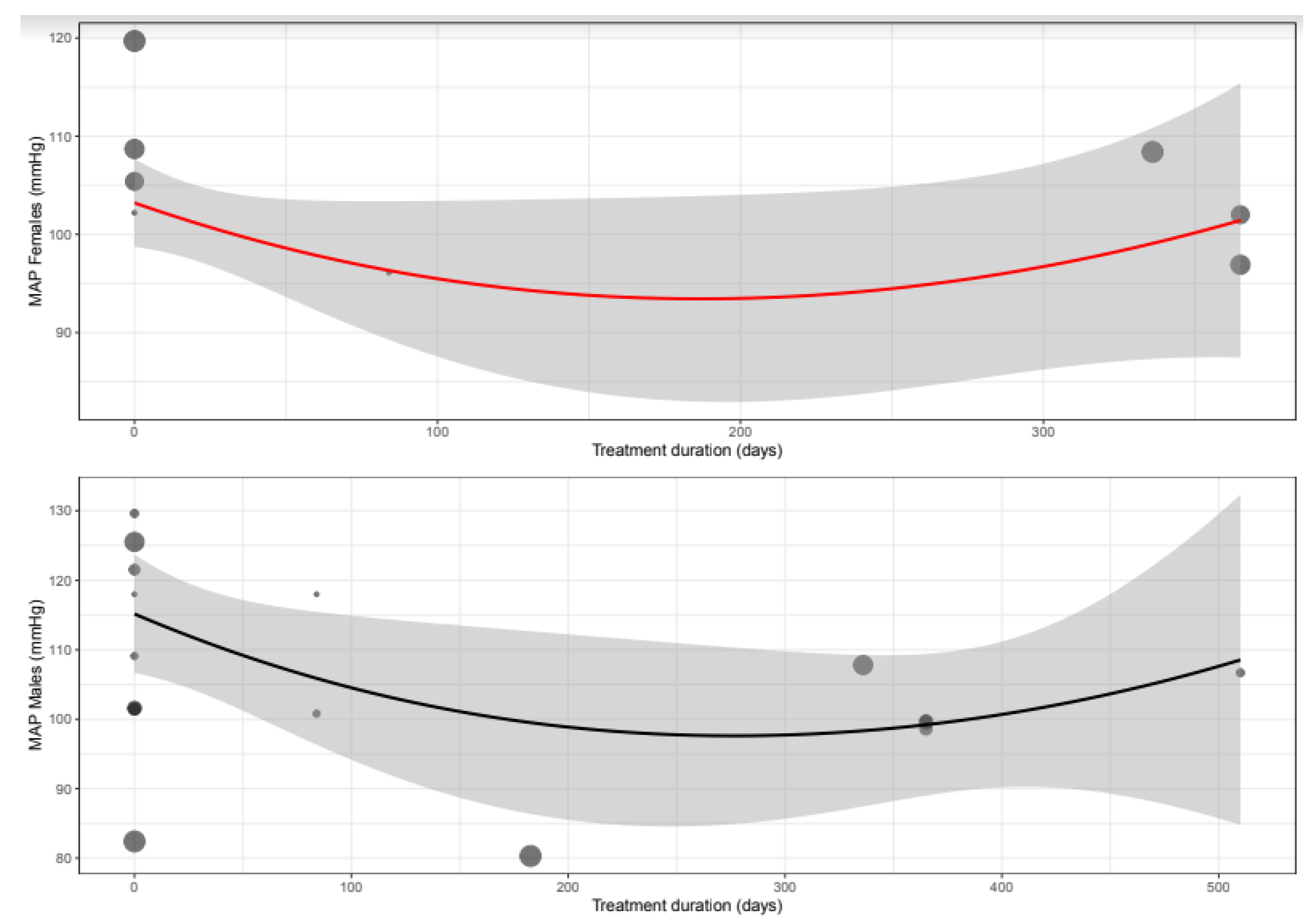
3.6. Mean Arterial Pressure
3.7. Heart Rate
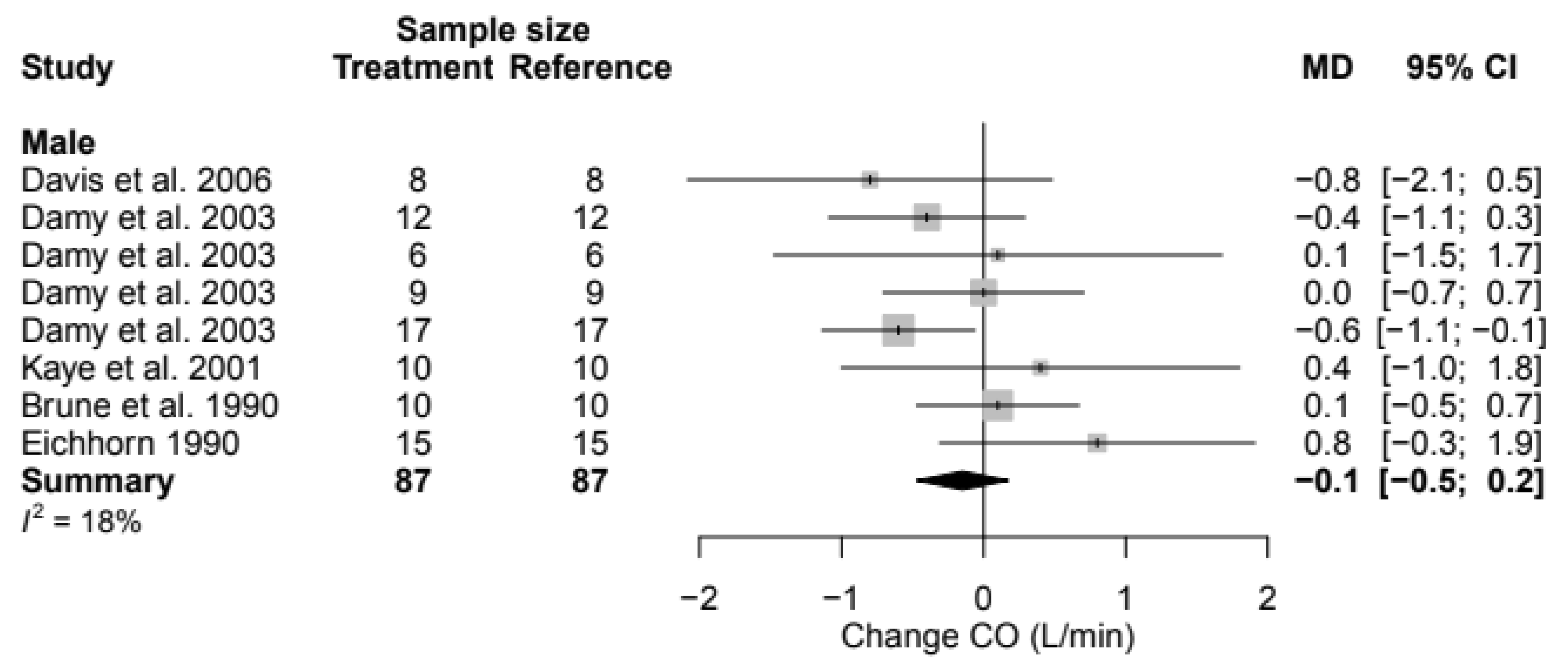
3.8. Cardiac Output
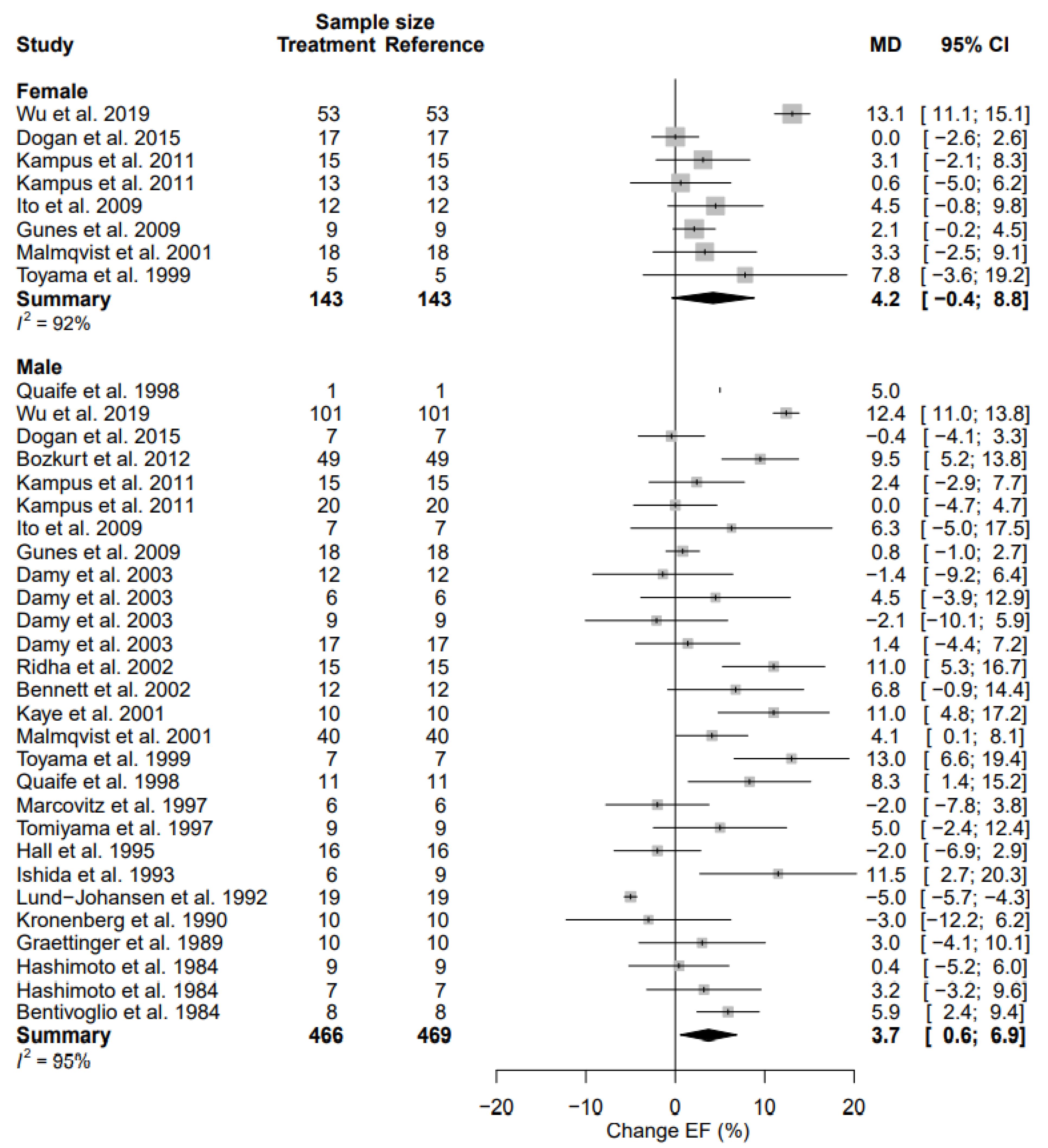
3.9. Left Ventricular Ejection Fraction
3.10. Left Ventricular Mass
4. Discussion
Strengths and Limitations
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 8 July 2022).
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef] [PubMed]
- Carey, R.M.; Muntner, P.; Bosworth, H.B.; Whelton, P.K. Prevention and Control of Hypertension: JACC Health Promotion Series. J. Am. Coll. Cardiol. 2018, 72, 1278–1293. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; Linfante, A.H.; Benjamin, E.J.; Berra, K.; Hayes, S.N.; Walsh, B.W.; Fabunmi, R.P.; Kwan, J.; Mills, T.; Simpson, S.L. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation 2005, 111, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Muiesan, M.L.; Salvetti, M.; Rosei, C.A.; Paini, A. Gender Differences in Antihypertensive Treatment: Myths or Legends? High Blood Press. Cardiovasc. Prev. 2016, 23, 105–113. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Calhoun, D.A.; Jones, D.; Textor, S.; Goff, D.C.; Murphy, T.P.; Toto, R.D.; White, A.; Cushman, W.C.; White, W.; Sica, D.; et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Circulation 2008, 117, e510–e526. [Google Scholar] [CrossRef]
- Oertelt-Prigione, S.; Regitz-Zagrosek, V. Sex and Gender Aspects in Clinical Medicine; Springer: London, UK, 2012; 201p. [Google Scholar]
- Schenck-Gustafsson, K. Handbook of Clinical Gender Medicine; Karger: Basel, Switzerland, 2012. [Google Scholar]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D.J. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007, 335, 974. [Google Scholar] [CrossRef]
- Sederholm Lawesson, S.; Isaksson, R.M.; Thylen, I.; Ericsson, M.; Angerud, K.; Swahn, E.; SymTime Study, G. Gender differences in symptom presentation of ST-elevation myocardial infarction—An observational multicenter survey study. Int. J. Cardiol. 2018, 264, 7–11. [Google Scholar] [CrossRef]
- Jin, X.; Chandramouli, C.; Allocco, B.; Gong, E.; Lam, C.S.P.; Yan, L.L. Women’s Participation in Cardiovascular Clinical Trials From 2010 to 2017. Circulation 2020, 141, 540–548. [Google Scholar] [CrossRef]
- Scott, P.E.; Unger, E.F.; Jenkins, M.R.; Southworth, M.R.; McDowell, T.Y.; Geller, R.J.; Elahi, M.; Temple, R.J.; Woodcock, J. Participation of Women in Clinical Trials Supporting FDA Approval of Cardiovascular Drugs. J. Am. Coll. Cardiol. 2018, 71, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharm. 2009, 48, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 6.1. [Google Scholar]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Chapter 10: Analysing data and untertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2020; Volume 6.1. [Google Scholar]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Schwarzer, G. meta: An R package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Bekheit, S.; Tangella, M.; el-Sakr, A.; Rasheed, Q.; Craelius, W.; el-Sherif, N. Use of heart rate spectral analysis to study the effects of calcium channel blockers on sympathetic activity after myocardial infarction. Am. Heart J. 1990, 119, 79–85. [Google Scholar] [CrossRef]
- Bennett, S.K.; Smith, M.F.; Gottlieb, S.S.; Fisher, M.L.; Bacharach, S.L.; Dilsizian, V. Effect of metoprolol on absolute myocardial blood flow in patients with heart failure secondary to ischemic or nonischemic cardiomyopathy. Am. J. Cardiol. 2002, 89, 1431–1434. [Google Scholar] [CrossRef]
- Cocco, G.; Chu, D. The anti-ischemic effect of metoprolol in patients with chronic angina pectoris is gender-specific. Cardiology 2006, 106, 147–153. [Google Scholar] [CrossRef]
- Damy, T.; Pousset, F.; Caplain, H.; Hulot, J.S.; Lechat, P. Pharmacokinetic and pharmacodynamic interactions between metoprolol and dronedarone in extensive and poor CYP2D6 metabolizers healthy subjects. Fundam. Clin. Pharmacol. 2004, 18, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.E.; Richards, A.M.; Nicholls, M.G.; Yandle, T.G.; Frampton, C.M.; Troughton, R.W. Introduction of metoprolol increases plasma B-type cardiac natriuretic peptides in mild, stable heart failure. Circulation 2006, 113, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Hall, S.A.; Cigarroa, C.G.; Marcoux, L.; Risser, R.C.; Grayburn, P.A.; Eichhorn, E.J. Time course of improvement in left ventricular function, mass and geometry in patients with congestive heart failure treated with beta-adrenergic blockade. J. Am. Coll. Cardiol. 1995, 25, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Heesch, C.M.; Marcoux, L.; Hatfield, B.; Eichhorn, E.J. Hemodynamic and energetic comparison of bucindolol and metoprolol for the treatment of congestive heart failure. Am. J. Cardiol. 1995, 75, 360–364. [Google Scholar] [CrossRef]
- Ishida, S.; Makino, N.; Masutomo, K.; Hata, T.; Yanaga, T. Effect of metoprolol on the beta-adrenoceptor density of lymphocytes in patients with dilated cardiomyopathy. Am. Heart J. 1993, 125, 1311–1315. [Google Scholar] [CrossRef]
- Kampus, P.; Serg, M.; Kals, J.; Zagura, M.; Muda, P.; Karu, K.; Zilmer, M.; Eha, J. Differential effects of nebivolol and metoprolol on central aortic pressure and left ventricular wall thickness. Hypertension 2011, 57, 1122–1128. [Google Scholar] [CrossRef]
- Kronenberg, M.W.; Beard, J.T.; Stein, S.M.; Sandler, M.P. Effects of beta-adrenergic blockade in acute myocardial infarction: Evaluation by radionuclide ventriculography. J. Nucl. Med. 1990, 31, 557–566. [Google Scholar]
- Marcovitz, P.A.; Armstrong, W.F. Impact of metoprolol on heart rate, blood pressure, and contractility in normal subjects during dobutamine stress echocardiography. Am. J. Cardiol. 1997, 80, 386–388. [Google Scholar] [CrossRef]
- Osadchuk, M.A.; Vasil’eva, I.N.; Mironova, E.D.; Khudarova, A.A.; Korzhenkov, N.P. Corrective effect of angiotensin-converting enzyme inhibitors on the daily profile of blood pressure and somnological characteristics in elderly patients with combined cardiac pathology. Med. News North Cauc. 2019, 14, 448–453. [Google Scholar] [CrossRef]
- Renard, M.B.; Bernard, R.M.; Ewalenko, M.B.; Englert, M. Hemodynamic effects of concurrent administration of metoprolol and tocainide in acute myocardial infarction. J. Cardiovasc. Pharmacol. 1983, 5, 116–120. [Google Scholar] [CrossRef]
- Silke, B.; Thompson, A.; Riddell, J.G. Contrasting actions of celiprolol and metoprolol on cardiac performance in normal volunteers. Cardiovasc. Drugs Ther. 1997, 11, 57–61. [Google Scholar] [CrossRef]
- Silvestre, O.M.; Farias, A.Q.; Ramos, D.S.; Furtado, M.S.; Rodrigues, A.C.; Ximenes, R.O.; de Campos Mazo, D.F.; Yoshimura Zitelli, P.M.; Diniz, M.A.; Andrade, J.L.; et al. beta-Blocker therapy for cirrhotic cardiomyopathy: A randomized-controlled trial. Eur. J. Gastroenterol. Hepatol. 2018, 30, 930–937. [Google Scholar] [CrossRef]
- Taniguchi, Y.; Ueshima, K.; Chiba, I.; Segawa, I.; Kobayashi, N.; Saito, M.; Hiramori, K. A new method using pulmonary gas-exchange kinetics to evaluate efficacy of beta-blocking agents in patients with dilated cardiomyopathy. Chest 2003, 124, 954–961. [Google Scholar] [CrossRef] [PubMed]
- Toyama, T.; Aihara, Y.; Iwasaki, T.; Hasegawa, A.; Suzuki, T.; Nagai, R.; Endo, K.; Hoshizaki, H.; Oshima, S.; Taniguchi, K. Cardiac sympathetic activity estimated by 123I-MIBG myocardial imaging in patients with dilated cardiomyopathy after beta-blocker or angiotensin-converting enzyme inhibitor therapy. J. Nucl. Med. 1999, 40, 217–223. [Google Scholar] [PubMed]
- Wu, L.; Zhang, Q.; Shu, Q.; Zhang, R.; Meng, Y. Sex-dependent changes in physical, mental, and quality of life outcomes in metoprolol-treated Chinese chronic heart failure patients. Medicine 2019, 98, e18331. [Google Scholar] [CrossRef] [PubMed]
- Corea, L.; Bentivoglio, M.; Verdecchia, P.; Provvidenza, M.; Motolese, M. Left ventricular hypertrophy regression in hypertensive patients treated with metoprolol. Int. J. Clin. Pharmacol. Ther. Toxicol. 1984, 22, 365–370. [Google Scholar]
- Frais, M.A.; Silke, B.; Ahuja, R.C.; Verma, S.P.; Nelson, G.I.; Taylor, S.H. Cardioselective beta-blockade with atenolol and acebutolol following acute myocardial infarction: A multiple-dose haemodynamic comparison. J. Cardiovasc. Pharmacol. 1985, 7, 80–85. [Google Scholar] [CrossRef]
- Gottdiener, J.S.; Reda, D.J.; Williams, D.W.; Materson, B.J.; Cushman, W.; Anderson, R.J. Effect of single-drug therapy on reduction of left atrial size in mild to moderate hypertension: Comparison of six antihypertensive agents. Circulation 1998, 98, 140–148. [Google Scholar] [CrossRef]
- Graettinger, W.F.; Lipson, J.L.; Klein, R.C.; Cheung, D.G.; Weber, M.A. Comparison of antihypertensive therapies by noninvasive techniques. Chest 1989, 96, 74–79. [Google Scholar] [CrossRef]
- Herman, R.B.; Jesudason, P.J.; Mustafa, A.M.; Husain, R.; Choy, A.M.; Lang, C.C. Differential effects of carvedilol and atenolol on plasma noradrenaline during exercise in humans. Br. J. Clin. Pharmacol. 2003, 55, 134–138. [Google Scholar] [CrossRef]
- Kyriakides, Z.S.; Kremastinos, D.; Karavolias, G.; Papadopoulos, C.; Apostolou, T.; Paraskevaidis, J.; Toutouzas, P. Intravenous atenolol in elderly patients in the early phase of acute myocardial infarction. Cardiovasc. Drugs Ther. 1992, 6, 475–479. [Google Scholar] [CrossRef]
- Malmqvist, K.; Kahan, T.; Edner, M.; Held, C.; Hagg, A.; Lind, L.; Muller-Brunotte, R.; Nystrom, F.; Ohman, K.P.; Osbakken, M.D.; et al. Regression of left ventricular hypertrophy in human hypertension with irbesartan. J. Hypertens. 2001, 19, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Myreng, Y.; Ihlen, H.; Nitter-Hauge, S. Effects of beta-adrenergic blockade on left ventricular relaxation and filling dynamics in coronary artery disease: A pulsed Doppler echocardiographic study. Eur. Heart J. 1988, 9, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Otterstad, J.E.; Froeland, G.; Erikssen, J. Left ventricular end-diastolic dimensions measured at the P wave and Q wave during a randomized, double-blind one-year follow-up study comparing the effect of atenolol vs. hydrochlorothiazide + amiloride on blood pressure in men with mild to moderate hypertension. Scand. J. Clin. Lab. Investig. 1993, 53, 155–162. [Google Scholar] [CrossRef]
- Todd, I.C.; Ballantyne, D. Antianginal efficacy of exercise training: A comparison with beta blockade. Br. Heart J. 1990, 64, 14–19. [Google Scholar] [CrossRef]
- Zemel, M.B.; Zemel, P.C.; Bryg, R.J.; Sowers, J.R. Dietary calcium induces regression of left ventricular hypertrophy in hypertensive non-insulin-dependent diabetic blacks. Am. J. Hypertens. 1990, 3, 458–463. [Google Scholar] [CrossRef]
- Aronow, W.S.; Spivacek, N.; Laverty, W.; Warren, M. Effect of tolamolol and propranolol on exercise heart rate and angina. Clin. Pharmacol. Ther. 1975, 17, 379–384. [Google Scholar] [CrossRef]
- Dogan, A.; Orscelik, O.; Kocyigit, M.; Elcik, D.; Baran, O.; Cerit, N.; Inanc, M.T.; Kalay, N.; Ismailogullari, S.; Saka, T.; et al. The effect of prophylactic migraine treatment on arterial stiffness. Blood Press. 2015, 24, 222–229. [Google Scholar] [CrossRef]
- Ledwich, J.R. A trial of propranolol in myocardial infarction. Can. Med. Assoc. J. 1968, 98, 988–994. [Google Scholar]
- Maekawa, K.; Saito, D.; Kobayashi, H.; Mizuo, K.; Obayashi, N.; Uchida, S.; Haraoka, S. Effects of nipradilol on venous hemodynamics: Evaluation with a Doppler blood flow method. Acta Med. Okayama 1994, 48, 87–91. [Google Scholar] [CrossRef]
- Mosley, C.; O’Connor, D.T.; Taylor, A.; Slutsky, R.A.; Cervenka, J. Comparative effects of antihypertensive therapy with guanabenz and propranolol on renal vascular resistance and left ventricular mass. J. Cardiovasc. Pharmacol. 1984, 6, S757–S761. [Google Scholar] [CrossRef] [PubMed]
- Silke, B.; Frais, M.A.; Verma, S.P.; Reynolds, G.; Taylor, S.H. Differences in haemodynamic response to beta-blocking drugs between stable coronary artery disease and acute myocardial infarction. Eur. J. Clin. Pharmacol. 1986, 29, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Silke, B.; Nelson, G.I.; Ahuja, R.C.; Walker, C.; Taylor, S.H. Comparison of haemodynamic dose-response effects of beta- and alpha-beta-blockade in acute myocardial infarction. Int. J. Cardiol. 1984, 5, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Silke, B.; Nelson, G.I.; Verma, S.P.; Hussain, M.; Ahuja, R.C.; Walker, C.; Taylor, S.H. Enhanced haemodynamic effects of propranolol in acute myocardial infarction. Eur. Heart. J. 1984, 5, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.G.; Nelson, R.R.; Nordstrom, L.A.; Diefenthal, H.C.; Gobel, F.L. Comparative effect of nadolol and propranolol on exercise tolerance in patients with angina pectoris. Br. Heart J. 1978, 40, 1361–1370. [Google Scholar] [CrossRef]
- Yegnanarayan, R.; Sangle, S.A.; Sirsikar, S.S.; Mitra, D.K. Regression of cardiac hypertrophy in hypertensive patients—Comparison of Abana with propranolol. Phytother. Res. Int. J. Devoted Med. Sci. Res. Plants Plant Prod. 1997, 11, 257–259. [Google Scholar] [CrossRef]
- Bozkurt, B.; Bolos, M.; Deswal, A.; Ather, S.; Chan, W.; Mann, D.L.; Carabello, B. New insights into mechanisms of action of carvedilol treatment in chronic heart failure patients--a matter of time for contractility. J. Card. Fail. 2012, 18, 183–193. [Google Scholar] [CrossRef]
- de Boer, R.A.; Siebelink, H.J.; Tio, R.A.; Boomsma, F.; van Veldhuisen, D.J. Carvedilol increases plasma vascular endothelial growth factor (VEGF) in patients with chronic heart failure. Eur. J. Heart Fail. 2001, 3, 331–333. [Google Scholar] [CrossRef]
- Ito, T.; Kawanishi, Y.; Futai, R.; Terasaki, F.; Kitaura, Y. Usefulness of carvedilol to abolish myocardial postsystolic shortening in patients with idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2009, 104, 1568–1573. [Google Scholar] [CrossRef]
- Kaye, D.M.; Johnston, L.; Vaddadi, G.; Brunner-LaRocca, H.; Jennings, G.L.; Esler, M.D. Mechanisms of carvedilol action in human congestive heart failure. Hypertension 2001, 37, 1216–1221. [Google Scholar] [CrossRef]
- Lund-Johansen, P.; Omvik, P.; Nordrehaug, J.E.; White, W. Carvedilol in hypertension: Effects on hemodynamics and 24-hour blood pressure. J. Cardiovasc. Pharmacol. 1992, 19, S27–S34. [Google Scholar] [CrossRef] [PubMed]
- Quaife, R.A.; Christian, P.E.; Gilbert, E.M.; Datz, F.L.; Volkman, K.; Bristow, M.R. Effects of carvedilol on right ventricular function in chronic heart failure. Am. J. Cardiol. 1998, 81, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Ridha, M.; Makikallio, T.H.; Lopera, G.; Pastor, J.; de Marchena, E.; Chakko, S.; Huikuri, H.V.; Castellanos, A.; Myerburg, R.J. Effects of carvedilol on heart rate dynamics in patients with congestive heart failure. Ann. Noninvasive Electrocardiol. 2002, 7, 133–138. [Google Scholar] [CrossRef]
- Yeoh, T.; Hayward, C.; Benson, V.; Sheu, A.; Richmond, Z.; Feneley, M.P.; Keogh, A.M.; Macdonald, P.; Fatkin, D. A randomised, placebo-controlled trial of carvedilol in early familial dilated cardiomyopathy. Heart Lung Circ. 2011, 20, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Silke, B.; Nelson, G.; Verma, S.P.; Clarke, J.; Baliga, G.; Taylor, S.H. Circulatory effects of intravenous and oral acebutolol in acute myocardial infarction. Clin. Pharmacol. Ther. 1985, 38, 266–272. [Google Scholar] [CrossRef]
- Tomiyama, H.; Doba, N.; Kushiro, T.; Yamashita, M.; Yoshida, H.; Kanmatsuse, K.; Kajiwara, N.; Hinohara, S. Effects of long-term antihypertensive therapy on physical fitness of men with mild hypertension. Hypertens. Res. 1997, 20, 105–111. [Google Scholar] [CrossRef]
- Frishman, W.H.; Strom, J.A.; Kirschner, M.; Poland, M.; Klein, N.; Halprin, S.; LeJemtel, T.H.; Kram, M.; Sonnenblick, E.H. Labetalol therapy in patients with systemic hypertension and angina pectoris: Effects of combined alpha and beta adrenoceptor blockade. Am. J. Cardiol. 1981, 48, 917–928. [Google Scholar] [CrossRef]
- Nelson, G.I.; Silke, B.; Ahuja, R.C.; Hussain, M.; Taylor, S.H. Haemodynamic dose-response effects of intravenous labetalol in acute myocardial infarction. Postgrad. Med. J. 1983, 59, 98–103. [Google Scholar]
- Renard, M.; Jacobs, P.; Melot, C.; Haardt, R.; Vainsel, H.; Bernard, R. Effect of labetalol on preload in acute myocardial infarction with systemic hypertension. J. Cardiovasc. Pharmacol. 1984, 6, 90–93. [Google Scholar] [CrossRef]
- Brune, S.; Schmidt, T.; Tebbe, U.; Kreuzer, H. Hemodynamic effects of nebivolol at rest and on exertion in patients with heart failure. Angiology 1990, 41, 696–701. [Google Scholar] [CrossRef]
- Gunes, Y.; Tuncer, M.; Guntekin, U.; Ceylan, Y.; Sahin, M.; Simsek, H. Regional functions of the left ventricle in patients with coronary slow flow and the effects of nebivolol. Ther. Adv. Cardiovasc. Dis. 2009, 3, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, T.; Heise, T.; Nosek, L.; Eckers, U.; Sawicki, P.T. Influence of nebivolol and enalapril on metabolic parameters and arterial stiffness in hypertensive type 2 diabetic patients. J. Hypertens. 2006, 24, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Bett, N.; Dryburgh, L.; Boyle, C.; Hawley, C. Hemodynamic properties of bucindolol, a beta-adrenoreceptor blocking drug with vasodilator activity. Am. J. Cardiol. 1986, 57, 678–681. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, E.J.; Bedotto, J.B.; Malloy, C.R.; Hatfield, B.A.; Deitchman, D.; Brown, M.; Willard, J.E.; Grayburn, P.A. Effect of beta-adrenergic blockade on myocardial function and energetics in congestive heart failure. Improvements in hemodynamic, contractile, and diastolic performance with bucindolol. Circulation 1990, 82, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Silke, B.; Verma, S.P.; Ahuja, R.C.; Hussain, M.; Hafizullah, M.; Reynolds, G.; Nelson, G.I.; Taylor, S.H. Is the intrinsic sympathomimetic activity (ISA) of beta-blocking compounds relevant in acute myocardial infarction? Eur. J. Clin. Pharmacol. 1984, 27, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, C.L.; Kokkas, B.A.; Kotridis, P.S.; Gitsios, C.T.; Sakadamis, G.C.; Kanonidis, J.E.; Kotoula, M.I.; Paradelis, A.G. The effect of β1-blocker bisoprolol on atrial natriuretic peptide plasma levels in hypertensive patients. Int. J. Angiol. 1995, 4, 165–168. [Google Scholar] [CrossRef]
- Kinhal, V.; Kulkarni, A.; Pozderac, R.; Cubbon, J. Hemodynamic effects of dilevalol in patients with systemic hypertension and left ventricular dysfunction. Am. J. Cardiol. 1989, 63, 64I–68I. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Tamaki, H.; Hosaka, T.; Maezawa, H. The hemodynamics and hemodynamic effects of indenolol in mild hypertension. Jpn. Heart J. 1984, 25, 1029–1045. [Google Scholar] [CrossRef]
- Littler, W.A. Use of nifedipine as monotherapy in the management of hypertension. Am. J. Med. 1985, 79, 36–40. [Google Scholar] [CrossRef]
- Rosales, O.R.; Sander, G.E.; Roffidal, L.; Given, M.B.; Giles, T.D. Carteolol, an antihypertensive beta-blocker with intrinsic sympathomimetic activity, reduces ECG evidence of left ventricular hypertrophy. Chest 1989, 95, 43–47. [Google Scholar] [CrossRef]
- National Health Care Institute. Metoprolol. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/m/metoprolol#dosering (accessed on 11 June 2021).
- National Health Care Institute. Labetalol p.o. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/l/labetalol (accessed on 11 June 2021).
- Labetalol IV. Available online: https://www.pdr.net/drug-summary/Labetalol-Hydrochloride-Injection-labetalol-hydrochloride-1568 (accessed on 11 June 2021).
- National Health Care Institute. Atenolol. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/a/atenolol (accessed on 11 June 2021).
- National Health Care Institute. Carvedilol. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/c/carvedilol (accessed on 11 June 2021).
- National Health Care Institute. Acebutolol. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/a/acebutolol (accessed on 11 June 2021).
- National Health Care Institute. Bisoprolol. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/b/bisoprolol (accessed on 11 June 2021).
- National Health Care Institute. Pindolol p.o. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/p/pindolol (accessed on 11 June 2021).
- National Health Care Institute. Nebivolol. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/n/nebivolol (accessed on 11 June 2021).
- National Center for Advancing Translational Sciences. Indenolol. Available online: https://drugs.ncats.io/drug/BRV874RC9S (accessed on 11 June 2021).
- National Health Care Institute. Propranolol p.o. Available online: https://www.farmacotherapeutischkompas.nl/bladeren/preparaatteksten/p/propranolol__cardiovasculair_of_neurologisch (accessed on 11 June 2021).
- Propranolol IV. Available online: https://www.pdr.net/drug-summary/Propranolol-Hydrochloride-Injection-propranolol-hydrochloride-1734.8469#topPage (accessed on 11 June 2021).
- Drugs.com. Nadolol Dosage. Available online: https://www.drugs.com/dosage/nadolol.html (accessed on 11 June 2021).
- Nipradilol. Available online: http://www.jodrugs.com/products/38009-nipradilol.aspx (accessed on 11 June 2021).
- Eichhorn, E.J.; Domanski, M.J.; Krause-Steinrauf, H.; Bristow, M.R.; Lavori, P.W. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N. Engl. J. Med. 2001, 344, 1659–1667. [Google Scholar] [CrossRef] [PubMed]
- Luther, R.R.; Maurath, C.J.; Klepper, M.J.; Peckinpaugh, R.O.; Ringham, G.L. Carteolol treatment of essential hypertension: A long-term study of safety and efficacy. J. Int. Med. Res. 1986, 14, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Chrisp, P.; Goa, K.L. Dilevalol. Drugs 1990, 39, 234–263. [Google Scholar] [CrossRef] [PubMed]
- Routledge, P.A.; Davies, D.M.; Rawlins, M.D. Pharmacokinetics of tolamolol in the treatment of hypertension. Eur. J. Clin. Pharmacol. 1977, 12, 171–174. [Google Scholar] [CrossRef]
- Celiprolol. Available online: https://www.medicines.org.uk/emc/product/4338/smpc#gref (accessed on 11 June 2021).
- Drugs.com. Timolol. Available online: https://www.drugs.com/dosage/timolol.html (accessed on 11 June 2021).
- Thomopoulos, C.; Parati, G.; Zanchetti, A. Effects of blood pressure lowering on outcome incidence in hypertension: 4. Effects of various classes of antihypertensive drugs--overview and meta-analyses. J. Hypertens. 2015, 33, 195–211. [Google Scholar] [CrossRef]
- Fletcher, A.; Beevers, D.G.; Bulpitt, C.; Butler, A.; Coles, E.C.; Hunt, D.; Munro-Faure, A.D.; Newson, R.B.; O’Riordan, P.W.; Petrie, J.C.; et al. Beta adrenoceptor blockade is associated with increased survival in male but not female hypertensive patients: A report from the DHSS Hypertension Care Computing Project (DHCCP). J. Hum. Hypertens. 1988, 2, 219–227. [Google Scholar]
- Beta-Blocker Heart Attack Study Group. The beta-blocker heart attack trial. JAMA 1981, 246, 2073–2074. [Google Scholar] [CrossRef]
- Bugiardini, R.; Yoon, J.; Kedev, S.; Stankovic, G.; Vasiljevic, Z.; Miličić, D.; Manfrini, O.; Schaar, M.V.D.; Gale, C.P.; Badimon, L.; et al. Prior Beta-Blocker Therapy for Hypertension and Sex-Based Differences in Heart Failure Among Patients With Incident Coronary Heart Disease. Hypertension 2020, 76, 819–826. [Google Scholar] [CrossRef]
- Farzam, K.; Jan, A. Beta Blockers. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Tamargo, J.; Rosano, G.; Walther, T.; Duarte, J.; Niessner, A.; Kaski, J.C.; Ceconi, C.; Drexel, H.; Kjeldsen, K.; Savarese, G.; et al. Gender differences in the effects of cardiovascular drugs. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 163–182. [Google Scholar] [CrossRef]
- Luzier, A.B.; Killian, A.; Wilton, J.H.; Wilson, M.F.; Forrest, A.; Kazierad, D.J. Gender-related effects on metoprolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin. Pharmacol. Ther. 1999, 66, 594–601. [Google Scholar] [CrossRef]
- Jochmann, N.; Stangl, K.; Garbe, E.; Baumann, G.; Stangl, V. Female-specific aspects in the pharmacotherapy of chronic cardiovascular diseases. Eur. Heart J. 2005, 26, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Kendall, M.J.; Quarterman, C.P.; Jack, D.B.; Beeley, L. Metoprolol pharmacokinetics and the oral contraceptive pill. Br. J. Clin. Pharmacol. 1982, 14, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, D.A.; Gal, J.; Gerber, J.G.; Nies, A.S. Age and gender influence the stereoselective pharmacokinetics of propranolol. J. Pharmacol. Exp. Ther. 1992, 261, 1181–1186. [Google Scholar] [PubMed]
- Olsson, G.; Wikstrand, J.; Warnold, I.; Manger Cats, V.; McBoyle, D.; Herlitz, J.; Hjalmarson, A.; Sonneblick, E.H. Metoprolol-induced reduction in postinfarction mortality: Pooled results from five double-blind randomized trials. Eur. Heart J. 1992, 13, 28–32. [Google Scholar] [CrossRef]
- Packer, M.; Coats, A.J.; Fowler, M.B.; Katus, H.A.; Krum, H.; Mohacsi, P.; Rouleau, J.L.; Tendera, M.; Castaigne, A.; Roecker, E.B.; et al. Effect of carvedilol on survival in severe chronic heart failure. N. Engl. J. Med. 2001, 344, 1651–1658. [Google Scholar] [CrossRef] [PubMed]
- MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999, 353, 2001–2007. [Google Scholar] [CrossRef]
- Simon, T.; Mary-Krause, M.; Funck-Brentano, C.; Jaillon, P. Sex Differences in the Prognosis of Congestive Heart Failure. Circulation 2001, 103, 375–380. [Google Scholar] [CrossRef]
- Packer, M.; Fowler, M.B.; Roecker, E.B.; Coats, A.J.; Katus, H.A.; Krum, H.; Mohacsi, P.; Rouleau, J.L.; Tendera, M.; Staiger, C.; et al. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: Results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation 2002, 106, 2194–2199. [Google Scholar] [CrossRef]
- Ghali, J.K.; Piña, I.L.; Gottlieb, S.S.; Deedwania, P.C.; Wikstrand, J.C. Metoprolol CR/XL in female patients with heart failure: Analysis of the experience in Metoprolol Extended-Release Randomized Intervention Trial in Heart Failure (MERIT-HF). Circulation 2002, 105, 1585–1591. [Google Scholar] [CrossRef]
- Leizorovicz, A.; Lechat, P.; Cucherat, M.; Bugnard, F. Bisoprolol for the treatment of chronic heart failure: A meta-analysis on individual data of two placebo-controlled studies—CIBIS and CIBIS II. Cardiac Insufficiency Bisoprolol Study. Am. Heart J. 2002, 143, 301–307. [Google Scholar] [CrossRef]
- Ghali, J.K. Sex-related differences in heart failure and beta-blockers. Heart Fail. Rev. 2004, 9, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ghali, J.K.; Krause-Steinrauf, H.J.; Adams, K.F.; Khan, S.S.; Rosenberg, Y.D.; Yancy, C.W.; Young, J.B.; Goldman, S.; Peberdy, M.A.; Lindenfeld, J. Gender differences in advanced heart failure: Insights from the BEST study. J. Am. Coll. Cardiol. 2003, 42, 2128–2134. [Google Scholar] [CrossRef]
- Shekelle, P.G.; Rich, M.W.; Morton, S.C.; Atkinson, C.S.W.; Tu, W.; Maglione, M.; Rhodes, S.; Barrett, M.; Fonarow, G.C.; Greenberg, B.; et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status. J. Am. Coll. Cardiol. 2003, 41, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Manzano, L.; Krum, H.; Rosano, G.; Holmes, J.; Altman, D.G.; Collins, P.D.; Packer, M.; Wikstrand, J.; Coats, A.J.S.; et al. Effect of age and sex on efficacy and tolerability of β blockers in patients with heart failure with reduced ejection fraction: Individual patient data meta-analysis. BMJ 2016, 353, i1855. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Bairey Merz, N.; Barnes, P.J.; Brinton, R.D.; Carrero, J.J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef] [PubMed]

| Study | Patient | Ethnicity | Beta Blocker Treatment | Mean Dose (mg/Day) | % Max Dose * | Subjects Beta Blockers (n) | Control Group ** | Controls (n) | Age | Intervention Duration (Days) | Study Design | Extracted Variables | Mentioned Methods of Measurement | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | M | F | Total | M | F | (Years + SD) | ||||||||||||
| Bozkurt et al. (2012) [63] | HFrEF | W, B, H | Carvedilol | 28.9 | 57.8 | 49 | 49 | 0 | Baseline *** | 49 | 49 | 0 | 63.9 | 9 | 182.63 | PCS | SBP, DBP, MAP, HR, LVEF, LVMI, LVEDVI, LVESVI, E/A ratio, CI, LVESD, LVEDD | Echo |
| Damy et al. (2003) [27] | He | W | Metoprolol | 200 | 1 | 36 | 36 | 0 | Placebo | 13 | 13 | 0 | 29.4 | 4.0 | 5 | RCT | SBP, DBP, HR, CO, LVEF | Echo, ECG, phonocardiogram, carotidogram, TEIC |
| Kaiser et al. (2006) [78] | T2D, HTN | - | Nebivolol | 5 | 100 | 10 | 7 | 3 | Enalapril *** | 10 | 7 | 3 | 54.4 | 8.8 | 84 | RCT | SBP, DBP, MAP, HR | Sphygmomanometer |
| Kampus et al. (2011) [32] | HTN | - | Nebivolol | 5 | 100 | 80 | 41 | 39 | Baseline *** | 80 | 41 | 39 | 46.5 | 9.9 | 365 | RCT | SBP, DBP, MAP, HR, LVEF, LVMI, E/A ratio | Echo, oscillometric |
| Metroprolol (succinate) | 75 | 38 | ||||||||||||||||
| Malmqvist et al. (2001) [48] | HTN, LVH | W | Atenolol | 75 | 75 | 58 | 40 | 18 | Irbesartan | 56 | 36 | 20 | 54.3 | 9.5 | 336 | RCT | SBP, DBP, MAP, HR, LVEF, LVMI, E/A ratio, LVEDD, | Sphygmomanometer, echo, ECG |
| Hashimoto et al. (1984) [84] | HTN | - | Indenolol | 30 | 25 | 16 | 16 | 0 | Baseline *** | 16 | 16 | 0 | 39.8 | 10.7 | 7 | PCS | SBP, DBP, HR, LVEF, LVDd, LVDs, CI, MBP | Sphygmomanometer, ECG, echo |
| Turner et al. (1978) [61] | AP | W | Propranolol or nadolol with placebo | 160 | 50 | 28 | 28 | 0 | Baseline *** | 28 | 28 | 0 | 53.0 | 8.0 | 28 | PCS | SBP, HR | Sphygmomanometer, ECG |
| Bentivoglio et al. (1984) [42] | HTN, LVH | - | Metoprolol (tartrate) | 200 | 50 | 8 | 8 | 0 | Baseline *** | 8 | 8 | 0 | 37.4 | 9.0 | 510 | PCS | SBP, DBP, MAP, HR, LVEF | Sphygmomanometer |
| Papadopoulos et al. (1995) [82] | HTN | - | Bisoprolol | 10 | 50 | 28 | 28 | 0 | Baseline *** | 28 | 28 | 0 | 52.5 | - | 30 | PCS | SBP, DBP, HR, LVD, LAD | Sphygmomanometer, echo |
| Gottdiener et al. (1998) [44] | HTN | W, B | Atenolol | 62.5 | 63 | 76 | 76 | 0 | Baseline *** | 76 | 76 | 0 | 58.8 | 10.0 | 56 | RCT | SBP, LA | Sphygmomanometer, echo |
| Tomiyama et al. (1997) [72] | HTN | A | Acebutolol | 250 | 21 | 9 | 9 | 0 | Nifedipine | 13 | 13 | 0 | 44.8 | 6.3 | 1095 | RCS | SBP, DBP, LVEF, LVMI, RWTd | Sphygmomanometer, echo |
| Yegnanarayan et al. (1997) [62] | HTN, LVH | - | Propranolol | 80 | 25 | 20 | 20 | 0 | Abana | 20 | 20 | 0 | - | - | 420 | RCT | SBP, DBP, IVST, LVPWT, FS, LVIDs, LVIDd | Sphygmomanometer, echo |
| Maekawa et al. (1994) [56] | He | - | Propranolol | 20 | 6 | 15 | 15 | 0 | Baseline *** | 15 | 15 | 0 | - | - | 0.08 | PCS | SBP, DBP, HR | Sphygmomanometer, echo, ECG |
| Nipradilol | 6 | 33 | ||||||||||||||||
| De Boer et al. (2001) [64] | HFrEF | - | Carvedilol | 61.5 | 123 | 12 | 12 | 0 | Placebo | 5 | 5 | 0 | 60.5 | 8.2 | 90 | RCT | SBP, DBP, HR | - |
| Otterstad et al. (1993) [50] | HTN | - | Atenolol | 75 | 75 | 31 | 31 | 0 | Co-amiloride | 49 | 48 | 1 | 45.9 | 11.5 | 360 | RCT | SBP, DBP, HR, LVM, LVMI, IVST, LVPWT, RWT, LVID | ECG, echo |
| Ledwich (1968) [55] | AMI | - | Propranolol | 60 and 120 | 19 and 38 | 20 | 20 | 0 | Placebo | 20 | 20 | 0 | 60.4 | 8.0 | 7 | RCT | SBP, DBP, HR | ECG |
| Bett et al. (1986) [79] | He | - | Bucindolol | 50 and 200 | 25 and 100 | 13 | 13 | 0 | Placebo *** | 13 | 13 | 0 | 22.0 | 1.0 | 0.167 | RCT | SBP, DBP, HR, CI, FS | Sphygmomanometer, echo, ECG |
| Frishman et al. (1981) [73] | HTN, AP | - | Labetalol | 1050 | 44 | 10 | 10 | 0 | Baseline *** | 10 | 10 | 0 | 59.4 | 5.78 | 28 | PCS | SBP, DBP, HR, LVEF, LVEDVI, LVESVI, CI, IVSTd, LVPWT | Sphygmomanometer, ECG, echo |
| Davis et al. (2006) [28] | HFrEF | - | Metoprolol (succinate) | 118.75 | 59 | 8 | 8 | 0 | Unchanged medication | 8 | 8 | 0 | 64.2 | 2.5 | 42 | RCT | SBP, DBP, HR, CO | ECG, oscillometric |
| Dogan et al. (2015) [54] | Migraine | - | Propranolol | 80 | 25 | 24 | 7 | 17 | No medication | 80 | 25 | 55 | 33.3 | 9.6 | 30 | PCS | SBP, DBP, HR, LVEF, IVST, LVPWT, LVESD, LVEDD | Echo, oscillometric |
| Myreng et al. (1988) [49] | AP | - | Atenolol | 100 | 100 | 20 | 20 | 0 | Healthy subjects | 18 | 16 | 2 | - | - | 126 | PCS | SBP, DBP, HR, desceleraton, E/A ratio, E, A, SV, IVRT | echo, ECG |
| Cocco et al. (2006) [26] | AP | - | Metoprolol (succinate) | 119 | 60 | 142 | 70 | 72 | Baseline *** | 142 | 70 | 72 | 58.0 | 9.2 | 84 | RCT | SBP, DBP, HR | ECG |
| Bekheit (1990) [24] | MI | - | Metoprolol | 200 | 100 | 8 | 8 | 0 | Diltiazem, nifedipine | 19 | 19 | 0 | 62.0 | 13.0 | 6 | PCS | SBP, DBP, HR | Sphygmomanometer, ECG |
| Rosales et al. (1989) [86] | HTN, LVH | - | Carteolol | 14.8 | 25 | 16 | 16 | 0 | Baseline *** | 16 | 16 | 0 | 58.9 | 4.0 | 365 | RCS | SBP, DBP, MAP | ECG |
| Marcovitz (1997) [34] | He | - | Metoprolol | 50 | 25 | 6 | 6 | 0 | Baseline *** | 6 | 6 | 0 | - | - | 3 | PCS | SBP, HR, LVEF, FS | ECG |
| Hall et al. (1995) [29] | HF | B, W | Metoprolol | 56.25 | 28 | 16 | 16 | 0 | Standard Therapy | 10 | 10 | 0 | 54.0 | 3.6 | 90 | RCT | SBP, HR, LVEF, LVM, LVEDV, LVESV | Echo |
| Kinhal et al. (1989) [83] | HTN, HFrEF | B, W | Dilevalol | 400 | 25 | 9 | 9 | 0 | Baseline *** | 9 | 9 | 0 | 60.0 | - | 39 | PCS | SBP, DBP, HR | Sphygmomanometer, ECG |
| Mosley et al. (1984) [57] | HTN | B, W | Propranolol | 167.5 | 52 | 12 | 12 | 0 | Guanabenz | 14 | 14 | 0 | 48.2 | 4.8 | 42 | RCT | SBP, DBP, HR, CO, LVM, SVR | Sphygmomanometer, echo |
| Graettinger et al. (1989) [45] | HTN | W | Atenolol | 156 | 156 | 10 | 10 | 0 | Lisinopril | 9 | 9 | 0 | 56.0 | - | 84 | RCT | SBP, DBP, HR, LVEF, LVM, IVST, LVPWT, LVIDd, RWT, RVD | Sphygmomanometer, echo |
| Silvestre et al. (2018) [38] | Cirrhotic cardiomyopathy | W, O | Metoprolol (succinate) | 120 | 60 | 41 | 18 | 23 | Placebo | 37 | 14 | 23 | 50.4 | - | 180 | RCT | SBP, DBP, HR | Echo |
| Wu et al. (2019) [41] | CHF | A | Metoprolol | 99.75 | 50 | 154 | 101 | 53 | Baseline *** | 154 | 101 | 53 | 66.4 | - | 270 | PCS | SBP, HR, LVEF, CI | Sphygmomanometer, echo, ECG |
| Osadchuk et al. (2019) [35] | HTN, CHD | - | Metoprolol (succinate) | 56.1 | 28 | 21 | 7 | 14 | Ramipril | 20 | 8 | 12 | 70.6 | 7.2 | 70 | RCT | SBP, DBP, HR | Oscillometric, ECG |
| Herman et al. (2003) [46] | He | - | Carvedilol | 18.75 | 38 | 12 | 12 | 0 | Baseline *** | 12 | 12 | 0 | 21.6 | 0.3 | 14 | RCT | DBP, HR | Sphygmomanometer |
| Atenolol | 37.5 | 38 | ||||||||||||||||
| Zemel et al. (1990) [52] | HTN, LVH | B | Atenolol | 50 | 50 | 6 | 6 | 0 | Calcium supplements *** | 6 | 6 | 0 | - | - | 84 | CCS | MAP, E/A ratio, LVPWT, FS | Oscillometric, ECG, echo |
| Ridha et al. (2002) [69] | CHF | W, B | Carvedilol | 32 | 64 | 15 | 15 | 0 | Baseline *** | 15 | 15 | 0 | 62.0 | 11.0 | 84 | PCS | HR, LVEF, MBP | Oscillometric, ECG |
| Silke et al. (1997) [37] | He | - | Metoprolol | 50 | 25 | 9 | 9 | 0 | Placebo *** | 9 | 9 | 0 | 22.1 | - | 0.33 | RCT | HR | ECG |
| Celiprolol | 200 | 50 | ||||||||||||||||
| Silke et al. (1986) [58] | AP, MI | - | Propranolol (i.v.) | 8 | 2.5 | 32 | 32 | 0 | Baseline *** | 32 | 32 | 0 | 52.8 | 7.0 | 0.0087 | PCS | HR, CI, SVRI | Catheter |
| Pindolol (i.v.) | 0.8 | 11 | ||||||||||||||||
| Silke et al. (1985) [71] | MI | - | Acebutolol (i.v.) | 25 and 50 | 4 and 8 | 24 | 24 | 0 | Baseline *** | 24 | 24 | 0 | 45.0 | - | 0.17 | RCT | HR, SVRI, CI | ECG, catheter |
| Acebutolol | 200 and 400 | 16 and 32 | ||||||||||||||||
| Silke et al. (1984) [59] | MI | - | Propranolol (i.v.) | 8 | 12 | 16 | 16 | 0 | Baseline *** | 16 | 16 | 0 | 54.0 | 1.8 | 0.01 | PCS | HR, SVRI, CI | Catheter |
| Labetalol (i.v.) | 40 | 14 | ||||||||||||||||
| Silke et al. (1984) [60] | AP, MI | - | Propranolol (i.v.) | 8 | 8 | 16 | 16 | 0 | Baseline *** | 16 | 16 | 0 | 51.5 | 3.3 | 0.00868 | PCS | HR, SVRI, CI | Catheter |
| Silke et al. (1984) [81] | AP, MI | - | Propranolol (i.v.) | 0.8 | 11 | 12 | 12 | 0 | Baseline *** | 12 | 12 | 0 | 52.8 | 5.5 | 0.0087 | PCS | HR, CI, SVRI | Catheter |
| Pindolol (i.v.) | 8 | 11 | ||||||||||||||||
| Taniguchi et al. (2003) [39] | CHF | - | Metoprolol | 30 and 60 | 15 and 30 | 12 | 10 | 2 | Baseline *** | 12 | 10 | 2 | 54.0 | 12.0 | 432 | PCS | HR, MBP | Echo |
| Atenolol | 25 | 25 | ||||||||||||||||
| Carteolol | 10 and 20 | 17 and 33 | ||||||||||||||||
| Kyriakides et al. (1992) [47] | AMI | - | Atenolol (i.v.) | 5 | 5 | 28 | 28 | 0 | Baseline *** | 28 | 28 | 0 | 53.0 | 6.0 | 0.0069 | PCS | HR, CI, MBP, SVR | ECG, catheter |
| Bennett et al. (2002) [25] | CHF | - | Metoprolol (succinate) | 106.25 | 53 | 12 | 12 | 0 | Baseline *** | 12 | 12 | 0 | 62.0 | 10.0 | 180 | PCS | HR, LVEF | PET |
| Brune et al. (1990) [76] | CHD | - | Nebivolol | 5 | 100 | 10 | 10 | 0 | Baseline *** | 10 | 10 | 0 | 56.7 | 4.8 | 7 | PCS | HR, CO, RAP, SV | Catheter |
| Renard et al. (1983) [36] | MI | - | Metoprolol (tartrate) | 150 | 38 | 9 | 9 | 0 | Baseline *** | 9 | 9 | 0 | 53.0 | - | 1.04 | PCS | HR | Catheter |
| Renard et al. (1984) [75] | AMI, HTN | - | Labetalol (i.v.) | 126 | 5 | 18 | 18 | 0 | Baseline *** | 18 | 18 | 0 | 56.8 | - | 0.04 | PCS | HR, CI | Catheter |
| Kaye et al. (2001) [66] | HF | - | Carvedilol | 42.5 | 85 | 10 | 10 | 0 | Baseline *** | 10 | 10 | 0 | 55 | 3.0 | 90 | PCS | HR, CO, LVEF, RVEF | Catheter |
| Frais et al. (1985) [43] | AMI | - | Atenolol (i.v.) | 8 | - | 16 | 16 | 0 | Baseline | 16 | 16 | 0 | - | - | 0.0087 | RCT | HR, SVRI, CI | ECG, catheter |
| Acebutolol (i.v.) | 80 | - | ||||||||||||||||
| Aronow et al. (1975) [53] | AP, CHD | - | Tolamolol | 10 and 20 | 1 and 2 | 45 | 45 | 0 | Saline | 15 | 15 | 0 | 51.1 | - | 0.0035 | RCT | HR | ECG |
| Propranolol | 10 | 3 | ||||||||||||||||
| Heesch et al. (1995) [30] | HF | - | Metoprolol | 100 | 50 | 30 | 30 | 0 | Baseline *** | 30 | 30 | 0 | 48.0 | 11.0 | 90 | RCT | HR, CI, LVEDP, LVESP | Catheter |
| Bucindolol | 175 | 88 | ||||||||||||||||
| Ishida et al. (1993) [31] | CHF | - | Metoprolol | 45.6 | 23 | 9 | 9 | 0 | Baseline *** | 9 | 9 | 0 | 52.6 | 10.7 | 180 | PCS | HR, LVEF, LVESD, LVEDD, FS | Echo |
| Todd et al. (1990) [51] | AP | - | Atenolol | 100 | 100 | 20 | 20 | 0 | No beta-blocker | 20 | 20 | 0 | 52.0 | - | 7 | RCT | HR | ECG |
| Kronenberg et al. (1990) [33] | AMI | - | Metoprolol (i.v.) | 12.5 | 19 | 10 | 10 | 0 | Normal subjects | 13 | - | - | 42.1 | 14.2 | 0.0069 | PCS | HR, LVEF, RVEF, LVEDV, LVESV, SV | Sphygmomanometer, catheter, radionuclide studies |
| Nelson et al. (1983) [74] | AMI | - | Labetalol (i.v.) | 242 | 10 | 21 | 21 | 0 | Baseline *** | 21 | 21 | 0 | 53.0 | 1.5 | 0.1146 | PCS | HR, CI, SVRI | ECG, catheter |
| Littler (1985) [85] | HTN | - | Timolol | 30 | 50 | 9 | 9 | 0 | Nifedipine, indapamide | 17 | 11 | 6 | 39.7 | 10.6 | 112 | PCS | HR, LVMI | Echo, |
| Yeoh et al. (2011) [70] | Early DCM | - | Carvedilol | 15.63 | 31 | 16 | 9 | 7 | Placebo | 16 | 8 | 8 | 39.5 | 11.3 | 180 | RCT | HR, E, A, E/A ratio, LVESD, LVEDD, FS | Echo |
| Ito et al. (2009) [65] | Idiopathic DCM | - | Carvedilol | 7.4 | 15 | 19 | 7 | 12 | Baseline *** | 19 | 7 | 12 | 47.9 | 10.3 | 60 | PCS | HR, LVEF, E/A ratio, LVEDD, DT, Ea mean | Echo |
| Eichhorn et al. (1990) [80] | HFrEF | - | Bucindolol | 175 | 88 | 15 | 15 | 0 | Baseline *** | 15 | 15 | 0 | 50.0 | 11.0 | 90 | PCS | HR, CO, SV, LVEDP, SVR | Catheter |
| Toyama et al. (1999) [40] | DCM, HF | - | Metoprolol | 31.25 | 0.16 | 12 | 7 | 5 | Enalapril *** | 12 | 7 | 5 | 58.0 | 12.0 | 365 | PCS | LVEF, LVESD, LVEDD | Echo |
| Quaife et al. (1998) [68] | HFrEF | - | Carvedilol | 56.25 | 1.13 | 11 | 10 | 1 | Placebo | 11 | 10 | 1 | 53.5 | 11.8 | 120 | RCT | LVEF, RVEF | Radio-nuclide ventriculography, catheter |
| Gunes et al. (2009) [77] | CSF | - | Nebivolol | 5 | 1 | 27 | 18 | 9 | Subjects without CSF | 27 | 16 | 11 | 54.7 | 10.9 | 90 | PCS | LVEF, LVEDV, LVESV, LVEDD, DT, IVRT | Echo |
| Lund-Johansen et al. (1992) [67] | HTN | - | Carvedilol | 62 | 1.24 | 19 | 19 | 0 | Baseline *** | 19 | 19 | 0 | 44.0 | - | 224 | PCS | LVEF, E, A, E/A ratio, IVST, LVPWT, LVESD, LVEDD, FS | Echo |
| Male | Female | cMD Male | cMD Female | |
|---|---|---|---|---|
| DBP | 0.4947 | 0.7065 | ||
| SBP | 0.5928 | 0.9189 | ||
| MAP | 0.3027 | 0.8006 | ||
| CO | 0.3867 | - | ||
| HR | 0.0341 | 0.7028 | −12.1 [−13.5; −10.7] | |
| LVEF | 0.266 | 0.4353 | ||
| LVM | 0.1025 | - |
| Random Sequence Allocation (Selection Bias) | Allocation Concealment (Selection Bias) | Incomplete Outcome Data (Attrition Bias) | Measurements Outcomes (Detection Bias) | Selective Reporting (Reporting Bias) | Overall Bias | |
|---|---|---|---|---|---|---|
| Bozkurt et al. (2012) [63] | High | Low | Low | Low | Low | High |
| Damy et al. (2003) [27] | Low | Low | Low | Low | Low | Low |
| Kaiser et al. (2006) [78] | Low | Low | Low | Low | Low | Low |
| Kampus et al. (2011) [32] | Low | High | Low | Low | Some concerns | High |
| Malmqvist et al. (2001) [48] | Low | Low | Low | Low | Low | Low |
| Hashimoto et al. (1984) [84] | High | Some concerns | Low | Low | Low | High |
| Turner et al. (1978) [61] | Low | Low | Low | Low | Low | Low |
| Bentivoglio et al. (1984) [42] | High | Low | Low | Low | Low | High |
| Papadopoulos et al. (1995) [82] | High | Some concerns | Low | Low | Low | High |
| Gottdiener et al. (1998) [44] | Low | Low | Low | Low | Low | Low |
| Tomiyama et al. (1997) [72] | Some concerns | Low | Low | Low | Low | Some concerns |
| Yegnanarayan et al. (1997) [62] | Some concerns | Low | Low | Low | Low | Some concerns |
| Maekawa et al. (1994) [56] | High | Low | Low | Low | Low | High |
| De Boer et al. (2001) [64] | Low | Low | Low | Low | Low | Low |
| Otterstad et al. (1993) [50] | Low | Low | Low | Low | Low | Low |
| Ledwich (1968) [55] | High | Low | Low | Low | Some concerns | High |
| Bett et al. (1986) [79] | Some concerns | Low | Low | Low | Low | Some concerns |
| Frishman et al. (1981) [73] | Some concerns | Low | Low | Low | Low | Some concerns |
| Davis et al. (2006) [28] | Low | Some concerns | Low | Low | Low | Some concerns |
| Dogan et al. (2015) [54] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Myreng et al. (1988) [49] | High | Some concerns | Low | Low | Low | High |
| Cocco et al. (2006) [26] | Some concerns | Low | Low | Low | Low | Some concerns |
| Bekheit (1990) [24] | High | Low | Low | Low | Low | High |
| Rosales et al. (1989) [86] | High | Some concerns | Low | Low | Low | High |
| Marcovitz (1997) [34] | High | Low | Low | Some concerns | Low | High |
| Hall et al. (1995) [29] | Some concerns | Low | Low | Low | Low | Some concerns |
| Kinhal et al. (1989) [83] | High | Low | Low | Low | Low | High |
| Mosley et al. (1984) [57] | Some concerns | High | High | Low | Low | High |
| Graettinger et al. (1989) [45] | Low | Low | High | Low | Low | High |
| Silvestre et al. (2018) [38] | Low | Low | Low | Low | Low | Low |
| Wu et al. (2019) [41] | High | Low | Low | Low | Low | High |
| Osadchuk et al. (2019) [35] | Low | Low | Low | Low | Low | Low |
| Herman et al. (2003) [46] | Some concerns | Low | Low | Low | Low | Some concerns |
| Zemel et al. (1990) [52] | High | Low | Low | Low | Low | High |
| Ridha et al. (2002) [69] | High | Low | Low | Low | Low | High |
| Silke et al. (1997) [37] | Low | Low | Low | Low | Low | Low |
| Silke et al. (1986) [58] | Low | Low | Low | Low | Low | Low |
| Silke et al. (1985) [71] | Some concerns | Low | Low | Low | Low | Some concerns |
| Silke et al. (1984) [59] | Low | Some concerns | Low | Low | Low | Some concerns |
| Silke et al. (1984) [60] | High | Low | Low | Low | Low | High |
| Silke et al. (1984) [81] | High | Low | Low | Low | Low | High |
| Taniguchi et al. (2003) [39] | High | Low | Low | Low | Low | High |
| Kyriakides et al. (1992) [47] | High | Low | Low | Low | Low | High |
| Bennett et al. (2002) [25] | High | Low | Low | Low | Low | High |
| Brune et al. (1990) [76] | High | Low | Low | Low | Low | High |
| Renard et al. (1983) [36] | Some concerns | Low | Low | Low | Low | Some concerns |
| Renard et al. (1984) [75] | High | Some concerns | Low | Low | Low | High |
| Kaye et al. (2001) [66] | Some concerns | Low | Low | Low | Low | Some concerns |
| Frais et al. (1985) [43] | Low | Low | Low | Low | Low | Low |
| Aronow et al. (1975) [53] | Low | Low | Low | Low | Some concerns | Some concerns |
| Heesch et al. (1995) [30] | Low | Low | Low | Low | Low | Low |
| Ishida et al. (1993) [31] | High | Some concerns | Low | Low | Low | High |
| Todd et al. (1990) [51] | High | Low | High | Low | Low | High |
| Kronenberg et al. (1990) [33] | High | Low | Low | Low | Low | High |
| Nelson et al. (1983) [74] | Some concerns | Low | Low | Low | Low | Some concerns |
| Littler (1985) [85] | Some concerns | Low | Low | Low | Low | Some concerns |
| Yeoh et al. (2011) [70] | Low | Low | Low | Low | Low | Low |
| Ito et al. (2009) [65] | High | Low | Low | Low | Low | High |
| Eichhorn et al. (1990) [80] | High | Low | Low | Low | Low | High |
| Toyama et al. (1999) [40] | Some Concerns | Low | Low | Low | Low | Some Concerns |
| Quaife et al. (1998) [68] | Low | Low | Low | Low | Low | Low |
| Gunes et al. (2009) [77] | High | Low | Low | Low | Low | High |
| Lund-Johansen et al. (1992) [67] | High | Low | Low | Low | Low | High |
| Variable | Females | I2 | Males | I2 | p | |
|---|---|---|---|---|---|---|
| SBP | MD | −11.1 (95% CI, −14.5; −7.8) | 85% | −11.1 (95% CI, −14.0; −8.2) | 86% | 0.9767 |
| % | −7.9% (95% CI, −10.4; −5.4) | −8.2% (95% CI, −10.4; −6.1) | ||||
| DBP | MD | −8.0 (95% CI, −10.6; −5.3) | 83% | −8.0 (95% CI, −10.1; −6.0) | 91% | 0.9715 |
| % | −9.4% (95% CI, −12.5; −6.2) | −9.7% (95% CI, −12.2; −7.3) | ||||
| MAP | MD | −8.1 (95% CI, −11.7; −4.5) | 15% | −9.9 (95% CI, −17.0; −2.8) | 92% | 0.6594 |
| % | −7.5% (95% CI, −10.9; −4.2) | −8.9% (95% CI, −10.9; −4.2) | ||||
| HR | MD | −10.8 (95% CI, −17.4; −4.2) | 98% | −9.8 (95% CI, −11.1; −8.4) | 78% | 0.7585 |
| % | −14.2% (95% CI, −22.8; −5.5) | −13.2% (95% CI, −15.1; −11.4) | ||||
| CO | MD | N.A. | N.A. | −0.1 (95% CI, −0.5; 0.2) | 18% | N.A. |
| % | N.A. | −2.9% (95% CI, −9.2; 3.4) | ||||
| LVEF | MD | 4.2 (95% CI, −0.4; 8.8) | 92% | 3.7 (95% CI, 0.6; 6.9) | 95% | 0.8583 |
| % | 8.0% (95% CI, −0.7; 16.8) | 7.2% (95% CI, 1.1; 13.4) | ||||
| LVM | MD | N.A | N.A. | −20.6 (95% CI, −56.3; 15.1) | 74% | N.A. |
| % | N.A. | −7.4% (95% CI, −20.2; 5.4) |
| Sources of Heterogeneity | SBP | DBP | HR | CO | LVEF | MAP | LVM |
|---|---|---|---|---|---|---|---|
| Atenolol | 0.7715 | 0.3898 | 0.8726 | - | 0.8072 | - | - |
| Bisoprolol | 0.1544 | 0.1912 | 0.9143 | - | - | - | - |
| Bucindolol | 0.5205 | 0.6509 | 0.4296 | - | - | - | - |
| Carteolol | 0.6041 | 0.1132 | - | - | - | 0.2227 | - |
| Carvedilol | 0.2460 | 0.9885 | 0.7797 | 0.6611 | 0.9052 | 0.3838 | - |
| Celiprolol | - | - | 0.4035 | ||||
| Dilevalol | 0.7909 | 0.0744 | 0.4172 | - | - | - | - |
| Indenolol | 0.3395 | 0.9763 | 0.7211 | - | 0.7071 | - | - |
| Labetalol | 0.3795 | 0.1292 | 0.8784 | - | 0.6314 | - | - |
| Metoprolol | 0.4296 | 0.5674 | 0.6810 | 0.0442 | 0.8987 | 0.9291 | 0.2770 |
| Nadolol | 0.9331 | - | 0.2502 | - | - | - | - |
| Nebivolol | 0.7113 | 0.6799 | 0.6141 | 0.2718 | 0.6424 | 0.6786 | - |
| Nipradilol | 0.6047 | 0.9661 | 0.3412 | - | - | - | - |
| Pindolol | - | - | 0.5646 | - | - | - | - |
| Propanolol | 0.6904 | 0.6387 | 0.5360 | - | 0.4853 | - | - |
| Timolol | - | - | 0.6154 | - | - | - | - |
| Tolamolol | 0.4957 | - | - | - | - | ||
| Low quality | 0.3656 | 0.1203 | 0.0465 | 0.0545 | 0.7036 | 0.6320 | 0.0072 |
| Medium quality | 0.1475 | 0.1570 | 0.3808 | 0.3622 | 0.9941 | - | 0.0063 |
| Maximum treatment duration | 0.3398 | 0.3340 | 0.6555 | 0.0996 | 0.3549 | 0.0584 | 0.4968 |
| Percentage of maximum dose | 0.9874 | 0.6394 | 0.5207 | 0.8426 | 0.3179 | 0.7050 | 0.0010 |
| Variable | Females | Males | |
|---|---|---|---|
| SBP (mmHg) | MD acute MD sub-acute MD chronic | N.A. −5.4 (−10.4; −0.4) −11.7 (−15.4; −8.0) | −7.8 (−10.0; −5.5) −17.7 (−32.3; −3.1) −11.5 (−25.4; −7.7) |
| DBP (mmHg) | MD acute MD sub-acute MD chronic | N.A. −0.9 (−25.4; 2.5) −8.9 (−11.4; −6.5) | −5.0 (−6.7; −3.3) −12.0 (−19.7; −4.3) −9.4 (−12.3; −6.4) |
| HR (bpm) | MD acute MD sub-acute MD chronic | N.A. −11.8 (−16.5; −7.1) −10.7 (−17.8; −3.7) | −8.2 (−10.1; −6.4) −13.1 (−15.7; −10.6) −11.0 (−13.2; −8.8) |
| CO (L/min) | MD acute MD sub-acute MD chronic | N.A. N.A. N.A. | −0.2 (−0.5; 0.1) N.A. 0.2 (−0.8; 1.1) |
| LVEF (%) | MD acute MD sub-acute MD chronic | N.A. 0.0 (−2.6; 2.6) 4.9 (0.0; 9.9) | 0.3 (−2.1; 2.7) −0.4 (−3.9; 3.1) 5.6 (1.4; 9.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilmes, N.; van Luik, E.M.; Vaes, E.W.P.; Vesseur, M.A.M.; Laven, S.A.J.S.; Mohseni-Alsalhi, Z.; Meijs, D.A.M.; Dikovec, C.J.R.; de Haas, S.; Spaanderman, M.E.A.; et al. Exploring Sex Differences of Beta-Blockers in the Treatment of Hypertension: A Systematic Review and Meta-Analysis. Biomedicines 2023, 11, 1494. https://doi.org/10.3390/biomedicines11051494
Wilmes N, van Luik EM, Vaes EWP, Vesseur MAM, Laven SAJS, Mohseni-Alsalhi Z, Meijs DAM, Dikovec CJR, de Haas S, Spaanderman MEA, et al. Exploring Sex Differences of Beta-Blockers in the Treatment of Hypertension: A Systematic Review and Meta-Analysis. Biomedicines. 2023; 11(5):1494. https://doi.org/10.3390/biomedicines11051494
Chicago/Turabian StyleWilmes, Nick, Eveline M. van Luik, Esmée W. P. Vaes, Maud A. M. Vesseur, Sophie A. J. S. Laven, Zenab Mohseni-Alsalhi, Daniek A. M. Meijs, Cédric J. R. Dikovec, Sander de Haas, Marc E. A. Spaanderman, and et al. 2023. "Exploring Sex Differences of Beta-Blockers in the Treatment of Hypertension: A Systematic Review and Meta-Analysis" Biomedicines 11, no. 5: 1494. https://doi.org/10.3390/biomedicines11051494
APA StyleWilmes, N., van Luik, E. M., Vaes, E. W. P., Vesseur, M. A. M., Laven, S. A. J. S., Mohseni-Alsalhi, Z., Meijs, D. A. M., Dikovec, C. J. R., de Haas, S., Spaanderman, M. E. A., & Ghossein-Doha, C. (2023). Exploring Sex Differences of Beta-Blockers in the Treatment of Hypertension: A Systematic Review and Meta-Analysis. Biomedicines, 11(5), 1494. https://doi.org/10.3390/biomedicines11051494