Chemerin and Chemokine-like Receptor 1 Expression Are Associated with Hepatocellular Carcinoma Progression in European Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemistry
2.3. Histological Scores
2.4. Statistics
3. Results
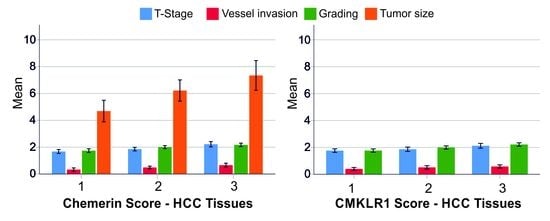
3.1. Chemerin Expression in HCC Tissues
3.2. Sex-Specific Differences of Chemerin Expression in HCC Tissues
3.3. CMKLR1 Expression in HCC Tissues
3.4. Sex-Specific Differences of CMKLR1 Expression in HCC Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buechler, C.; Feder, S.; Haberl, E.M.; Aslanidis, C. Chemerin Isoforms and Activity in Obesity. Int. J. Mol. Sci. 2019, 20, 1128. [Google Scholar] [CrossRef] [PubMed]
- Bondue, B.; Wittamer, V.; Parmentier, M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011, 22, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Mariani, F.; Roncucci, L. Chemerin/chemR23 axis in inflammation onset and resolution. Inflamm. Res. 2015, 64, 85–95. [Google Scholar] [CrossRef]
- Goralski, K.B.; Jackson, A.E.; McKeown, B.T.; Sinal, C.J. More Than an Adipokine: The Complex Roles of Chemerin Signaling in Cancer. Int. J. Mol. Sci. 2019, 20, 4778. [Google Scholar] [CrossRef]
- Jacenik, D.; Fichna, J. Chemerin in immune response and gastrointestinal pathophysiology. Clin. Chim. Acta 2020, 504, 146–153. [Google Scholar] [CrossRef]
- Pachynski, R.K.; Zabel, B.A.; Kohrt, H.E.; Tejeda, N.M.; Monnier, J.; Swanson, C.D.; Holzer, A.K.; Gentles, A.J.; Sperinde, G.V.; Edalati, A.; et al. The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J. Exp. Med. 2012, 209, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Haberl, E.M.; Pohl, R.; Rein-Fischboeck, L.; Feder, S.; Sinal, C.J.; Bruckmann, A.; Hoering, M.; Krautbauer, S.; Liebisch, G.; Buechler, C. Overexpression of Hepatocyte Chemerin-156 Lowers Tumor Burden in a Murine Model of Diethylnitrosamine-Induced Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 21, 252. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Yin, H.K.; Guan, D.X.; Zhao, J.S.; Feng, Y.X.; Deng, Y.Z.; Wang, X.; Li, N.; Wang, X.F.; Cheng, S.Q.; et al. Chemerin suppresses hepatocellular carcinoma metastasis through CMKLR1-PTEN-Akt axis. Br. J. Cancer 2018, 118, 1337–1348. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yang, X.; Liu, W.; Li, B.; Yin, W.; Shi, Y.; He, R. Chemerin has a protective role in hepatocellular carcinoma by inhibiting the expression of IL-6 and GM-CSF and MDSC accumulation. Oncogene 2017, 36, 3599–3608. [Google Scholar] [CrossRef]
- Lin, W.; Chen, Y.L.; Jiang, L.; Chen, J.K. Reduced expression of chemerin is associated with a poor prognosis and a lowed infiltration of both dendritic cells and natural killer cells in human hepatocellular carcinoma. Clin. Lab. 2011, 57, 879–885. [Google Scholar] [PubMed]
- Haberl, E.M.; Feder, S.; Pohl, R.; Rein-Fischboeck, L.; Durholz, K.; Eichelberger, L.; Wanninger, J.; Weiss, T.S.; Buechler, C. Chemerin Is Induced in Non-Alcoholic Fatty Liver Disease and Hepatitis B-Related Hepatocellular Carcinoma. Cancers 2020, 12, 2967. [Google Scholar] [CrossRef]
- Graham, K.L.; Zabel, B.A.; Loghavi, S.; Zuniga, L.A.; Ho, P.P.; Sobel, R.A.; Butcher, E.C. Chemokine-like receptor-1 expression by central nervous system-infiltrating leukocytes and involvement in a model of autoimmune demyelinating disease. J. Immunol. 2009, 183, 6717–6723. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.D.; Aolymat, I.; Tiszlavicz, L.; Reisz, Z.; Garalla, H.M.; Beynon, R.; Simpson, D.; Dockray, G.J.; Varro, A. Chemerin acts via CMKLR1 and GPR1 to stimulate migration and invasion of gastric cancer cells: Putative role of decreased TIMP-1 and TIMP-2. Oncotarget 2019, 10, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Cai, Q.; Luo, Y.; Li, B.; Chen, Y.; Yang, X.; Xuan, Y.; Yang, H.; He, R. Epithelial chemerin-CMKLR1 signaling restricts microbiota-driven colonic neutrophilia and tumorigenesis by up-regulating lactoperoxidase. Proc. Natl. Acad. Sci. USA 2022, 119, e2205574119. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Oppenheim, J.J. Chemokine-like receptor 1 (CMKLR1) and chemokine (C-C motif) receptor-like 2 (CCRL2); two multifunctional receptors with unusual properties. Exp. Cell Res. 2011, 317, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. CMKLR1 and GPR1 mediate chemerin signaling through the RhoA/ROCK pathway. Mol. Cell. Endocrinol. 2015, 417, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Wanninger, J.; Bauer, S.; Eisinger, K.; Weiss, T.S.; Walter, R.; Hellerbrand, C.; Schaffler, A.; Higuchi, A.; Walsh, K.; Buechler, C. Adiponectin upregulates hepatocyte CMKLR1 which is reduced in human fatty liver. Mol. Cell. Endocrinol. 2012, 349, 248–254. [Google Scholar] [CrossRef]
- Available online: https://de.statista.com/statistik/daten/studie/1221/umfrage/anzahl-der-auslaender-in-deutschland-nach-herkunftsland/ (accessed on 21 February 2023).
- Mirlacher, M.; Simon, R. Recipient block TMA technique. Methods Mol. Biol. 2010, 664, 37–44. [Google Scholar] [CrossRef]
- Sobin, L.H.; Fleming, I.D. TNM Classification of Malignant Tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997, 80, 1803–1804. [Google Scholar] [CrossRef]
- Available online: https://www.lsbio.com/resources/lsbio-advantage#lsbio-ratings (accessed on 21 February 2023).
- Weber, F.; Jung, R.; Treeck, O.; Buechler, C. Chemerin and Chemokine-like Receptor 1 Are Not Prognostic in Colorectal Carcinoma. Anticancer. Res. 2023, 43, 831–839. [Google Scholar] [CrossRef]
- Rizzardi, A.E.; Johnson, A.T.; Vogel, R.I.; Pambuccian, S.E.; Henriksen, J.; Skubitz, A.P.; Metzger, G.J.; Schmechel, S.C. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn. Pathol. 2012, 7, 42. [Google Scholar] [CrossRef]
- Ishak, K.; Baptista, A.; Bianchi, L.; Callea, F.; De Groote, J.; Gudat, F.; Denk, H.; Desmet, V.; Korb, G.; MacSween, R.N.; et al. Histological grading and staging of chronic hepatitis. J. Hepatol. 1995, 22, 696–699. [Google Scholar] [CrossRef]
- Wu, E.M.; Wong, L.L.; Hernandez, B.Y.; Ji, J.F.; Jia, W.; Kwee, S.A.; Kalathil, S. Gender differences in hepatocellular cancer: Disparities in nonalcoholic fatty liver disease/steatohepatitis and liver transplantation. Hepatoma Res. 2018, 4, 66. [Google Scholar] [CrossRef]
- Choo, S.P.; Tan, W.L.; Goh, B.K.P.; Tai, W.M.; Zhu, A.X. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 2016, 122, 3430–3446. [Google Scholar] [CrossRef]
- Han, S.X.; Wang, J.L.; Guo, X.J.; He, C.C.; Ying, X.; Ma, J.L.; Zhang, Y.Y.; Zhao, Q.; Zhu, Q. Serum SALL4 is a novel prognosis biomarker with tumor recurrence and poor survival of patients in hepatocellular carcinoma. J. Immunol. Res. 2014, 2014, 262385. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.C.; Vachharajani, N.; Chapman, W.C.; Brunt, E.M. SALL4 immunoreactivity predicts prognosis in Western hepatocellular carcinoma patients but is a rare event: A study of 236 cases. Am. J. Surg. Pathol. 2014, 38, 966–972. [Google Scholar] [CrossRef]
- Park, J.W.; Chen, M.; Colombo, M.; Roberts, L.R.; Schwartz, M.; Chen, P.J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S.; et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Fingas, C.D.; Best, J.; Sowa, J.P.; Canbay, A. Epidemiology of nonalcoholic steatohepatitis and hepatocellular carcinoma. Clin. Liver Dis. 2016, 8, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Asselah, T.; Rubbia-Brandt, L.; Marcellin, P.; Negro, F. Steatosis in chronic hepatitis C: Why does it really matter? Gut 2006, 55, 123–130. [Google Scholar] [CrossRef]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 2004, 114, 147–152. [Google Scholar] [CrossRef]
- Horn, P.; von Loeffelholz, C.; Forkert, F.; Stengel, S.; Reuken, P.; Aschenbach, R.; Stallmach, A.; Bruns, T. Low circulating chemerin levels correlate with hepatic dysfunction and increased mortality in decompensated liver cirrhosis. Sci. Rep. 2018, 8, 9242. [Google Scholar] [CrossRef] [PubMed]
- Peschel, G.; Grimm, J.; Gulow, K.; Muller, M.; Buechler, C.; Weigand, K. Chemerin Is a Valuable Biomarker in Patients with HCV Infection and Correlates with Liver Injury. Diagnostics 2020, 10, 974. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Takai, K.; Hanai, T.; Shiraki, M.; Suzuki, Y.; Hayashi, H.; Naiki, T.; Nishigaki, Y.; Tomita, E.; Shimizu, M.; et al. Impact of serum chemerin levels on liver functional reserves and platelet counts in patients with hepatocellular carcinoma. Int. J. Mol. Sci. 2014, 15, 11294–11306. [Google Scholar] [CrossRef] [PubMed]
- Feder, S.; Kandulski, A.; Schacherer, D.; Weiss, T.S.; Buechler, C. Serum Chemerin Does Not Differentiate Colorectal Liver Metastases from Hepatocellular Carcinoma. Int. J. Mol. Sci. 2019, 20, 3919. [Google Scholar] [CrossRef]
- Kirstein, M.M.; Vogel, A. The pathogenesis of hepatocellular carcinoma. Dig. Dis. 2014, 32, 545–553. [Google Scholar] [CrossRef]
- Naguib, A.; Bencze, G.; Cho, H.; Zheng, W.; Tocilj, A.; Elkayam, E.; Faehnle, C.R.; Jaber, N.; Pratt, C.P.; Chen, M.; et al. PTEN functions by recruitment to cytoplasmic vesicles. Mol. Cell 2015, 58, 255–268. [Google Scholar] [CrossRef]
- Available online: https://www.novusbio.com/products/chemr23-cmklr1-antibody-bz194_nbp1-43233 (accessed on 21 February 2023).
- Available online: https://www.bosterbio.com/anti-chemr23-r249-cmklr1-antibody-a02960-1-boster.html (accessed on 21 February 2023).


| Low Chemerin | Moderate Chemerin | High Chemerin | p-Value | |
|---|---|---|---|---|
| Patients | 92 | 186 | 105 | |
| T stage | 1.69 ± 0.66 a | 1.85 ± 0.80 b | 2.21 ± 0.88 a,b | <0.001 a, 0.004 b |
| Lymph node inv | 0.03 ± 0.18 | 0.09 ± 0.29 | 0.07 ± 0.25 | not significant |
| Vessel inv | 0.32 ± 0.53 a | 0.50 ± 0.62 | 0.67 ± 0.69 a | <0.001 a |
| Grading | 1.70 ± 0.62 a,c | 1.99 ± 0.72 c | 2.10 ± 0.63 a | <0.001 a, 0.003 c |
| Tumor size (cm) | 4.92 ± 3.75 a,c | 6.47 ± 4.77 c | 7.62 ± 5.50 a | <0.001 a, 0.02 c |
| UICC score | 1.71 ± 0.76 a | 1.92 ± 0.86 b | 2.22 ± 0.90 a,b | <0.001 a, 0.018 b |
| Steatosis grade % | 14 ± 15 | 15 ± 19 | 14 ± 19 | not significant |
| Inflammation grade | 0.70 ± 0.57 | 0.74 ± 0.70 | 0.85 ± 0.68 | not significant |
| Fibrosis grade | 4.30 ± 2.42 | 3.83 ± 2.41 | 3.99 ± 2.37 | not significant |
| Age (years) | 63.64 ± 10.32 | 64.7222 ± 12.53 | 64.19 ± 10.53 | not significant |
| CMKLR1 | 1.44 ± 0.72 a,c | 1.91 ± 0.78 b,c | 2.50 ± 0.66 a,b | <0.001 a,b,c |
| Low Chemerin | Moderate Chemerin | High Chemerin | p-Value | |
|---|---|---|---|---|
| Patients | 11 | 36 | 21 | |
| T stage | 1.80 ± 0.79 | 1.90 ± 0.85 | 2.17 ± 0.92 | not significant |
| Lymph node inv | 0.0 ± 0.0 | 0.11 ± 0.32 | 0.0 ± 0.0 | not significant |
| Vessel inv | 0.27 ± 0.65 | 0.56 ± 0.70 | 0.70 ± 0.80 | not significant |
| Grading | 1.64 ± 0.67 | 2.17 ± 0.70 | 2.10 ± 0.62 | not significant |
| Tumor size (cm) | 7.08 ± 6.98 | 9.08 ± 6.30 | 8.28 ± 5.36 | not significant |
| UICC score | 1.73 ± 0.79 | 1.94 ± 0.83 | 2.05 ± 0.86 | not significant |
| Steatosis grade % | 15 ± 15 | 10 ± 10 | 10 ± 9 | not significant |
| Inflammation grade | 0.80 ± 0.63 | 0.81 ± 0.82 | 0.76 ± 0.62 | not significant |
| Fibrosis grade | 3.00 ± 3.00 | 3.00 ± 2.73 | 3.78 ± 2.44 | not significant |
| Age (years) | 61.54 ± 12.88 | 63.41 ± 13.07 | 62.57 ± 10.39 | not significant |
| CMKLR1 | 1.55 ± 0.82 a,c | 1.92 ± 0.84 b,c | 2.76 ± 0.44 a,b | <0.001 a,b,c |
| Low Chemerin | Moderate Chemerin | High Chemerin | p-Value | |
|---|---|---|---|---|
| Patients | 81 | 150 | 84 | |
| T stage | 1.67 ± 0.65 a | 1.84 ± 0.80 b | 2.23 ± 0.87 a,b | <0.001 a, 0.006 b |
| Lymph node inv | 0.04 ± 0.19 | 0.09 ± 0.28 | 0.09 ± 0.28 | not significant |
| Vessel inv | 0.32 ± 0.52 a | 0.49 ± 0.60 | 0. 66 ± 0.67 a | 0.002 a |
| Grading | 1.70 ± 0.62 a,c | 1.95 ± 0.72 c | 2.11 ± 0.64 a | 0.001 a, 0.037 c |
| Tumor size (cm) | 4.62 ± 3.00 a | 5.83 ± 4.09 | 7.46 ± 5.55 a | 0.002 a |
| UICC score | 1.70 ± 0.77 a | 1.92 ± 0.87 b | 2.26 ± 0.91 a,b | <0.001 a, 0.012 b |
| Steatosis grade % | 14 ± 16 | 17 ± 20 | 15 ± 21 | not significant |
| Inflammation grade | 0.68 ± 0.57 | 0.73 ± 0.67 | 0.88 ± 0.70 | not significant |
| Fibrosis grade | 4.46 ± 2.31 | 4.01 ± 2.30 | 4.04 ± 2.37 | not significant |
| Age (years) | 63.93 ± 9.98 | 65.03 ± 12.42 | 64.60 ± 10.59 | not significant |
| CMKLR1 | 1.42 ± 0.70 a,c | 1.91 ± 0.76 b,c | 2.43 ± 0.68 a,b | <0.001 a,b,c |
| Histologic Measures | Low CMKLR1 | Moderate CMKLR1 | High CMKLR1 | p-Value |
|---|---|---|---|---|
| Patients | 138 | 123 | 121 | |
| T stage | 1.76 ± 0.73 a | 1.86 ± 0.81 | 2.12 ± 0.87 a | 0.006 a |
| Lymph node inv | 0.04 ± 0.19 | 0.08 ± 0.28 | 0.10 ± 0.30 | not significant |
| Vessel inv | 0.40 ± 0.59 a | 0.50 ± 0.63 | 0.62 ± 0.67 a | 0.015 a |
| Grading | 1.75 ± 0.69 a | 1.94 ± 0.65 b | 2.20 ± 0.65 a,b | <0.001 a, 0.012 b |
| Tumor size (cm) | 6.02 ± 4.58 | 6.17 ± 4.77 | 7.09 ± 5.20 | not significant |
| UICC | 1.84 ± 0.81 a | 1.88 ± 0.85 b | 2.17 ± 0.91 a,b | 0.012 a, 0.037 b |
| Steatosis grade % | 19 ± 21 | 13 ± 16 | 11 ± 13 | not significant |
| Inflammation grade | 0.78 ± 0.61 | 0.65 ± 0.63 | 0.87 ± 0.74 | not significant |
| Fibrosis grade | 3.90 ± 2.50 | 4.00 ± 2.48 | 4.05 ± 2.24 | not significant |
| Age (years) | 64.66 ± 12.08 | 63.88 ± 11.00 | 64.31 ± 11.37 | not significant |
| Chemerin | 1.60 ± 0.61 a,c | 2.15 ± 0.62 b,c | 2.41 ± 0.67 a,b | <0.001 a,b, 0.013 c |
| Histologic Measures | Low CMKLR1 | Moderate CMKLR1 | High CMKLR1 | p-Value |
|---|---|---|---|---|
| Patients | 21 | 18 | 29 | |
| T stage | 2.11 ± 0.90 | 1.65 ± 0.70 | 2.09 ± 0.90 | not significant |
| Lymph node inv | 0.10 ± 0.30 | 0.06 ± 0.24 | 0.04 ± 0.19 | not significant |
| Vessel inv | 0.48 ± 0.68 | 0.44 ± 0.70 | 0.68 ± 0.77 | not significant |
| Grading | 1.76 ± 0.77 | 2.22 ± 0.65 | 2.17 ± 0.60 | not significant |
| Tumor size | 9.19 ± 6.20 | 8.14 ± 6.88 | 8.24 ± 5.67 | not significant |
| UICC | 2.10 ± 0.83 | 1.72 ± 0.75 | 1.97 ± 0.87 | not significant |
| Steatosis grade % | 15 ± 13 | 13 ± 12 | 9 ± 8 | not significant |
| Inflammation grade | 0.78 ± 0.65 | 0.83 ± 0.79 | 0.78 ± 0.75 | not significant |
| Fibrosis grade | 2.88 ± 2.64 | 2.38 ± 2.75 | 4.04 ± 2.49 | not significant |
| Age (years) | 65.48 ± 14.73 | 62.72 ± 8.09 | 61.03 ± 12.16 | not significant |
| Chemerin | 1.66 ± 0.48 a | 2.17 ± 0.62 | 2.48 ± 0.63 a | <0.001 a |
| Histologic Measures | Low CMKLR1 | Moderate CMKLR1 | High CMKLR1 | p-Value |
|---|---|---|---|---|
| Patients | 117 | 105 | 92 | |
| T stage | 1.70 ± 0.68 a | 1.91 ± 0.83 | 2.13 ± 0.87 a | 0.002 a |
| Lymph node inv | 0.03 ± 0.16 a | 0.09 ± 0.28 | 0.12 ± 0.33 a | 0.028 a |
| Vessel inv | 0.39 ± 0.57 a | 0.51 ± 0.62 | 0.60 ± 0.63 a | 0.028 a |
| Grading | 1.74 ± 0.68 a | 1.89 ± 0.64 b | 2.21 ± 0.67 a,b | <0.001 a, 0.006 b |
| Tumor size | 5.43 ± 4.00 | 5.83 ± 4.25 | 6.72 ± 5.01 | not significant |
| UICC | 1.80 ± 0.80 a | 1.90 ± 0.86 b | 2.23 ± 0.92 a,b | 0.002 a, 0.036 b |
| Steatosis grade % | 20 ± 22 | 13 ± 17 | 11 ± 15 | not significant |
| Inflammation grade | 0.78 ± 0.61 | 0.62 ± 0.60 b | 0.90 ± 0.74 b | 0.026 b |
| Fibrosis grade | 4.07 ± 2.45 | 4.27 ± 2.34 | 4.05 ± 2.17 | not significant |
| Age (years) | 64.51 ± 11.61 | 64.08 ± 11.44 | 65.34 ± 10.98 | not significant |
| Chemerin | 1.59 ± 0.63 a,c | 2.14 ± 0.63 c | 2.38 ± 0.68 a | <0.001 a, 0.001 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weber, F.; Utpatel, K.; Evert, K.; Treeck, O.; Buechler, C. Chemerin and Chemokine-like Receptor 1 Expression Are Associated with Hepatocellular Carcinoma Progression in European Patients. Biomedicines 2023, 11, 737. https://doi.org/10.3390/biomedicines11030737
Weber F, Utpatel K, Evert K, Treeck O, Buechler C. Chemerin and Chemokine-like Receptor 1 Expression Are Associated with Hepatocellular Carcinoma Progression in European Patients. Biomedicines. 2023; 11(3):737. https://doi.org/10.3390/biomedicines11030737
Chicago/Turabian StyleWeber, Florian, Kirsten Utpatel, Katja Evert, Oliver Treeck, and Christa Buechler. 2023. "Chemerin and Chemokine-like Receptor 1 Expression Are Associated with Hepatocellular Carcinoma Progression in European Patients" Biomedicines 11, no. 3: 737. https://doi.org/10.3390/biomedicines11030737
APA StyleWeber, F., Utpatel, K., Evert, K., Treeck, O., & Buechler, C. (2023). Chemerin and Chemokine-like Receptor 1 Expression Are Associated with Hepatocellular Carcinoma Progression in European Patients. Biomedicines, 11(3), 737. https://doi.org/10.3390/biomedicines11030737