Evaluation of Human-Induced Pluripotent Stem Cells Derived from a Patient with Schwartz–Jampel Syndrome Revealed Distinct Hyperexcitability in the Skeletal Muscles
Abstract
1. Introduction
2. Materials and Methods
2.1. Establishing MyoD-hiPSCs
2.2. Myotube Differentiation Using MyoD-hiPSCs
2.3. Experimental Animals
2.4. Satellite Cell Culture and Differentiation
2.5. Immunofluorescence Analysis
2.6. Quantitative Real-Time PCR (qPCR)
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Calcium Imaging
2.9. Statistical Analysis
3. Results
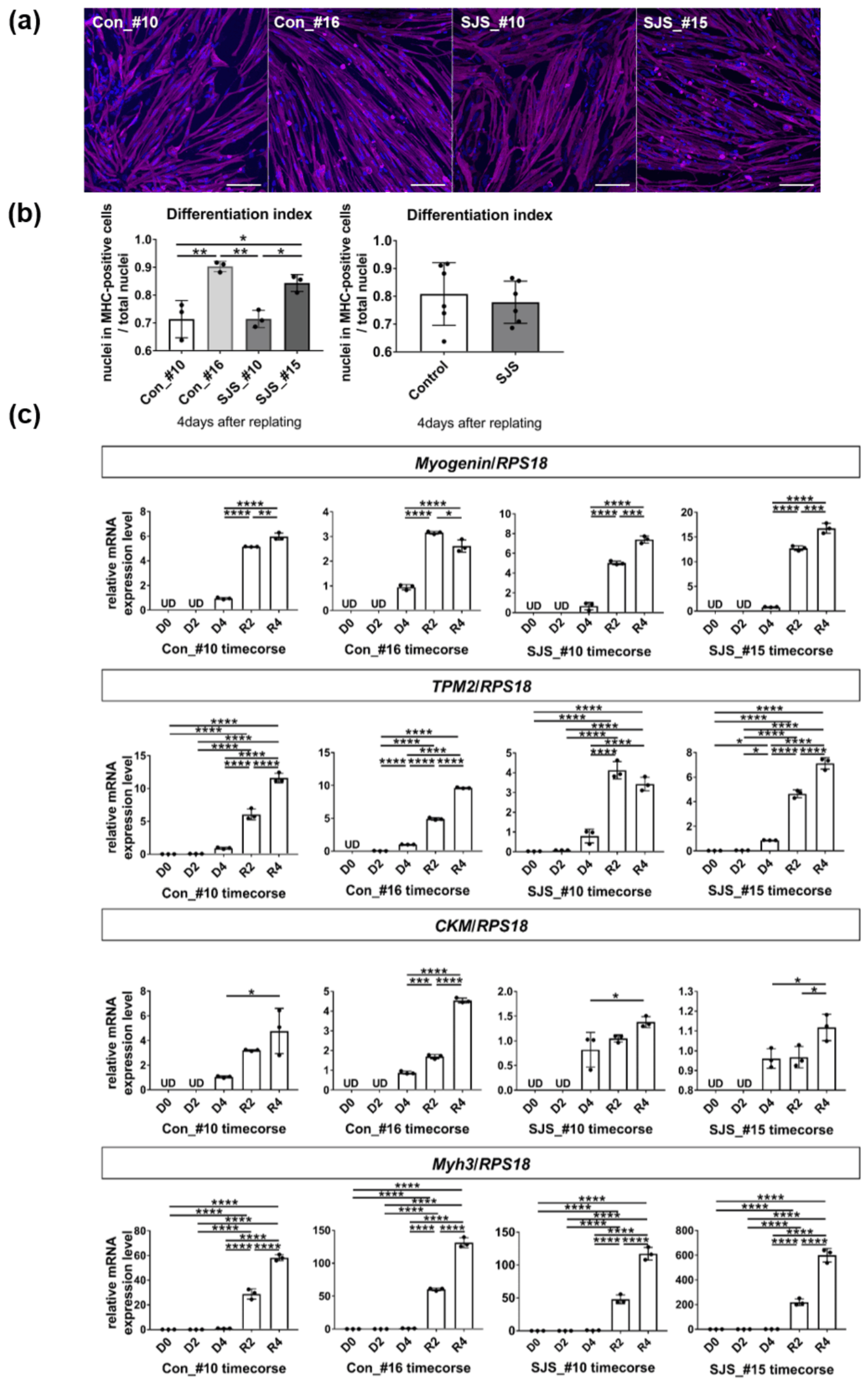
3.1. Differentiation of Myotubes Derived from Control and SJS-hiPSCs
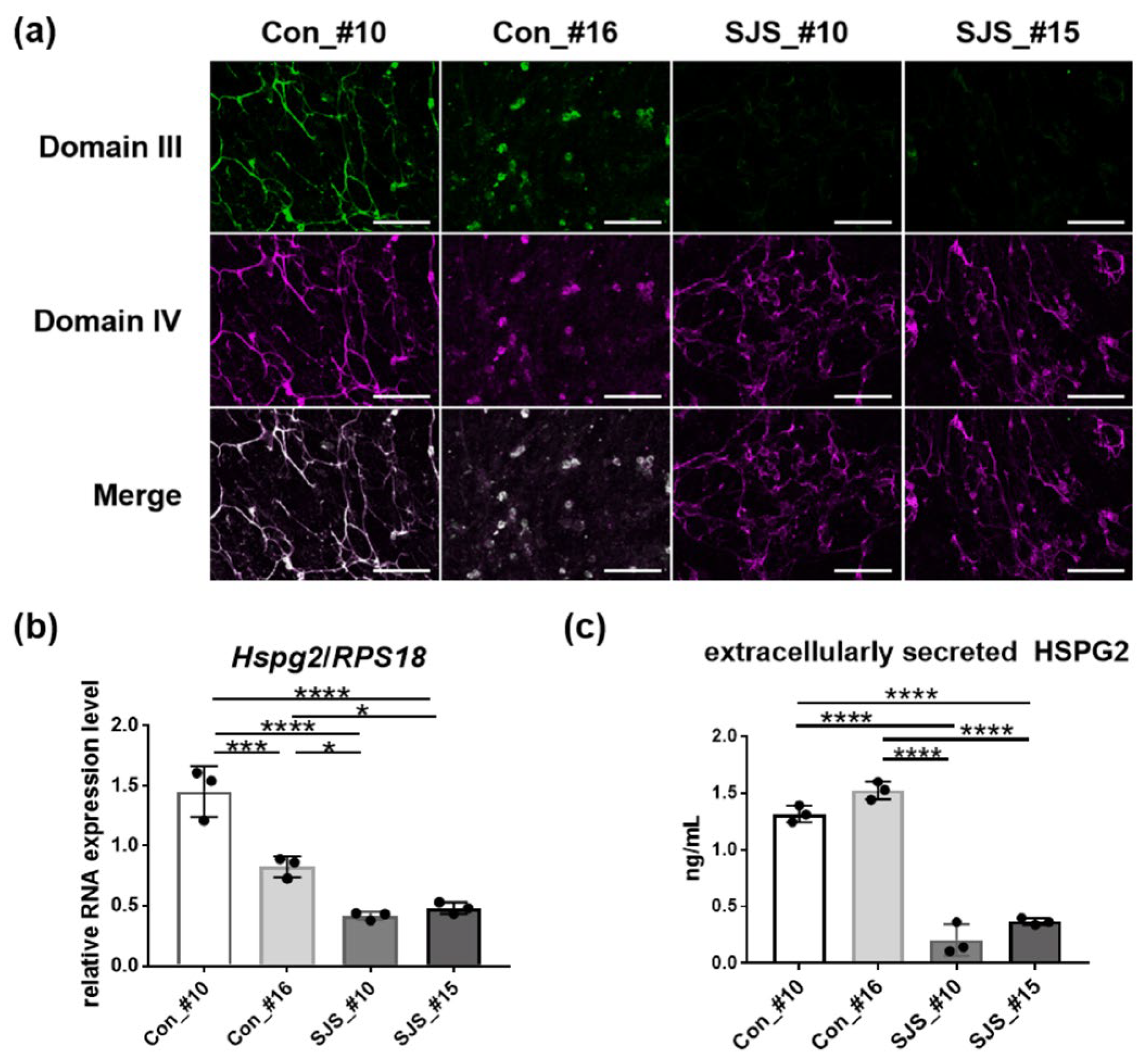
3.2. Expression Level of Perlecan in Control- and SJS-hiPSCs
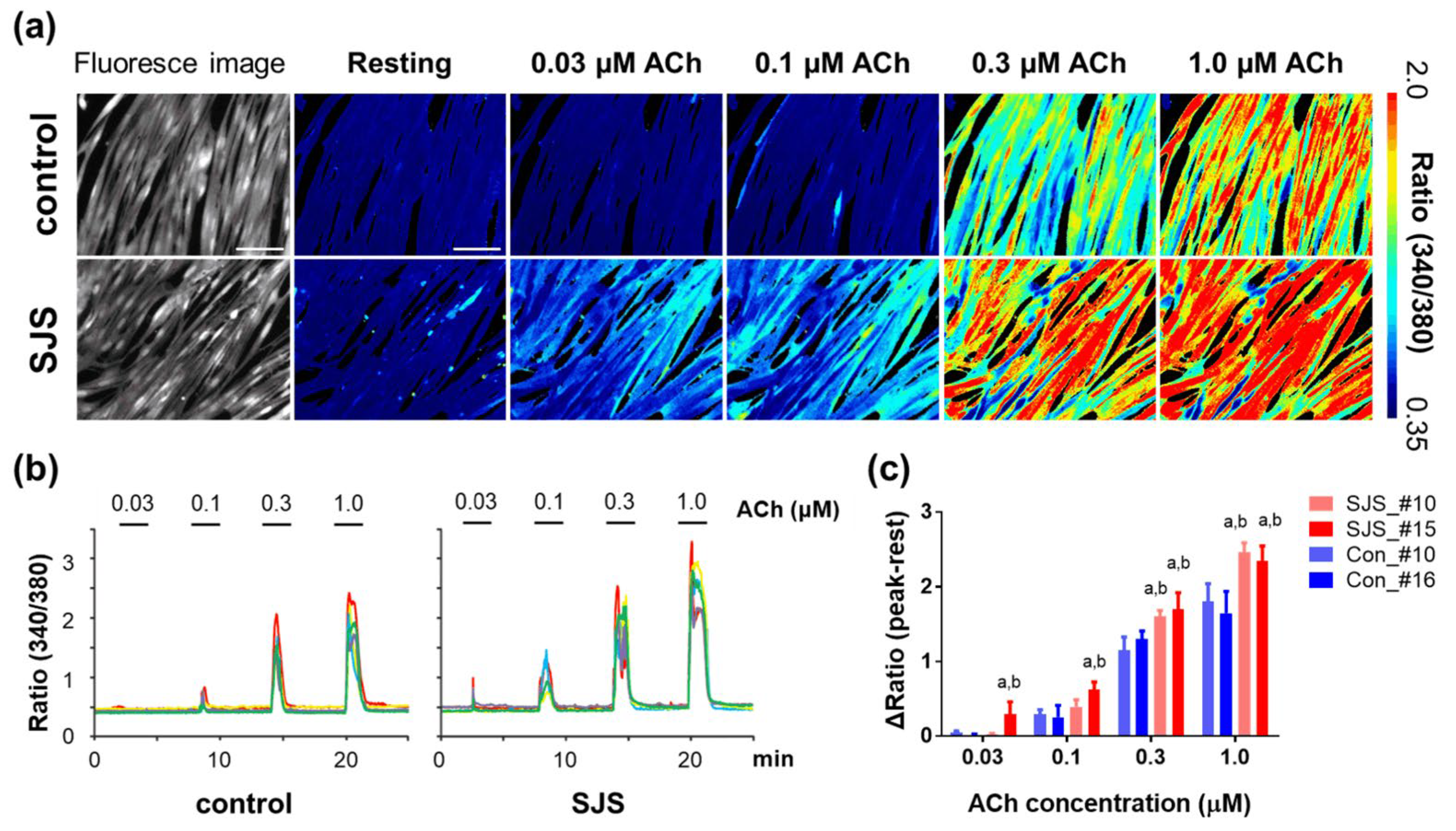
3.3. Calcium Ion Dynamics in SJS-hiPSCs
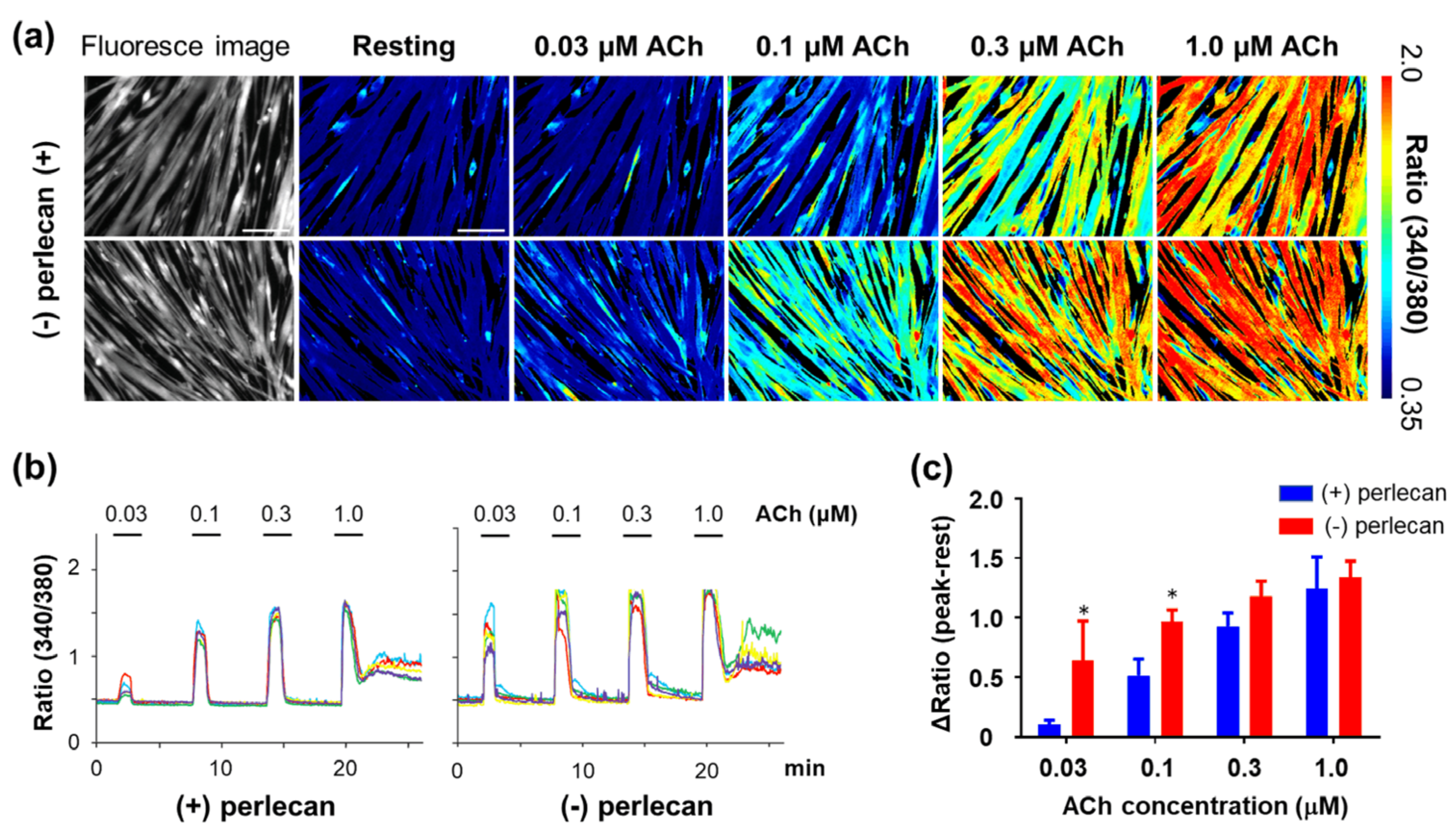
3.4. Calcium Ion Dynamics in Hspg2−/−-Tg Mouse Satellite Cell-Derived Myotubes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, O.; Jampel, R.S. Congenital blepharophimosis associated with a unique generalized myopathy. Arch. Ophthalmol. 1962, 68, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Giedion, A.; Boltshauser, E.; Briner, J.; Eich, G.; Exner, G.; Fendel, H.; Kaufmann, L.; Steinmann, B.; Spranger, J.; Superti-Furga, A. Heterogeneity in Schwartz-Jampel chondrodystrophic myotonia. Eur. J. Pediatr. 1997, 156, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Nicole, S.; Davoine, C.S.; Topaloglu, H.; Cattolico, L.; Barral, D.; Beighton, P.; Hamida, C.B.; Hammouda, H.; Cruaud, C.; White, P.S.; et al. Perlecan, the major proteoglycan of basement membranes, is altered in patients with Schwartz-Jampel syndrome (chondrodystrophic myotonia). Nat. Genet. 2000, 26, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Dagoneau, N.; Scheffer, D.; Huber, C.; Al-Gazali, L.I.; Di Rocco, M.; Godard, A.; Martinovic, J.; Raas-Rothschild, A.; Sigaudy, S.; Unger, S.; et al. Null leukemia inhibitory factor receptor (LIFR) mutations in Stuve-Wiedemann/Schwartz-Jampel type 2 syndrome. Am. J. Hum. Genet. 2004, 74, 298–305. [Google Scholar] [CrossRef]
- Arikawa-Hirasawa, E.; Le, A.H.; Nishino, I.; Nonaka, I.; Ho, N.C.; Francomano, C.A.; Govindraj, P.; Hassell, J.R.; Devaney, J.M.; Spranger, J.; et al. Structural and functional mutations of the perlecan gene cause Schwartz-Jampel syndrome, with myotonic myopathy and chondrodysplasia. Am. J. Hum. Genet. 2002, 70, 1368–1375. [Google Scholar] [CrossRef]
- Fasanelli, S.; Kozlowski, K.; Reiter, S.; Sillence, D. Dyssegmental dysplasia (report of two cases with a review of the literature). Skeletal. Radiol. 1985, 14, 173–177. [Google Scholar] [CrossRef]
- Nakazawa, H.; Tamura, M.; Nako, T.; Iida, T.; Yamada, T.; Murotsuki, J.; Nakazawa, Y.; Ando, A.; Nishizima, C.; Suzuki, K.; et al. Prenatal diagnosis of dyssegmental dysplasia using 3D CT: A case report. Kanto J. Obstet. Gynecol. 2016, 53, 647–653. [Google Scholar]
- Cao, A.; Cianchetti, C.; Calisti, L.; de Virgiliis, S.; Ferreli, A.; Tangheroni, W. Schwartz-Jampel syndrome. Clinical, electrophysiological and histopathological study of a severe variant. J. Neurol. Sci. 1978, 35, 175–187. [Google Scholar] [CrossRef]
- Pavone, L.; Mollica, F.; Grasso, A.; Cao, A.; Gullotta, F. Schwartz-Jampel syndrome in two daughters of first cousins. J. Neurol. Neurosurg. Psychiatry 1978, 41, 161–169. [Google Scholar] [CrossRef]
- Ho, N.C.; Sandusky, S.; Madike, V.; Francomano, C.A.; Dalakas, M.C. Clinico-pathogenetic findings and management of chondrodystrophic myotonia (Schwartz-Jampel syndrome): A case report. BMC Neurol. 2003, 3, 3. [Google Scholar] [CrossRef]
- Padmanabha, H.; Suthar, R.; Sankhyan, N.; Singhi, P. Stiffness, facial dysmorphism, and skeletal abnormalities: Schwartz-Jampel syndrome 1A. J. Pediatr. 2018, 200, 286. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Alciati, A.; Reggiani, A.; Caldirola, D.; Perna, G. Human-induced pluripotent stem cell technology: Toward the future of personalized psychiatry. J. Pers. Med. 2022, 12, 1340. [Google Scholar] [CrossRef]
- Zhang, J.; Lian, Q.; Zhu, G.; Zhou, F.; Sui, L.; Tan, C.; Mutalif, A.; Navasankari, R.; Zhang, Y.; Tse, H.; et al. A human iPSC model of Hutchinson Gilford Progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 2011, 8, 31–45. [Google Scholar] [CrossRef]
- Cannell, M.B.; Berlin, J.R.; Lederer, W.J. Intracellular calcium in cardiac myocytes: Calcium transients measured using fluorescence imaging. Soc. Gen. Physiol. Ser. 1987, 42, 201–214. [Google Scholar]
- Guatimosim, S.; Guatimosim, C.; Song, L.S. Imaging calcium sparks in cardiac myocytes. Methods Mol. Biol. 2011, 689, 205–214. [Google Scholar]
- Tsugorka, A.; Rios, E.; Blatter, L.A. Imaging elementary events of calcium release in skeletal muscle cells. Science 1995, 269, 1723–1726. [Google Scholar] [CrossRef]
- Wang, X.; Weisleder, N.; Collet, C.; Zhou, J.; Chu, Y.; Hirata, Y.; Zhao, X.; Pan, Z.; Brotto, M.; Cheng, H.; et al. Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat. Cell Biol. 2005, 7, 525–530. [Google Scholar] [CrossRef]
- Dana, H.; Sun, Y.; Mohar, B.; Hulse, B.K.; Kerlin, A.M.; Hasseman, J.P.; Tsegaye, G.; Tsang, A.; Wong, A.; Patel, R.; et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 2019, 16, 649–657. [Google Scholar] [CrossRef]
- Barreto-Chang, O.L.; Dolmetsch, R.E. Calcium imaging of cortical neurons using Fura-2 AM. J. Vis. Exp. 2009. [Google Scholar] [CrossRef]
- Okita, K.; Matsumura, Y.; Sato, Y.; Okada, A.; Morizane, A.; Okamoto, S.; Hong, H.; Nakagawa, M.; Tanabe, K.; Tezuka, K.I.; et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods 2011, 8, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.D.; Campbell, M.J.; Kejariwal, A.; Mi, H.; Karlak, B.; Daverman, R.; Diemer, K.; Muruganujan, A.; Narechania, A. PANTHER: A library of protein families and subfamilies indexed by function. Genome Res. 2003, 13, 2129–2141. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Sims, G.E.; Murphy, S.; Miller, J.R.; Chan, A.P. Predicting the functional effect of amino acid substitutions and indels. PLoS ONE 2012, 7, e46688. [Google Scholar] [CrossRef]
- Kumar, P.; Henikoff, S.; Ng, P.C. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nat. Protoc. 2009, 4, 1073–1081. [Google Scholar] [CrossRef]
- Iwata, S.; Ito, M.; Nakata, T.; Noguchi, Y.; Okuno, T.; Ohkawara, B.; Masuda, A.; Goto, T.; Adachi, M.; Osaka, H.; et al. A missense mutation in domain III in HSPG2 in Schwartz-Jampel syndrome compromises secretion of perlecan into the extracellular space. Neuromuscul. Disord. 2015, 25, 667–671. [Google Scholar] [CrossRef]
- Tanaka, A.; Woltjen, K.; Miyake, K.; Hotta, A.; Ikeya, M.; Yamamoto, T.; Nishino, T.; Shoji, E.; Sehara-Fujisawa, A.; Manabe, Y.; et al. Efficient and reproducible myogenic differentiation from human iPS cells: Prospects for modeling Miyoshi Myopathy in vitro. PLoS ONE 2013, 8, e61540. [Google Scholar] [CrossRef]
- Uchimura, T.; Otomo, J.; Sato, M.; Sakurai, H. A human iPS cell myogenic differentiation system permitting high-throughput drug screening. Stem Cell Res. 2017, 25, 98–106. [Google Scholar] [CrossRef]
- Arikawa-Hirasawa, E.; Watanabe, H.; Takami, H.; Hassell, J.R.; Yamada, Y. Perlecan is essential for cartilage and cephalic development. Nat. Genet. 1999, 23, 354–358. [Google Scholar] [CrossRef]
- Tsumaki, N.; Tanaka, K.; Arikawa-Hirasawa, E.; Nakase, T.; Kimura, T.; Thomas, J.T.; Ochi, T.; Luyten, F.P.; Yamada, Y. Role of CDMP-1 in skeletal morphogenesis: Promotion of mesenchymal cell recruitment and chondrocyte differentiation. J. Cell Biol. 1999, 144, 161–173. [Google Scholar] [CrossRef]
- Fukada, S.I.; Higuchi, S.; Segawa, M.; Koda, K.I.; Yamamoto, Y.; Tsujikawa, K.; Kohama, Y.; Uezumi, A.; Imamura, M.; Miyagoe-Suzuki, Y.; et al. Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Exp. Cell Res. 2004, 296, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Penton, C.M.; Badarinarayana, V.; Prisco, J.; Powers, E.; Pincus, M.; Allen, R.E.; August, P.R. Laminin 521 maintains differentiation potential of mouse and human satellite cell-derived myoblasts during long-term culture expansion. Skelet. Muscle 2016, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Shahini, A.; Vydiam, K.; Choudhury, D.; Rajabian, N.; Nguyen, T.; Lei, P.; Andreadis, S.T. Efficient and high yield isolation of myoblasts from skeletal muscle. Stem Cell Res. 2018, 30, 122–129. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Hamada, K.; Terauchi, A.; Matsui, M.; Nakamura, T.; Okada, T.; Mikoshiba, K. Distinct roles of M1 and M3 muscarinic acetylcholine receptors controlling oscillatory and non-oscillatory [Ca2+]i increase. Cell Calcium. 2013, 54, 111–119. [Google Scholar] [CrossRef]
- Stum, M.; Davoine, C.S.; Vicart, S.; Guillot-Noël, L.; Topaloglu, H.; Carod-Artal, F.J.; Kayserili, H.; Hentati, F.; Merlini, L.; Urtizberea, J.A.; et al. Spectrum of HSPG2 (Perlecan) mutations in patients with Schwartz-Jampel syndrome. Hum. Mutat. 2006, 27, 1082–1091. [Google Scholar] [CrossRef]
- Lin, P.Y.; Hung, J.H.; Hsu, C.K.; Chang, Y.T.; Sun, Y.T. A novel pathogenic HSPG2 mutation in Schwartz-Jampel syndrome. Front. Neurol. 2021, 12, 632336. [Google Scholar] [CrossRef]
- Gubbiotti, M.A.; Neill, T.; Iozzo, R.V. A current view of perlecan in physiology and pathology: A mosaic of functions. Matrix Biol. 2017, 57-58, 285–298. [Google Scholar] [CrossRef]
- Peng, H.B.; Ali, A.A.; Daggett, D.F.; Rauvala, H.; Hassell, J.R.; Smalheiser, N.R. The relationship between perlecan and dystroglycan and its implication in the formation of the neuromuscular junction. Cell Adhes. Commun. 1998, 5, 475–489. [Google Scholar] [CrossRef]
- Hayashi, K.; Madri, J.A.; Yurchenco, P.D. Endothelial cells interact with the core protein of basement membrane perlecan through beta 1 and beta 3 integrins: An adhesion modulated by glycosaminoglycan. J. Cell Biol. 1992, 119, 945–959. [Google Scholar] [CrossRef]
- Christova, L.G.; Alexandrov, A.S.; Ishpekova, B.A. Single motor unit activity pattern in patients with Schwartz-Jampel syndrome. J. Neurol. Neurosurg. Psychiatry 1999, 66, 252–253. [Google Scholar] [CrossRef]
- Bangratz, M.; Sarrazin, N.; Devaux, J.; Zambroni, D.; Echaniz-Laguna, A.; René, F.; Boërio, D.; Davoine, C.S.; Fontaine, B.; Feltri, M.L.; et al. A mouse model of Schwartz-Jampel syndrome reveals myelinating Schwann cell dysfunction with persistent axonal depolarization in vitro and distal peripheral nerve hyperexcitability when perlecan is lacking. Am. J. Pathol. 2012, 180, 2040–2055. [Google Scholar] [CrossRef]
- Kamimura, K.; Ueno, K.; Nakagawa, J.; Hamada, R.; Saitoe, M.; Maeda, N. Perlecan regulates bidirectional Wnt signaling at the Drosophila neuromuscular junction. J. Cell Biol. 2013, 200, 219–233. [Google Scholar] [CrossRef]
- Brandan, E.; Maldonado, M.; Garrido, J.; Inestrosa, N.C. Anchorage of collagen-tailed acetylcholinesterase to the extracellular matrix is mediated by heparan sulfate proteoglycans. J. Cell. Biol. 1985, 101, 985–992. [Google Scholar] [CrossRef]
- Peng, H.B.; Xie, H.; Rossi, S.G.; Rotundo, R.L. Acetylcholinesterase clustering at the neuromuscular junction involves perlecan and dystroglycan. J. Cell Biol. 1999, 145, 911–921. [Google Scholar] [CrossRef]
- Arikawa-Hirasawa, E.; Rossi, S.G.; Rotundo, R.L.; Yamada, Y. Absence of acetylcholinesterase at the neuromuscular junctions of perlecan-null mice. Nat. Neurosci. 2002, 5, 119–123. [Google Scholar] [CrossRef]
- Smirnov, S.P.; Barzaghi, P.; McKee, K.K.; Ruegg, M.A.; Yurchenco, P.D. Conjugation of LG domains of agrins and perlecan to polymerizing laminin-2 promotes acetylcholine receptor clustering. J. Biol. Chem. 2005, 280, 41449–41457. [Google Scholar] [CrossRef]
- Xu, Z.; Ichikawa, N.; Kosaki, K.; Yamada, Y.; Sasaki, T.; Sakai, L.Y.; Kurosawa, H.; Hattori, N.; Arikawa-Hirasawa, E. Perlecan deficiency causes muscle hypertrophy, a decrease in myostatin expression, and changes in muscle fiber composition. Matrix Biol. 2010, 29, 461–470. [Google Scholar] [CrossRef]
- Yamashita, Y.; Nakada, S.; Yoshihara, T.; Nara, T.; Furuya, N.; Miida, T.; Hattori, N.; Arikawa-Hirasawa, E. Perlecan, a heparan sulfate proteoglycan, regulates systemic metabolism with dynamic changes in adipose tissue and skeletal muscle. Sci. Rep. 2018, 8, 7766. [Google Scholar] [CrossRef]
- Cho, C.H.; Woo, J.S.; Perez, C.F.; Lee, E.H. A focus on extracellular Ca(2+) entry into skeletal muscle. Exp. Mol. Med. 2017, 49, e378. [Google Scholar] [CrossRef]
- Szent-Gyorgyi, A.G. Calcium regulation of muscle contraction. Biophys. J. 1975, 15, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Calderon, J.C.; Bolanos, P.; Caputo, C. The excitation-contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef] [PubMed]
- Larrain, J.; Alvarez, J.; Hassell, J.R.; Brandan, E. Expression of perlecan, a proteoglycan that binds myogenic inhibitory basic fibroblast growth factor, is down regulated during skeletal muscle differentiation. Exp. Cell Res. 1997, 234, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Nakai, J.; Ohkura, M.; Imoto, K. A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein. Nat. Biotechnol. 2001, 19, 137–141. [Google Scholar] [CrossRef]
- Moeyaert, B.; Holt, G.; Madangopal, R.; Perez-Alvarez, A.; Fearey, B.C.; Trojanowski, N.F.; Ledderose, J.; Zolnik, T.A.; Das, A.; Patel, D.; et al. Improved methods for marking active neuron populations. Nat. Commun. 2018, 9, 4440. [Google Scholar] [CrossRef]
- Shoji, E.; Sakurai, H.; Nishino, T.; Nakahata, T.; Heike, T.; Awaya, T.; Fujii, N.; Manabe, Y.; Matsuo, M.; Sehara-Fujisawa, A. Early pathogenesis of Duchenne muscular dystrophy modelled in patient-derived human induced pluripotent stem cells. Sci Rep. 2015, 5, 12831. [Google Scholar] [CrossRef]
- Sudevan, S.; Takiura, M.; Kubota, Y.; Higashitani, N.; Cooke, M.; Ellwood, R.A.; Etheridge, T.; Szewczyk, N.J.; Higashitani, A. Mitochondrial dysfunction causes Ca(2+) overload and ECM degradation-mediated muscle damage in C. Elegans . FASEB J. 2019, 33, 9540–9550. [Google Scholar] [CrossRef]

| Target | Primary Antibodies | Secondary Antibodies |
|---|---|---|
| MHC | Mouse anti-MHC monoclonal antibody (clone MF20, MAB4470; R&D Systems), dilution: 1/100 | Alexa Fluor 647-labeled goat anti-mouse IgG (A21235; Thermo Fisher Scientific), dilution: 1/400 |
| Perlecan | Rat anti-mouse Heparan Sulfate Proteoglycan (Perlecan) antibody (clone A7L6, MAB1948P; Merck), dilution: 1/200 | Alexa Fluor 647-labeled goat anti-rat IgG (A11006; Thermo Fisher Scientific), dilution: 1/400 |
| Mouse anti-human perlecan monoclonal antibody (clone 7B5, 13-4400; Thermo Fisher Scientific), dilution: 1/200 | Alexa Fluor 488-labeled goat anti-mouse IgG (A21235; Thermo Fisher Scientific), dilution: 1/400 |
| Target | Sequence |
|---|---|
| Myogenin | Fw: TGGGCGTGTAAGGTGTGTAA |
| Rev: CGATGTACTGGATGGCACTG | |
| TPM2 | Fw: ACGTGAGGACGAGCATGTG |
| Rev: GTGCAGCGCTTGAGTGTCT | |
| CKM | Fw: ACATGGCCAAGGTACTGACC |
| Rev: TGATGGGGTCAAAGAGTTCC | |
| Myh3 | Fw: CTGGAGGATGAATGCTCAGAGC |
| Rev: CCCAGAGAGTTCCTCAGTAAGG | |
| HSPG2 | Fw: GGCTGAGGGCATACGATGGCT |
| Rev: CCCACTGCCCAGGTCGTCTCC | |
| RPS18 | Fw: GCAGAATCCACGCCAGTACAAG |
| Rev: GCTTGTTGTCCAGACCATTGGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamashita, Y.; Nakada, S.; Nakamura, K.; Sakurai, H.; Ohno, K.; Goto, T.; Mabuchi, Y.; Akazawa, C.; Hattori, N.; Arikawa-Hirasawa, E. Evaluation of Human-Induced Pluripotent Stem Cells Derived from a Patient with Schwartz–Jampel Syndrome Revealed Distinct Hyperexcitability in the Skeletal Muscles. Biomedicines 2023, 11, 814. https://doi.org/10.3390/biomedicines11030814
Yamashita Y, Nakada S, Nakamura K, Sakurai H, Ohno K, Goto T, Mabuchi Y, Akazawa C, Hattori N, Arikawa-Hirasawa E. Evaluation of Human-Induced Pluripotent Stem Cells Derived from a Patient with Schwartz–Jampel Syndrome Revealed Distinct Hyperexcitability in the Skeletal Muscles. Biomedicines. 2023; 11(3):814. https://doi.org/10.3390/biomedicines11030814
Chicago/Turabian StyleYamashita, Yuri, Satoshi Nakada, Kyoko Nakamura, Hidetoshi Sakurai, Kinji Ohno, Tomohide Goto, Yo Mabuchi, Chihiro Akazawa, Nobutaka Hattori, and Eri Arikawa-Hirasawa. 2023. "Evaluation of Human-Induced Pluripotent Stem Cells Derived from a Patient with Schwartz–Jampel Syndrome Revealed Distinct Hyperexcitability in the Skeletal Muscles" Biomedicines 11, no. 3: 814. https://doi.org/10.3390/biomedicines11030814
APA StyleYamashita, Y., Nakada, S., Nakamura, K., Sakurai, H., Ohno, K., Goto, T., Mabuchi, Y., Akazawa, C., Hattori, N., & Arikawa-Hirasawa, E. (2023). Evaluation of Human-Induced Pluripotent Stem Cells Derived from a Patient with Schwartz–Jampel Syndrome Revealed Distinct Hyperexcitability in the Skeletal Muscles. Biomedicines, 11(3), 814. https://doi.org/10.3390/biomedicines11030814








