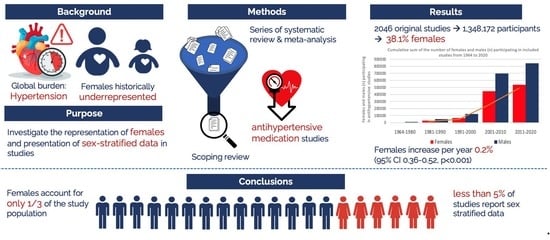
The Representation of Females in Studies on Antihypertensive Medication over the Years: A Scoping Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Scoping Review
2.2. Selection of Studies
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
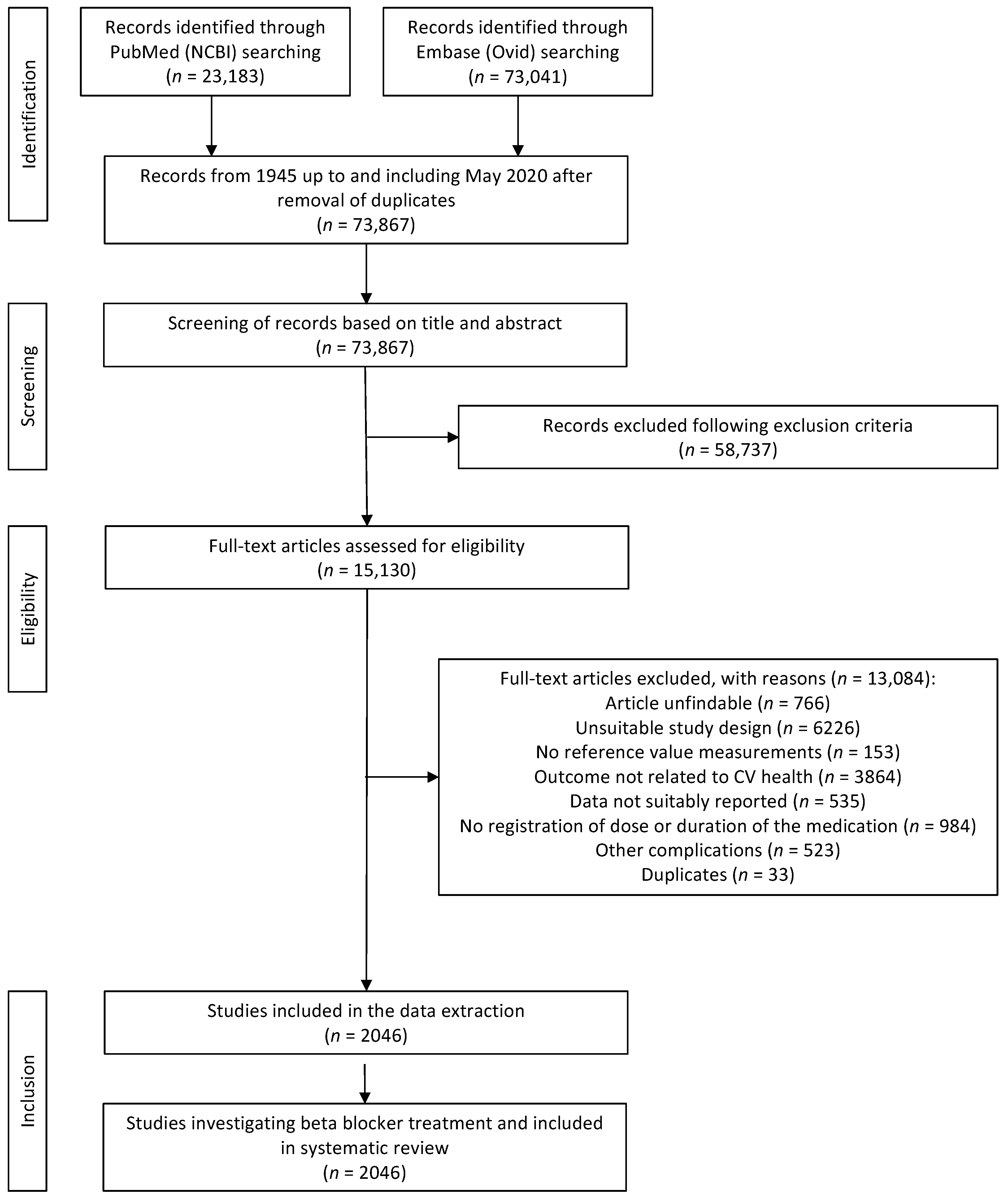
3.1. Study Selection
3.2. Study Characteristics
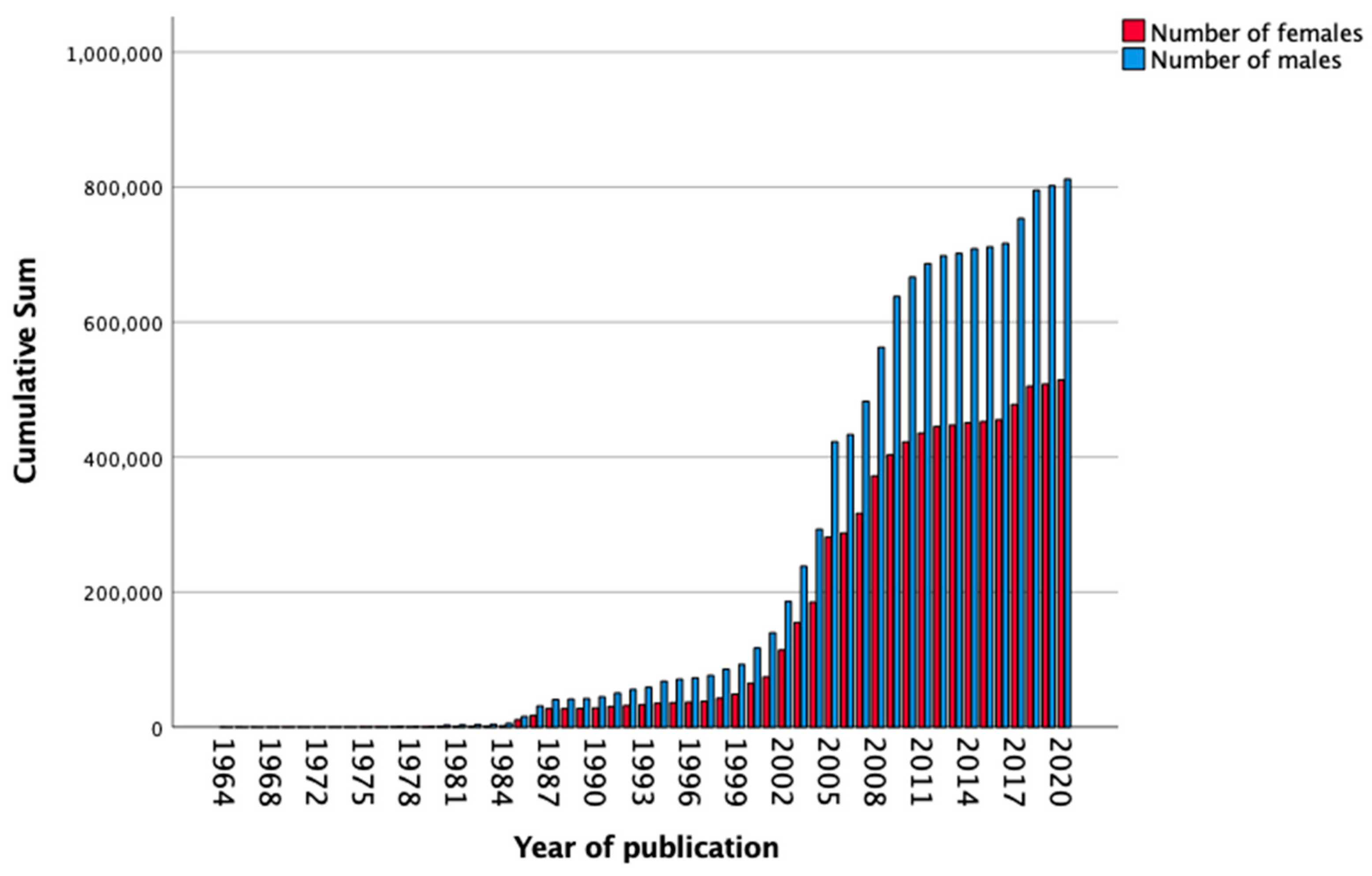
3.3. Prevalence of Included Females and Sex-Stratified Data
3.4. Prevalence of Females and Sex-Stratified Data Selective Antihypertensive Medication
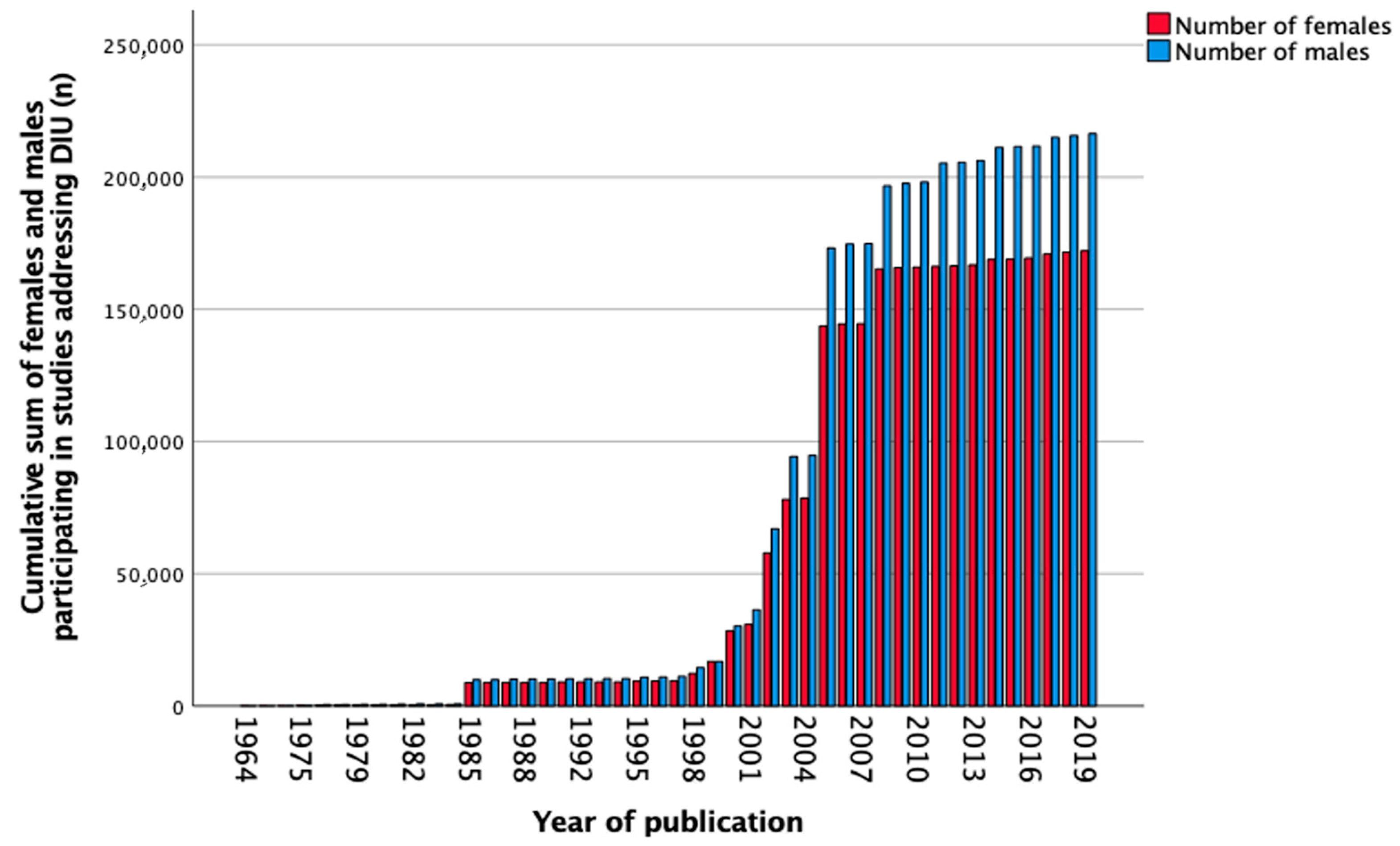
3.5. Diuretics
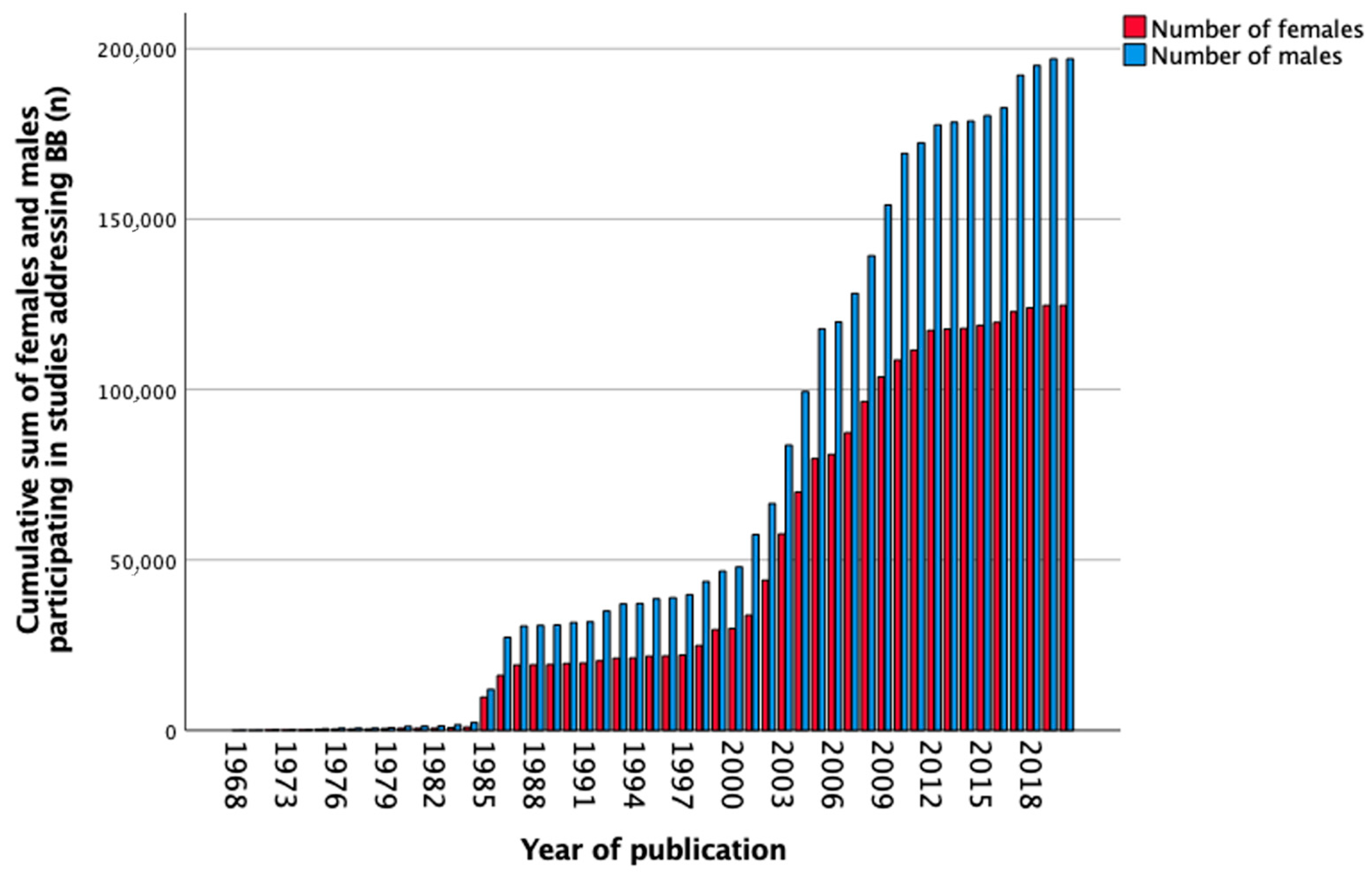
3.6. Beta-Blockers
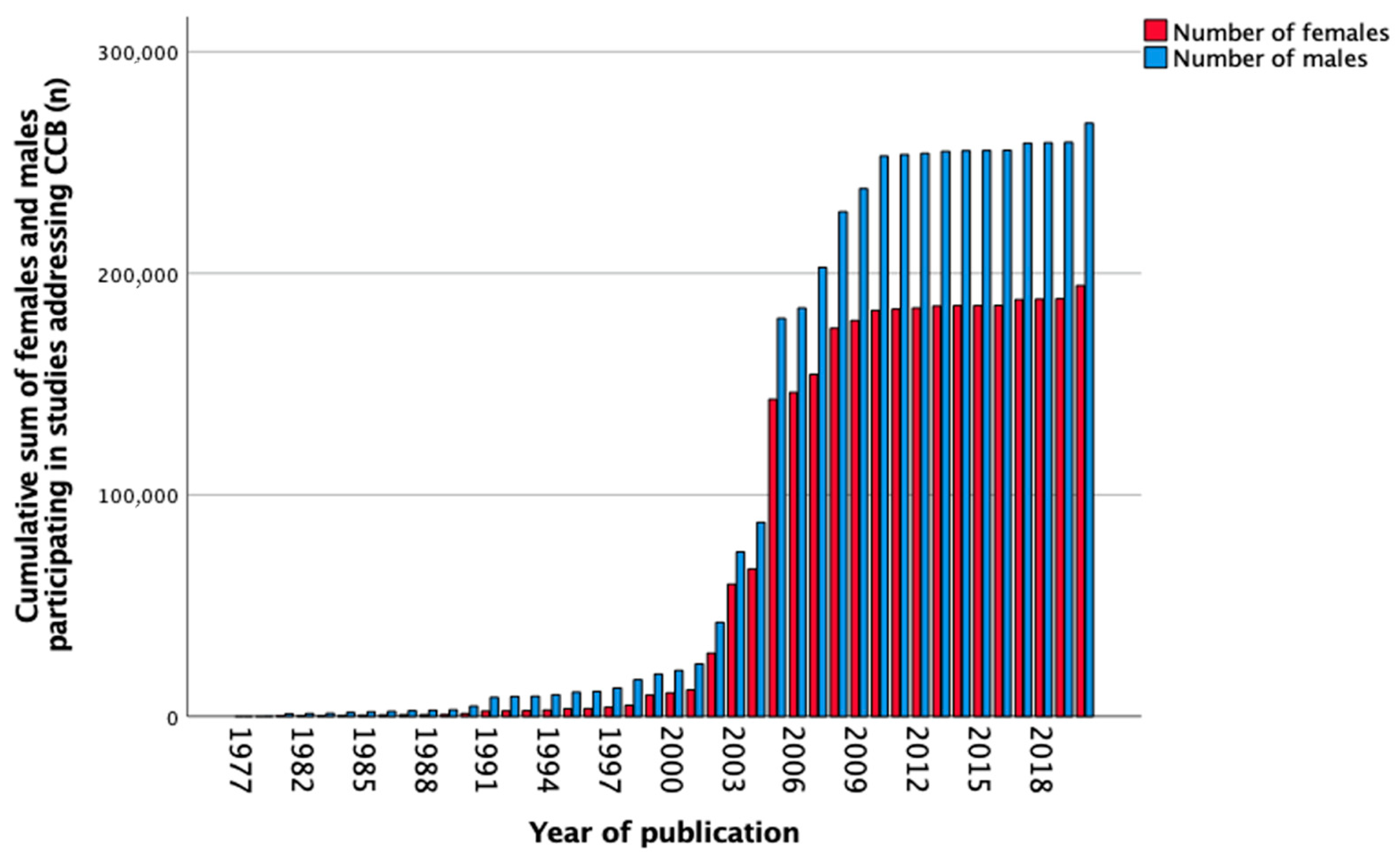
3.7. Calcium Channel Blockers
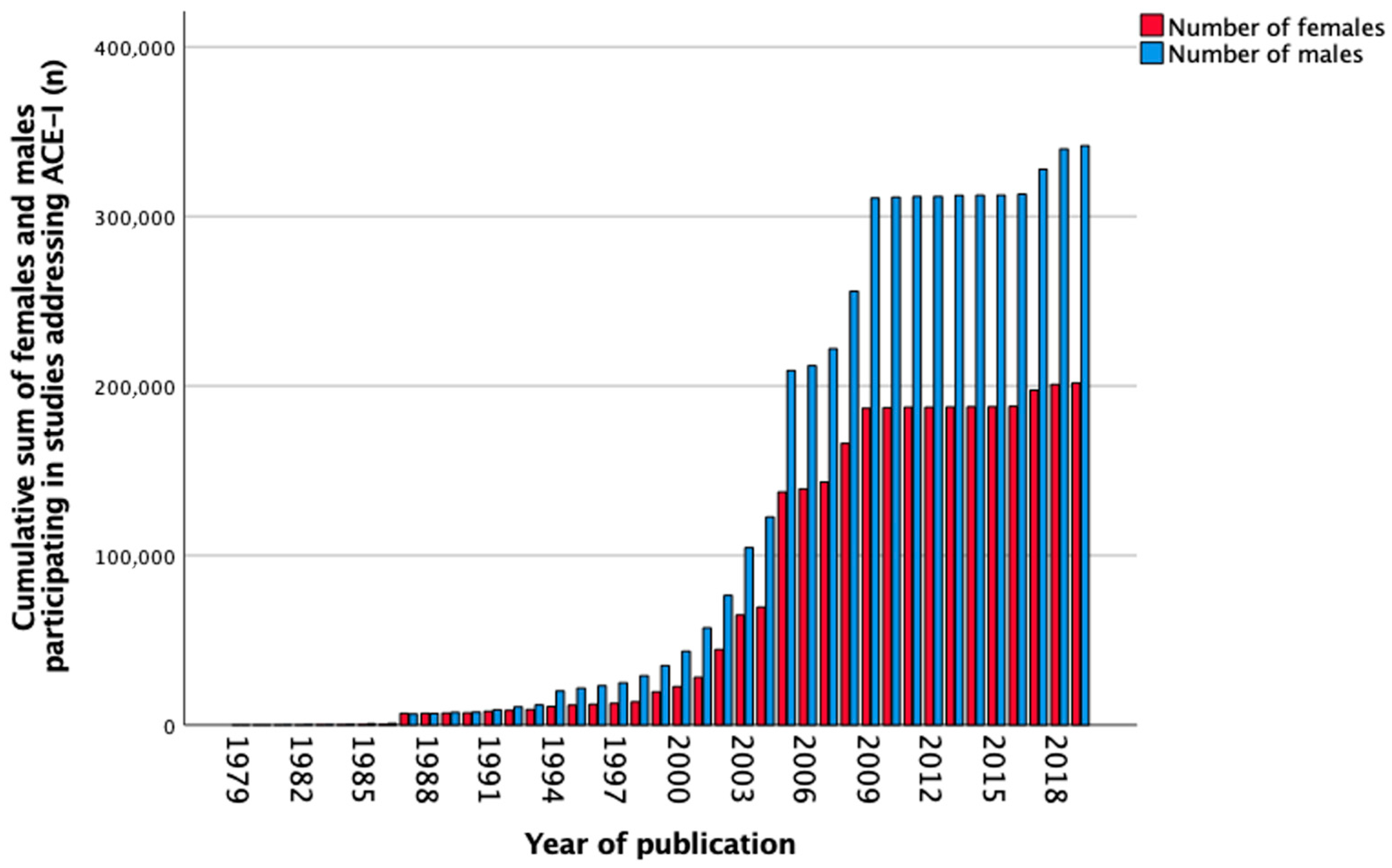
3.8. Angiotensin-Converting Enzyme Inhibitors
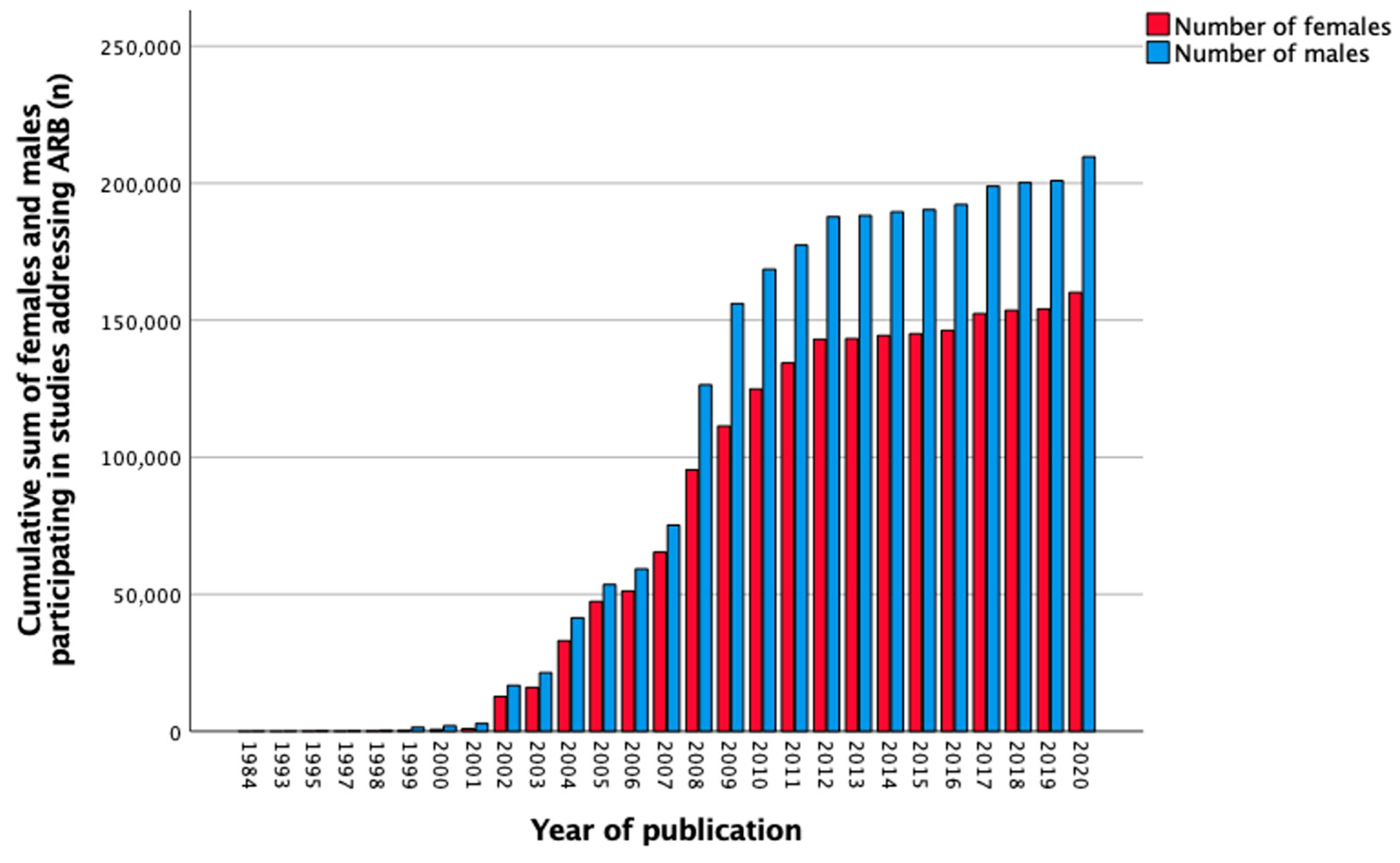
3.9. Angiotensin Receptor Blockers
4. Discussions
4.1. Clinical Implications and Recommendations for Future Research
4.2. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Non-Standard Abbreviations and Acronyms
| BB | Beta-blockers |
| ACEI | Angiotensin-converting enzyme inhibitors |
| ARB | Angiotensin receptor blockers |
| CCB | Calcium channel blockers |
| DIU | Diuretics |
| CI | Confidence interval |
| SD | Standard deviation |
| SE | Standard error |
| CVDs | Cardiovascular diseases |
| NIHc | National Institutes of Health |
| SPRINT trial | Randomized Trial of Intensive versus Standard Blood Pressure Control |
| ACC/AHA | The American College of Cardiology/American Heart Association |
| β | Beta coefficient |
| CV | Cardiovascular |
| RCTs | Randomized controlled trials |
References
- Gaziano, T.A.; Bitton, A.; Anand, S.; Abrahams-Gessel, S.; Murphy, A. Growing Epidemic of Coronary Heart Disease in Low- and Middle-Income Countries. Curr. Probl. Cardiol. 2010, 35, 72–115. [Google Scholar] [CrossRef] [PubMed]
- Leening, M.J.G.; Siregar, S.; Vaartjes, I.; Bots, M.L.; Versteegh, M.I.M.; Van Geuns, R.-J.M.; Koolen, J.J.; Deckers, J.W. Heart disease in the Netherlands: A quantitative update. Neth. Hear. J. 2013, 22, 3–10. [Google Scholar] [CrossRef]
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (dalys) for 359 diseases and injuries and healthy life expectancy (hale) for 195 countries and territories, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet 2018, 392, 1859–1922. [Google Scholar] [CrossRef]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Merz, C.N.B.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet women and cardiovascular disease Commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- World Health Organization. A Global Brief on Hypertension: Silent Killer, Global Public Health Crisis: World Health Day 2013; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Avery, E.; Clark, J. Sex-related reporting in randomised controlled trials in medical journals. Lancet 2016, 388, 2839–2840. [Google Scholar] [CrossRef]
- Clayton, J.A.; Tannenbaum, C. Reporting Sex, Gender, or Both in Clinical Research? JAMA 2016, 316, 1863–1864. [Google Scholar] [CrossRef] [PubMed]
- Meischke, H.; Larsen, M.P.; Eisenberg, M.S. Gender differences in reported symptoms for acute myocardial infarction: Impact on prehospital delay time interval. Am. J. Emerg. Med. 1998, 16, 363–366. [Google Scholar] [CrossRef]
- McSweeney, J.C.; Cody, M.; O’Sullivan, P.; Elberson, K.; Moser, D.K.; Garvin, B.J. Women’s Early Warning Symptoms of Acute Myocardial Infarction. Circulation 2003, 108, 2619–2623. [Google Scholar] [CrossRef]
- Möller-Leimkühler, A.M. Gender differences in cardiovascular disease and comorbid depression. Dialogues Clin. Neurosci. 2007, 9, 71–83. [Google Scholar] [CrossRef]
- Hage, F.G.; Mansur, S.J.; Xing, D.; Oparil, S. Hypertension in women. Kidney Int. Suppl. 2013, 3, 352–356. [Google Scholar] [CrossRef] [PubMed]
- Bentley-Lewis, R.; Seely, E.; Dunaif, A. Ovarian Hypertension: Polycystic Ovary Syndrome. Endocrinol. Metab. Clin. North Am. 2011, 40, 433–449. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.; Coffman, K.; Miller, V.M. Women-Specific Factors to Consider in Risk, Diagnosis and Treatment of Cardiovascular Disease. Women’s Health 2015, 11, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Mosca, L.; Benjamin, E.J.; Berra, K.; Bezanson, J.L.; Dolor, R.J.; Lloyd-Jones, D.M.; Newby, L.K.; Piña, I.L.; Roger, V.L.; Shaw, L.J.; et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women—2011 Update: A guideline from the American heart association. Circulation 2011, 123, 1243–1262. [Google Scholar] [CrossRef]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [CrossRef]
- The SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N. Engl. J. Med. 2015, 373, 2103–2116. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Tahhan, A.S.; Vaduganathan, M.; Greene, S.J.; Fonarow, G.C.; Fiuzat, M.; Jessup, M.; Lindenfeld, J.; O’connor, C.M.; Butler, J. Enrollment of older patients, women, and racial and ethnic minorities in contemporary heart failure clinical trials: A systematic review. JAMA Cardiol. 2018, 3, 1011–1019. [Google Scholar] [CrossRef]
- Heiat, A.; Gross, C.P.; Krumholz, H.M. Representation of the Elderly, Women, and Minorities in Heart Failure Clinical Trials. Arch. Intern. Med. 2002, 162, 1682–1688. [Google Scholar] [CrossRef]
- Jin, X.; Chandramouli, C.; Allocco, B.; Gong, E.; Lam, C.S.; Yan, L.L. Women’s Participation in Cardiovascular Clinical Trials From 2010 to 2017. Circulation 2020, 141, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Melloni, C.; Berger, J.S.; Wang, T.Y.; Gunes, F.; Stebbins, A.; Pieper, K.S.; Dolor, R.J.; Douglas, P.S.; Mark, D.B.; Newby, L.K.; et al. Representation of Women in Randomized Clinical Trials of Cardiovascular Disease Prevention. Circ. Cardiovasc. Qual. Outcomes 2010, 3, 135–142. [Google Scholar] [CrossRef]
- Bots, S.H.; Onland-Moret, N.C.; Tulevski, I.I.; van der Harst, P.; Cramer, M.J.M.; Asselbergs, F.W.; Somsen, G.A.; Ruijter, H.M.D. Heart failure medication dosage and survival in women and men seen at outpatient clinics. Heart 2021, 107, 1748–1755. [Google Scholar] [CrossRef] [PubMed]
- Santema, B.T.; Ouwerkerk, W.; Tromp, J.; Sama, I.E.; Ravera, A.; Regitz-Zagrosek, V.; Hillege, H.; Samani, N.J.; Zannad, F.; Dickstein, K.; et al. Identifying optimal doses of heart failure medications in men compared with women: A prospective, observational, cohort study. Lancet 2019, 394, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.; Lewis, B.; Agewall, S.; Wassmann, S.; Vitale, C.; Schmidt, H.H.H.W.; Drexel, H.; Patak, A.; Torp-Pedersen, C.; Kjeldsen, K.P.; et al. Gender differences in the effect of cardiovascular drugs: A position document of the Working Group on Pharmacology and Drug Therapy of the ESC. Eur. Hear. J. 2015, 36, 2677–2680. [Google Scholar] [CrossRef] [PubMed]
- Mas, S.; Gassò, P.; Álvarez, S.; Ortiz, J.; Sotoca, J.M.; Francino, A.; Carne, X.; Lafuente, A. Pharmacogenetic predictors of angiotensin-converting enzyme inhibitor-induced cough: The role of: ACE, ABO and BDKRB2 Genes. Pharmacogenet. Genom. 2011, 21, 531–538. [Google Scholar] [CrossRef]
- Wenger, N.K.; Arnold, A.; Merz, C.N.B.; Cooper-DeHoff, R.M.; Ferdinand, K.C.; Fleg, J.L.; Gulati, M.; Isiadinso, I.; Itchhaporia, D.; Light-McGroary, K.; et al. Hypertension Across a Woman’s Life Cycle. J. Am. Coll. Cardiol. 2018, 71, 1797–1813. [Google Scholar] [CrossRef]
- Bots, S.H.; Schreuder, M.M.; van Lennep, J.E.R.; Watson, S.; van Puijenbroek, E.; Onland-Moret, N.C.; Ruijter, H.M.D. Sex Differences in Reported Adverse Drug Reactions to Angiotensin-Converting Enzyme Inhibitors. JAMA Netw. Open 2022, 5, e228224. [Google Scholar] [CrossRef]
- Costa-Hong, V.A.; Muela, H.C.S.; Macedo, T.A.; Sales, A.R.K.; Bortolotto, L.A. Gender differences of aortic wave reflection and influence of menopause on central blood pressure in patients with arterial hypertension. BMC Cardiovasc. Disord. 2018, 18, 123. [Google Scholar] [CrossRef]
- Ljungman, C.; Kahan, T.; Schiöler, L.; Hjerpe, P.; Hasselström, J.; Wettermark, B.; Boström, K.B.; Manhem, K. Gender differences in antihypertensive drug treatment: Results from the Swedish Primary Care Cardiovascular Database (SPCCD). J. Am. Soc. Hypertens. 2014, 8, 882–890. [Google Scholar] [CrossRef]
- Wallentin, F.; Wettermark, B.; Kahan, T. Drug treatment of hypertension in Sweden in relation to sex, age, and comorbidity. J. Clin. Hypertens. 2017, 20, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef] [PubMed]
- Merone, L.; Tsey, K.; Russell, D.; Nagle, C. Sex Inequalities in Medical Research: A Systematic Scoping Review of the Literature. Women’s Health Rep. 2022, 3, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Benko, C.; Pelster, B. How Women Decide; Harvard Business Publishing: Boston, MA, USA, 2013. [Google Scholar]
- Ding, E.L.; Powe, N.R.; Manson, J.E.; Sherber, N.S.; Braunstein, J.B. Sex differences in perceived risks, distrust, and willingness to participate in clinical trials: A randomized study of cardiovascular prevention trials. Arch. Intern. Med. 2007, 167, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Mather, M.; Lighthall, N.R. Risk and Reward Are Processed Differently in Decisions Made Under Stress. Curr. Dir. Psychol. Sci. 2012, 21, 36–41. [Google Scholar] [CrossRef]
- Peterson, E.D.; Lytle, B.L.; Biswas, M.S.; Coombs, L. Willingness to participate in cardiac trials. Am. J. Geriatr. Cardiol. 2004, 13, 11–15. [Google Scholar] [CrossRef]
- Schiffer, V.M.; Janssen, E.B.; van Bussel, B.C.; Jorissen, L.L.; Tas, J.; Sels, J.-W.E.; Bergmans, D.C.; Dinh, T.H.; van Kuijk, S.M.; Hana, A.; et al. The “sex gap” in COVID-19 trials: A scoping review. Eclinicalmedicine 2020, 29–30, 100652. [Google Scholar] [CrossRef]
| PubMed | Embase |
|---|---|
| Component 1: Antihypertensive medication: “diuretics” OR “adrenergic beta-antagonists” OR “beta blockers” [Title/Abstract] OR “Antihypertensive agents” OR “blood pressure lowering therapy” [Title/Abstract] OR “antihypertensive medication” [Title/Abstract] OR “antihypertensive therapy” [Title/Abstract] OR “angiotensin-converting enzyme inhibitors” OR “ACE inhibitors” [Title/Abstract] OR “Angiotensin receptor antagonists” OR “angiotensin receptor blockers” [Title/Abstract] OR “sympatholytics” OR “Calcium Channel Blockers” | Component 1: Antihypertensive medication: exp diuretic agent/or exp beta adrenergic receptor blocking agent/or exp adrenergic receptor blocking agent/or exp antihypertensive agent/or exp dipeptidyl carboxypeptidase inhibitor/or exp angiotensin receptor antagonist/or exp calcium channel blocking agent.ti,ab. |
| Component 2: Cardiac geometry: “ventricular remodeling” OR “ventricular remodeling” [Title/Abstract] OR “cardiac remodeling” [Title/Abstract] OR “cardiac adaptation” [Title/Abstract] OR “LV geometry” [Title/Abstract] OR “left ventricular geometry” [Title/Abstract] OR “cardiac geometry” [Title/Abstract] OR “cardiac dimension” [Title/Abstract] OR “left ventricle remodeling” [Title/Abstract] OR “Hypertrophy, Left Ventricular” OR “left ventricular hypertrophy” [Title/Abstract] OR “echocardiography” OR Echocardiography [Title/Abstract] OR “left ventricular mass” [Title/Abstract] OR “left ventricular mass index” [Title/Abstract] OR “relative wall thickness” [Title/Abstract] OR “concentric cardiac remodeling” [Title/Abstract] OR “eccentric cardiac remodeling” [Title/Abstract] | Component 2: Cardiac geometry: exp heart ventricle remodeling/or (ventricular remodeling or cardiac remodeling or cardiac adaptation or LV geometry or left ventricular remodeling or cardiac geometry or cardiac dimension).ti,ab. or exp echocardiography/ or echocardiography.ti,ab. |
| Component 3: Heart failure: “Heart Failure” OR “Heart Failure, Systolic” | Component 3: Heart failure: exp heart failure.ti,ab. |
| Component 4: Diastolic dysfunction: “heart failure, diastolic” OR “diastolic dysfunction” [Title/Abstract] | Component 4: Diastolic dysfunction: exp diastolic dysfunction/or diastolic function.ti,ab. |
| Component 5: Myocardial infarction: “myocardial infarction” OR “myocardial infarction” [Title/Abstract] OR “acute myocardial infarction” [Title/Abstract] OR “heart attack” [Title/Abstract] | Component 5: Myocardial infarction: exp heart infarction.ti,ab. |
| Component 6: CVA: Stroke OR “cerebrovascular accident” [Title/Abstract] OR “acute cerebrovascular accident” [Title/Abstract] OR “acute cerebrovascular insult” [Title/Abstract] | Component 6: CVA: exp cerebrovascular accident.ti,ab. |
| Number of Participants n (%) | n = 2046 |
|---|---|
| Total | 1,348,172 (100%) |
| Females | 514,604 (38.2%) |
| Males | 812,397 (60.3%) |
| Unspecified | 21,171 (1.6%) |
| Mean age ± SD | 58.0 ± 8.8 |
| Sex stratification n (%) | |
| Not stratified | 1706 (83.4%) |
| Stratified | 75 (3.7%) |
| Only females | 8 (0.4%) |
| Only males | 131 (6.4%) |
| Not mentioned | 126 (6.2%) |
| Study design n (%) | |
| Randomized controlled trial | 1198 (58.6%) |
| Prospective cohort study | 737 (37.8%) |
| Retrospective cohort study | 43(2.1%) |
| Case–control study | 22 (1.1%) |
| Cross-sectional study | 4 (0.2%) |
| Other | 6 (0.3%) |
| Effect n (%) | |
| Acute | 419 (20.5%) |
| Chronic | 1627 (79.5%) |
| Total | 1964–1980 | 1981–1990 | 1991–2000 | 2001–2010 | 2011–2020 | |
|---|---|---|---|---|---|---|
| Number of studies | ||||||
| Total, n (%) | 2046 (100) | 58 (2.8) | 420 (20.5) | 544 (26.6) | 690 (33.7) | 334 (16.3) |
| Without mentioning sex distinction, n (%) | 126 (6.2) | 5 (8.6) | 60 (14.3) | 31 (5.7) | 21 (3.0) | 9 (2.7) |
| Without sex stratification, n (%) | 1706 (83.4) | 36 (62.1) | 281 (66.9) | 466 (85.7) | 629 (91.2) | 294 (88.0) |
| With sex stratification, n (%) | 75 (3.7) | 3 (5.2) | 14 (3.3) | 14 (2.6) | 20 (2.9) | 24 (7.2) |
| Only including females, n (%) | 8 (0.4) | 0 (0.0) | 0 (0.0) | 3 (0.6) | 1 (0·1) | 4 (1·2) |
| Only including males, n (%) | 131 (6.4) | 14 (24.1) | 65 (15.5) | 31(5.7) | 19 (2.8) | 3 (0.9) |
| Participants | ||||||
| Total, n (%) | 1,348,172 (100) | 4636 (0.3) | 77,070 (5.7) | 111,616 (8.3) | 917,034 (68.0) | 237,816 (17.6) |
| Females, n (%) | 514,604 (38.2) | 1132 (24.4) | 27,347 (35.5) | 36,423 (32.6) | 357,636 (39.0) | 92,066 (38.7) |
| Males, n (%) | 812,397 (60.3) | 3033 (65.4) | 41,765 (54.2) | 72,549 (65.0) | 549,607 (59.9) | 145,443 (61.1) |
| Sex not known, n (%) | 21,171 (1.6) | 471 (10.2) | 7958 (10.3) | 2644 (2.4) | 9791 (1.1) | 332(0.1) |
| Trends | ||||||
| Trend of increase in females per year | β = 0.9 (95% CI 0.361–0.517, p < 0.001 *) | β = −1.342 (95% CI −2.752–0.068, p = 0.062) | β = 0.449 (95% CI −0.368–1.266, p = 0.280) | β = 0.229 (95% CI −0.515–0.973, p = 0.545) | β = 0.515 (95% CI 0.076–0.954, p = 0.022 *) | β = 0.274 (95% CI −0.501–1.049, p = 0.487) |
| Trend of increase in females in studies stratifying based on sex | β = 0.517 (95% CI 0.064–0.969, p = 0.026 *) | β = −2.887 (95% CI −27.937–22.162, p = 0.381) | β = −2.167 (95% CI −7.567–3.234, p = 0.399) | β = −2.926 (95% CI −7.454–1.601, p = 0.183) | β = 2.496 (95% CI −0.520–5.511, p = 0.099) | β = 0.570 (95% CI −4.818–5.958, p = 0.828) |
| Age | ||||||
| Mean age (range, SD) | 58.0 (22–86, 8.8) | 53.6 (39–72, 6.4) | 54.7 (22–82, 8.7) | 56.6 (22–81, 8.9) | 59.5 (22–86, 8.4) | 61.1 (25–86, 9.1) |
| Medication groups | ||||||
| Diuretics, n (%) | 252 (10.8) | 11 (23.4) | 37 (8.9) | 48 (7.2) | 89 (10.0) | 67 (20.9) |
| Beta-blockers, n (%) | 652 (27.9) | 25 (53.2) | 120 (28.8) | 146 (22.0) | 265 (29.7) | 96 (30.9) |
| Calcium channel blockers, n (%) | 442 (18.9) | 4 (8.5) | 128 (30.7) | 149 (22.4) | 122 (13.7) | 39 (12.2) |
| Angiotensin-converting enzyme inhibitors, n (%) | 671 (28.7) | 7 (14.9) | 130 (31.2) | 294 (44.2) | 206 (23.1) | 34 (10.6) |
| Angiotensin receptor blockers, n (%) | 324 (13.8) | 0 (0.0) | 2 (0.5) | 28 (4.2) | 210 (23.5) | 84 (26.3) |
| Total | 1964–1980 | 1981–1990 | 1991–2000 | 2001–2010 | 2011–2020 | |
|---|---|---|---|---|---|---|
| Number of studies | ||||||
| Total, n (%) | 252 (100) | 11 (4.4) | 37 (14.7) | 48 (19.0) | 89 (35.3) | 67 (26.6) |
| Without mentioning sex distinction, n (%) | 13 (5.2) | 0 (0.0) | 5 (13.5) | 4 (8.3) | 3 (3.4) | 1 (1.5) |
| Without sex stratification, n (%) | 214 (84.9) | 9 (81.8) | 26 (70.3) | 40 (83.3) | 81 (91.0) | 58 (86.6) |
| With sex stratification, n (%) | 10 (4.0) | 1 (9.1) | 0 (0.0) | 1 (2.1) | 3 (3.4) | 5 (7.5) |
| Only including females, n (%) | 2 (0.8) | 0 (0.0) | 0 (0.0) | 1 (2.1) | 0 (0.0) | 1 (1.5) |
| Only including males, n (%) | 13 (5.2) | 1 (9.1) | 6 (16.2) | 2 (4.2) | 2 (2.2) | 2 (3.0) |
| Participants | ||||||
| Total, n (%) | 389,873 (100) | 956 (0.2) | 18,349 (4.7) | 40,554 (10.4) | 305,510 (78.4) | 24,504 (6.3) |
| Females, n (%) | 172,154 (44.2) | 385 (40.0) | 8621 (47.0) | 19,429 (47.9) | 137,542 (45.0) | 6177 (25.3) |
| Males, n (%) | 216,402 (55.5) | 572 (59.8) | 9683 (52.8) | 19,944 (49.2) | 167,892 (55.0) | 18,311 (74.7) |
| Sex not known, n (%) | 1317 (0.3) | 0 (0.0) | 45 (0.2) | 1181 (2.9) | 76 (0.0) | 16 (0.0) |
| Total participants in studies that stratified sex, n (%) | 8259 (100) | 53 (0.6) | 128 (1.5) | 1155 (14.0) | 268 (3.2) | 6655 (80.7) |
| Included females in studies that stratified sex, n (%) | 253 (3.1) | 22 (41.5) | 0 (0.0) | 18 (1.6) | 107 (40.0) | 106 (1.6) |
| Included males in studies that stratified sex, n (%) | 8006 (96.9.) | 31 (58.5) | 12 (100.0) | 1137 (98.4) | 161 (60.0) | 6549 (98.4) |
| Age | ||||||
| Mean age, (range, SD) | 60.8 (27–86, 9.0) | 50.1 (39–59, 9.6) | 56.8 (40–78, 8.7) | 58.6 (27–76, 7.4) | 61.6 (27–86, 9.2) | 64.3 (41–78, 10.0) |
| Total | 1968–1980 | 1981–1990 | 1991–2000 | 2001–2010 | 2011–2020 | |
|---|---|---|---|---|---|---|
| Number of studies | ||||||
| Total, n (%) | 652 (100) | 25 (3.8) | 120 (18.4) | 146 (22.4) | 265 (40.6) | 96 (14.7) |
| Without mentioning sex distinction, n (%) | 46 (7.1) | 3 (12.0) | 14 (11.7) | 13 (8.9) | 14 (5.3) | 2 (2.1) |
| Without sex stratification, n (%) | 533 (81.7) | 17 (68.0) | 78 (65.0) | 117 (80.1) | 234 (88.3) | 87 (90.6) |
| With sex stratification, n (%) | 19 (2.9) | 0 (0.0) | 3 (2.5) | 2 (1.4) | 9 (3.4) | 5 (5.2) |
| Only including females, n (%) | 3 (0.5) | 0 (0.0) | 0 (0.0) | 1 (0.7) | 1 (0.4) | 1 (1.0) |
| Only including males, n (%) | 51 (7.8) | 5 (20.0) | 25 (20.8) | 13 (8.9) | 7 (2.6) | 1 (1.0) |
| Participants | ||||||
| Total, n (%) | 340,718 (100) | 2211 (0.6) | 56,493 (16.6) | 28,568 (8.4) | 209,644 (61.5) | 43,802 (12.9) |
| Females, n (%) | 124,711 (36.6) | 586 (26.5) | 19,060 (33.7) | 10,253 (35.9) | 78,751 (37.6) | 16,061 (36.7) |
| Males, n (%) | 197,044 (57.8) | 1166 (52.7) | 30,475 (53.9) | 16,340 (57.2) | 121,322 (57.9) | 27,741 (63.3) |
| Sex not known, n (%) | 18,963 (5.6) | 459 (20.8) | 6958 (12.3) | 1975 (6.9) | 9571 (4.5) | 0 (0.0) |
| Total participants in studies that stratified sex, n (%) | 13,460 (100) | 103 (0.8) | 497 (3.7) | 1745 (13.0) | 10,640 (79.0) | 484 (3.6) |
| Included females in studies that stratified sex, n (%) | 5835 (43.4) | 0 (0.0) | 5 (1.0) | 31 (1.8) | 5511 (51.8) | 288 (59.5) |
| Included males in studies that stratified sex, n (%) | 7625 (56.6) | 103 (100) | 492 (99.0) | 1714(98.2) | 5129 (48.2) | 196 (40.5) |
| Age | ||||||
| Mean age (range, SD) | 56.3 (22–77, 8.6) | 53.6 (41–72, 7.7) | 51.2 (22–76, 8.5) | 55.3 (22–77, 7.6) | 58.1 (22–76, 8.8) | 58.5 (25–77, 9.8) |
| Total | 1977–1980 | 1981–1990 | 1991–2000 | 2001–2010 | 2011–2020 | |
|---|---|---|---|---|---|---|
| Number of studies | ||||||
| Total, n (%) | 442 (100) | 4 (0.9) | 128 (29.0) | 149 (33.7) | 122 (27.6) | 39 (8.8) |
| Without mentioning sex distinction, n (%) | 28 (6.3) | 1 (25.0) | 16 (12.5) | 9 (6.0) | 1 (0.8) | 1 (2.6) |
| Without sex stratification, n (%) | 368 (83.3) | 3 (75.0) | 86 (67.2) | 128 (85.9) | 119 (97.5) | 32 (82.1) |
| With sex stratification, n (%) | 14 (3.2) | 0 (0.0) | 6 (4.7) | 2 (1.3) | 2 (1.6) | 4 (10.3) |
| Only including females, n (%) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (5.1) |
| Only including males, n (%) | 30 (6.8) | 0 (0.0) | 20 (15.6) | 10 (6.7) | 0 (0.0) | 0 (0.0) |
| Participants | ||||||
| Total, n (%) | 464,084 (100) | 1514 (0.3) | 4830 (1.0) | 26699 (5.8) | 404,807 (87.2) | 26234 (5.7) |
| Females, n (%) | 194,514 (41.9) | 291 (19.2) | 1055 (21.8) | 9308 (34.9) | 172,626 (42.6) | 11234 (42.8) |
| Males, n (%) | 267,771 (57.7) | 1211 (80.0) | 3414 (70.7) | 16124 (60.4) | 232,153 (57.3) | 14869 (56.7) |
| Sex not known, n (%) | 1799 (0.4) | 12 (0.8) | 361 (7.5) | 1267 (4.7) | 28 (0.0) | 131 (0.5) |
| Total participants in studies that stratified sex, n (%) | 17,102 (100) | 0 (0.0) | 365 (2.1) | 1694 (9.9) | 24 (0.1) | 15,019 (87.8) |
| Included females in studies that stratified sex, n (%) | 6288 (36.8) | 0 (0.0) | 30 (8.2) | 4 (0.2) | 10 (41.7) | 6244 (41.6) |
| Included males in studies that stratified sex, n (%) | 10,814 (63.2) | 0 (0.0) | 335 (91.8) | 1690 (99.8) | 14 (58.3) | 8775 (58.4) |
| Age | ||||||
| Mean age (range, SD) | 56.1 (27–79, 8.8) | 54.0 (54–54) | 53.4 (36–74, 9.4) | 59.3 (27–74, 9.0) | 59.3 (27–74, 9.0) | 61.0 (27–78, 9.2) |
| Total | 1979–1980 | 1981–1990 | 1991–2000 | 2001–2010 | 2011–2020 | |
|---|---|---|---|---|---|---|
| Number of studies | ||||||
| Total, n (%) | 671 (100) | 7 (1.0) | 130 (19.4) | 294 (43.8) | 206 (30.7) | 34 (5.1) |
| Without mentioning sex distinction, n (%) | 48 (7.2) | 0 (0.0) | 26 (20.0) | 15 (5.1) | 6 (2.9) | 1 (2.9) |
| Without sex stratification, n (%) | 564 (84.1) | 4 (57.1) | 85 (65.4) | 259 (88.1) | 190 (92.2) | 26 (76.5) |
| With sex stratification, n (%) | 28 (4.2) | 1 (14.3) | 3 (2.3) | 11 (3.7) | 6 (2.9) | 7 (20.6) |
| Only including females, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Only including males, n (%) | 31 (4.6) | 2 (28.6) | 16 (12.3) | 9 (3.1) | 4 (1.9) | 0 (0.0) |
| Participants | ||||||
| Total, n (%) | 546,164 (100) | 72 (0.0) | 15,297 (2.8) | 53,251 (9.8) | 432,462 (79.2) | 45,082 (8.3) |
| Females, n (%) | 201,749 (36.9) | 15 (20.8) | 7138 (46.7) | 15,572 (29.2) | 164,447 (38.0) | 14,577 (32.3) |
| Males, n (%) | 341,789 (62.6) | 57 (79.2) | 7491 (49) | 35,960 (67.5) | 267,839 (61.9) | 30,442 (67.5) |
| Sex not known, n (%) | 2626 (0.5) | 0 (0.0) | 668 (4.4) | 1719 (3.2) | 176 (0.0) | 63 (0.1) |
| Total participants in studies that stratified sex, n (%) | 2738 (100) | 31 (0.9) | 256 (3.1) | 1641 (91.6) | 322 (2.8) | 488 (1.6) |
| Included females in studies that stratified sex, n (%) | 507 (18.5) | 1 (3.2) | 12 (4.7) | 120 (7.3) | 72 (22.4) | 302 (61.9) |
| Included males in studies that stratified sex, n (%) | 2231 (81.5) | 30 (96.8) | 244 (95.3) | 1521 (92.7) | 250 (77.6) | 186 (38.1) |
| Age | ||||||
| Mean age (range, SD) | 58.3 (23–82, 8.5) | 59.5 (54–65, 10.8) | 58.0 (38–82, 9.2) | 57.5 (23–81, 8.0) | 59.3 (23–81, 8.7) | 59.7 (47–71, 10.1) |
| Total | 1984–1990 | 1991–2000 | 2001–2010 | 2011–2020 | |
|---|---|---|---|---|---|
| Number of studies | |||||
| Total, n (%) | 324 (100) | 2 (0.6) | 28 (8.6) | 210 (64.8) | 84 (25.9) |
| Without mentioning sex distinction, n (%) | 8 (2.5) | 0 (0.0) | 1 (3.6) | 4 (1.9) | 3 (3.6) |
| Without sex stratification, n (%) | 295 (91.0) | 2 (100) | 24 (85.7) | 192 (91.4) | 77 (91.7) |
| With sex stratification, n (%) | 14 (4.3) | 0 (0.0) | 0 (0.0) | 10 (4.8) | 4 (4.8) |
| Only including females, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Only including males, n (%) | 7 (2.2) | 0 (0.0) | 3 (10.7) | 4 (1.9) | 0 (0.0) |
| Participants | |||||
| Total, n (%) | 379,302 (100) | 79 (0.0) | 2642 (0.7) | 300,251 (79.2) | 76,330 (20.1) |
| Females, n (%) | 160,182 (42.2) | 21 (26.6) | 638 (24.1) | 124,292 (41.4) | 35,231 (46.2) |
| Males, n (%) | 209,658 (55.3) | 58 (73.4) | 1991 (75.4) | 166,595 (55.5) | 41,014 (53.7) |
| Sex not known, n (%) | 9462 (2.5) | 0 (0.0) | 13 (0.5) | 9364 (3.1) | 85 (0.1) |
| Total participants in studies that stratified sex, n (%) | 28,420 (100) | 0 (0.0) | 129 (0.5) | 11,709 (41.2) | 16,582 (58.3) |
| Included females in studies that stratified sex, n (%) | 12,696 (44.7) | 0 (0.0) | 0 (0.0) | 5717 (48.8) | 6979 (42.1) |
| Included males in studies that stratified sex, n (%) | 15,724 (55.3) | 0 (0.0) | 129 (100) | 5992 (51.2) | 9603 (57.9) |
| Age | |||||
| Mean age (range, SD) | 59.7 (29–78, 8.9) | 60.5 (57–64) | 58.5 (42–73, 7.9) | 59.6 (29–76, 8.6) | 60.4 (39–78, 10.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohseni-Alsalhi, Z.; Vesseur, M.A.M.; Wilmes, N.; Laven, S.A.J.S.; Meijs, D.A.M.; van Luik, E.M.; Vaes, E.W.P.; Dikovec, C.J.R.; Wiesenberg, J.; Almutairi, M.F.; et al. The Representation of Females in Studies on Antihypertensive Medication over the Years: A Scoping Review. Biomedicines 2023, 11, 1435. https://doi.org/10.3390/biomedicines11051435
Mohseni-Alsalhi Z, Vesseur MAM, Wilmes N, Laven SAJS, Meijs DAM, van Luik EM, Vaes EWP, Dikovec CJR, Wiesenberg J, Almutairi MF, et al. The Representation of Females in Studies on Antihypertensive Medication over the Years: A Scoping Review. Biomedicines. 2023; 11(5):1435. https://doi.org/10.3390/biomedicines11051435
Chicago/Turabian StyleMohseni-Alsalhi, Zenab, Maud A. M. Vesseur, Nick Wilmes, Sophie A. J. S. Laven, Daniek A. M. Meijs, Eveline M. van Luik, Esmée W. P. Vaes, Cédric J. R. Dikovec, Jan Wiesenberg, Mohamad F. Almutairi, and et al. 2023. "The Representation of Females in Studies on Antihypertensive Medication over the Years: A Scoping Review" Biomedicines 11, no. 5: 1435. https://doi.org/10.3390/biomedicines11051435
APA StyleMohseni-Alsalhi, Z., Vesseur, M. A. M., Wilmes, N., Laven, S. A. J. S., Meijs, D. A. M., van Luik, E. M., Vaes, E. W. P., Dikovec, C. J. R., Wiesenberg, J., Almutairi, M. F., Janssen, E. B. N. J., de Haas, S., Spaanderman, M. E. A., & Ghossein-Doha, C. (2023). The Representation of Females in Studies on Antihypertensive Medication over the Years: A Scoping Review. Biomedicines, 11(5), 1435. https://doi.org/10.3390/biomedicines11051435