Lung microRNAs Expression in Lung Cancer and COPD: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Clinical Biochemistry Assays
2.3. Real-Time PCR (RT-PCR)
- hsa-miR-34a-5p: GGCCAGCUGUGAGUGUUUCUUUGGCAGUGUCUUAGCUGGUUGUUGUGAGCAAUAGUAAGGAAGCAAUCAGCAAGUAUACUGCCCUAGAAGUGCUGCACGUUGUGGGGCCC (Catalog number 478048_mir);
- hsa-miR-33a-5p: CUGUGGUGCAUUGUAGUUGCAUUGCAUGUUCUGGUGGUACCCAUGCAAUGUUUCCACAGUGCAUCACAG (Catalog number 478347_mir);
- hsa-miR-149-3p: GCCGGCGCCCGAGCUCUGGCUCCGUGUCUUCACUCCCGUGCUUGUCCGAGGAGGGAGGGAGGGACGGGGGCUGUGCUGGGGCAGCUGGA (Catalog number 478720_mir);
- hsa-miR-197-3p: GGCUGUGCCGGGUAGAGAGGGCAGUGGGAGGUAAGAGCUCUUCACCCUUCACCACCUUCUCCACCCAGCAUGGCC (Catalog number 477959_mir);
- hsa-miR-199a-5p: GCCAACCCAGUGUUCAGACUACCUGUUCAGGAGGCUCUCAAUGUGUACAGUAGUCUGCACAUUGGUUAGGC (Catalog number 478231_mir);
- hsa-miR-320a-3p: CUCCCCUCCGCCUUCUCUUCCCGGUUCUUCCCGGAGUCGGGAAAAGCUGGGUUGAGAGGGCGAAAAAGGAUG (Catalog number 478594_mir);
- Additionally, U6 snRNA: GTGCTCGCTTCGGCAGCACATATACTAAAATTGGAACGATACAGAGAAGAT CATGGCCCCTGCGCAAGGATGACACGCAAATTCGTGAAGCGTTCCATATTTT (Catalog number 001973) was selected as housekeeping miRNA.
2.4. In Silico Prediction of “hsa-miR” Target Genes
2.5. Statistical Analysis
3. Results
3.1. Patients
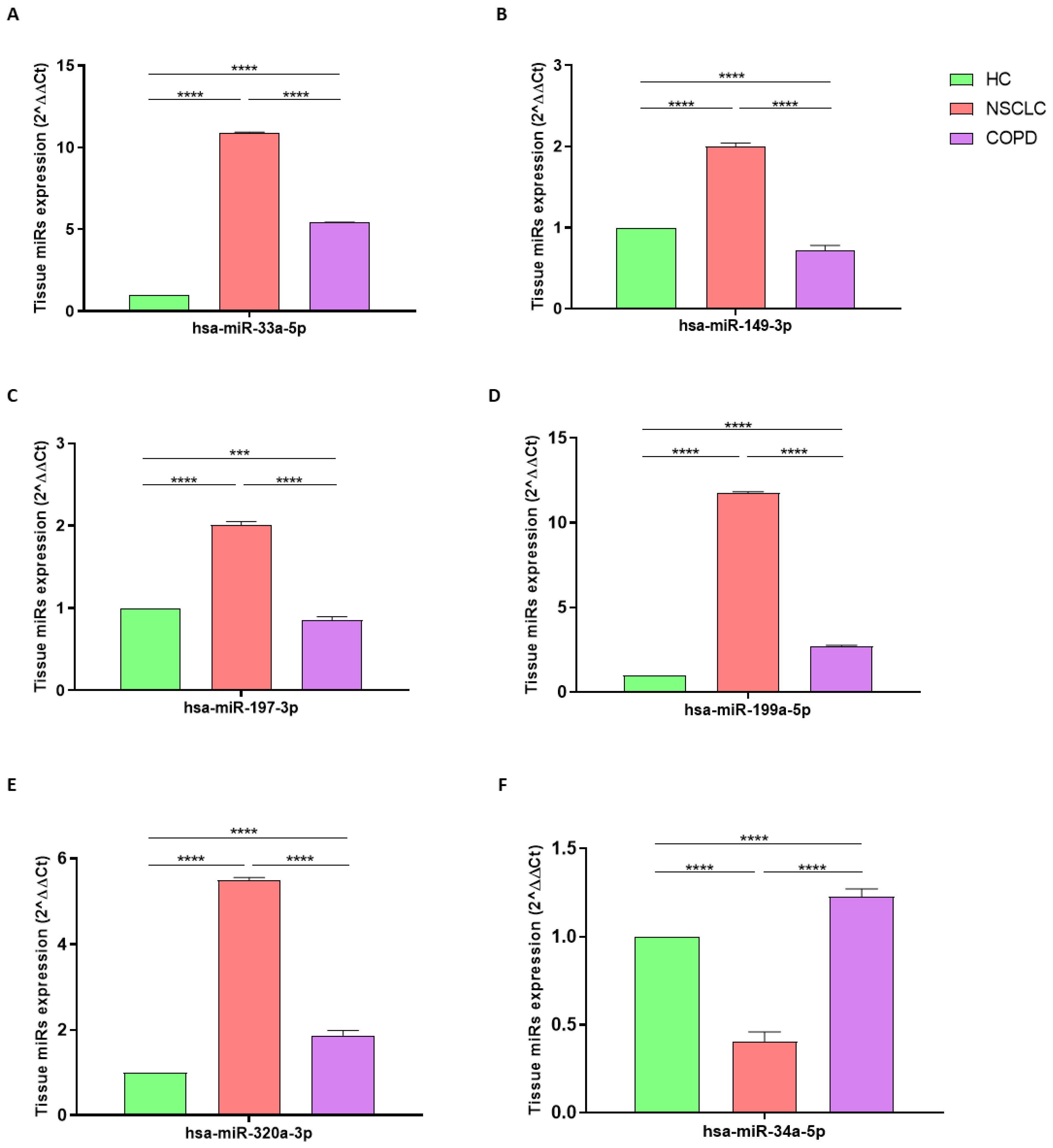
3.2. miRNA Expression in NSCLC
3.3. miRNA Expression in COPD
3.4. miRNA Expression in NSCLC vs. COPD
3.5. In Silico Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer. Global Cancer Observatory: Cancer Today. World Health Organization. Available online: https://gco.iarc.fr/today (accessed on 29 September 2022).
- Thai, A.A.; Solomon, B.J.; Sequist, L.V.; Gainor, J.F.; Heist, R.S. Lung Cancer. Lancet 2021, 398, 535–554. [Google Scholar] [CrossRef]
- Gallelli, L.; Falcone, D.; Scaramuzzino, M.; Pelaia, G.; D’Agostino, B.; Mesuraca, M.; Terracciano, R.; Spaziano, G.; Maselli, R.; Navarra, M.; et al. Effects of Simvastatin on Cell Viability and Proinflammatory Pathways in Lung Adenocarcinoma Cells Exposed to Hydrogen Peroxide. BMC Pharm. Toxicol. 2014, 15, 67. [Google Scholar] [CrossRef] [Green Version]
- Marcianò, G.; Palleria, C.; Casarella, A.; Rania, V.; Basile, E.; Catarisano, L.; Vocca, C.; Bianco, L.; Pelaia, C.; Cione, E.; et al. Effect of Statins on Lung Cancer Molecular Pathways: A Possible Therapeutic Role. Pharmaceuticals 2022, 15, 589. [Google Scholar] [CrossRef]
- Jiang, L.; Sun, Y.-Q.; Langhammer, A.; Brumpton, B.M.; Chen, Y.; Nilsen, T.I.; Leivseth, L.; Wahl, S.G.F.; Mai, X.-M. Asthma and Asthma Symptom Control in Relation to Incidence of Lung Cancer in the HUNT Study. Sci. Rep. 2021, 11, 4539. [Google Scholar] [CrossRef]
- D’Agostino, B.; Orlotti, D.; Calò, G.; Sullo, N.; Russo, M.; Guerrini, R.; De Nardo, M.; Mazzeo, F.; Candeletti, S.; Rossi, F. Nociceptin Modulates Bronchoconstriction Induced by Sensory Nerve Activation in Mouse Lung. Am. J. Respir. Cell Mol. Biol. 2010, 42, 250–254. [Google Scholar] [CrossRef]
- D’Agostino, B.; Marrocco, G.; De Nardo, M.; Calò, G.; Guerrini, R.; Gallelli, L.; Advenier, C.; Rossi, F. Activation of the Nociceptin/Orphanin FQ Receptor Reduces Bronchoconstriction and Microvascular Leakage in a Rabbit Model of Gastroesophageal Reflux: N/OFQ Effects in the Airways in a GER Animal Model. Br. J. Pharmacol. 2005, 144, 813–820. [Google Scholar] [CrossRef]
- Rouget, C.; Cui, Y.Y.; D’Agostino, B.; Faisy, C.; Naline, E.; Bardou, M.; Advenier, C. Nociceptin Inhibits Airway Microvascular Leakage Induced by HCl Intra-Oesophageal Instillation: Nociceptin and Gastro-Oesophageal Reflux. Br. J. Pharmacol. 2004, 141, 1077–1083. [Google Scholar] [CrossRef]
- Gallelli, L.; D’Agostino, B.; Marrocco, G.; De Rosa, G.; Filippelli, W.; Rossi, F.; Advenier, C. Role of Tachykinins in the Bronchoconstriction Induced by HCl Intraesophageal Instillation in the Rabbit. Life Sci. 2003, 72, 1135–1142. [Google Scholar] [CrossRef]
- D’Agostino, B.; Advenier, C.; De Palma, R.; Gallelli, L.; Marrocco, G.; Abbate, G.F.; Rossi, F. The Involvement of Sensory Neuropeptides in Airway Hyper-Responsiveness in Rabbits Sensitized and Challenged to Parietaria Judaica: Sensory Neuropeptides in Airway Hyper-Responsiveness. Clin. Exp. Allergy 2002, 32, 472–479. [Google Scholar] [CrossRef]
- Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2022. Available online: www.goldcopd.org (accessed on 15 October 2022).
- Caramori, G.; Casolari, P.; Barczyk, A.; Durham, A.L.; Di Stefano, A.; Adcock, I. COPD Immunopathology. Semin. Immunopathol. 2016, 38, 497–515. [Google Scholar] [CrossRef] [Green Version]
- Cappetta, D.; De Angelis, A.; Spaziano, G.; Tartaglione, G.; Piegari, E.; Esposito, G.; Ciuffreda, L.P.; Liparulo, A.; Sgambato, M.; Russo, T.P.; et al. Lung Mesenchymal Stem Cells Ameliorate Elastase-Induced Damage in an Animal Model of Emphysema. Stem Cells Int. 2018, 2018, 9492038. [Google Scholar] [CrossRef] [Green Version]
- Houghton, A.M. Mechanistic Links between COPD and Lung Cancer. Nat. Rev. Cancer 2013, 13, 233–245. [Google Scholar] [CrossRef]
- Durham, A.L.; Adcock, I.M. The Relationship between COPD and Lung Cancer. Lung Cancer 2015, 90, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Yang, I.A.; Relan, V.; Wright, C.M.; Davidson, M.R.; Sriram, K.B.; Savarimuthu Francis, S.M.; Clarke, B.E.; Duhig, E.E.; Bowman, R.V.; Fong, K.M. Common Pathogenic Mechanisms and Pathways in the Development of COPD and Lung Cancer. Expert Opin. Ther. Targets 2011, 15, 439–456. [Google Scholar] [CrossRef]
- Chang, J.T.; Anic, G.M.; Rostron, B.L.; Tanwar, M.; Chang, C.M. Cigarette Smoking Reduction and Health Risks: A Systematic Review and Meta-analysis. Nicotine Tob. Res. 2021, 23, 635–642. [Google Scholar] [CrossRef]
- De Cunto, G.; Brancaleone, V.; Riemma, M.A.; Cerqua, I.; Vellecco, V.; Spaziano, G.; Cavarra, E.; Bartalesi, B.; D’Agostino, B.; Lungarella, G.; et al. Functional Contribution of Sphingosine-1-phosphate to Airway Pathology in Cigarette Smoke-exposed Mice. Br. J. Pharm. 2020, 177, 267–281. [Google Scholar] [CrossRef]
- Parris, B.A.; O’Farrell, H.E.; Fong, K.M.; Yang, I.A. Chronic Obstructive Pulmonary Disease (COPD) and Lung Cancer: Common Pathways for Pathogenesis. J. Thorac. Dis. 2019, 11 (Suppl. S17), S2155–S2172. [Google Scholar] [CrossRef]
- Langevin, S.M.; Kratzke, R.A.; Kelsey, K.T. Epigenetics of Lung Cancer. Transl. Res. 2015, 165, 74–90. [Google Scholar] [CrossRef] [Green Version]
- Sundar, I.K.; Rahman, I. Gene Expression Profiling of Epigenetic Chromatin Modification Enzymes and Histone Marks by Cigarette Smoke: Implications for COPD and Lung Cancer. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 311, L1245–L1258. [Google Scholar] [CrossRef] [Green Version]
- de Torres, J.P.; Marín, J.M.; Casanova, C.; Cote, C.; Carrizo, S.; Cordoba-Lanus, E.; Baz-Dávila, R.; Zulueta, J.J.; Aguirre-Jaime, A.; Saetta, M.; et al. Lung Cancer in Patients with Chronic Obstructive Pulmonary Disease: Incidence and Predicting Factors. Am. J. Respir. Crit. Care Med. 2011, 184, 913–919. [Google Scholar] [CrossRef]
- Park, H.Y.; Kang, D.; Shin, S.H.; Yoo, K.-H.; Rhee, C.K.; Suh, G.Y.; Kim, H.; Shim, Y.M.; Guallar, E.; Cho, J.; et al. Chronic Obstructive Pulmonary Disease and Lung Cancer Incidence in Never Smokers: A Cohort Study. Thorax 2020, 75, 506–509. [Google Scholar] [CrossRef] [Green Version]
- Polverino, F.; Mirra, D.; Yang, C.X.; Esposito, R.; Spaziano, G.; Rojas-Quintero, J.; Sgambato, M.; Piegari, E.; Cozzolino, A.; Cione, E.; et al. Similar programmed death ligand 1 (PD-L1) expression profile in patients with mild COPD and lung cancer. Sci. Rep. 2022, 12, 22402. [Google Scholar] [CrossRef]
- Mirra, D.; Cione, E.; Spaziano, G.; Esposito, R.; Sorgenti, M.; Granato, E.; Cerqua, I.; Muraca, L.; Iovino, P.; Gallelli, L.; et al. Circulating MicroRNAs Expression Profile in Lung Inflammation: A Preliminary Study. JCM 2022, 11, 5446. [Google Scholar] [CrossRef]
- Gallelli, L.; Cione, E.; Caroleo, M.C.; Carotenuto, M.; Lagana, P.; Siniscalchi, A.; Guidetti, V. microRNAs to Monitor Pain-migraine and Drug Treatment. Microrna 2017, 6, 152–156. [Google Scholar] [CrossRef]
- Iannone, F.; Montesanto, A.; Cione, E.; Crocco, P.; Caroleo, M.C.; Dato, S.; Rose, G.; Passarino, G. Expression Patterns of Muscle-Specific MiR-133b and MiR-206 Correlate with Nutritional Status and Sarcopenia. Nutrients 2020, 12, 297. [Google Scholar] [CrossRef] [Green Version]
- Perri, M.; Lucente, M.; Cannataro, R.; De Luca, I.F.; Gallelli, L.; Moro, G.; De Sarro, G.; Caroleo, M.C.; Cione, E. Variation in Immune-Related microRNAs Profile in Human Milk Amongst Lactating Women. MicroRNA 2018, 7, 107–114. [Google Scholar] [CrossRef]
- Maltby, S.; Plank, M.; Tay, H.L.; Collison, A.; Foster, P.S. Targeting MicroRNA Function in Respiratory Diseases: Mini-Review. Front. Physiol. 2016, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Bartel, D.P. MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, M.A.; Arora, S.; Prakasam, G.; Calin, G.A.; Syed, M.A. MicroRNA in Lung Cancer: Role, Mechanisms, Pathways and Therapeutic Relevance. Mol. Asp. Med. 2019, 70, 3–20. [Google Scholar] [CrossRef]
- Espín-Pérez, A.; Krauskopf, J.; Chadeau-Hyam, M.; van Veldhoven, K.; Chung, F.; Cullinan, P.; Piepers, J.; van Herwijnen, M.; Kubesch, N.; Carrasco-Turigas, G.; et al. Short-Term Transcriptome and MicroRNAs Responses to Exposure to Different Air Pollutants in Two Population Studies. Environ. Pollut. 2018, 242, 182–190. [Google Scholar] [CrossRef]
- Peng, Y.; Croce, C.M. The Role of MicroRNAs in Human Cancer. Signal Transduct. Target 2016, 1, 15004. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Saikia, J.; Sharawat, S.K.; Malik, P.S.; Kumar, S.; Mohan, A. Analysis of MiR-375-3p, MiR-197-3p, and MiR-15a-5p Expression and Their Clinical Relevance as Biomarkers in Lung Cancer. Technol. Cancer Res. Treat. 2022, 21, 153303382210809. [Google Scholar] [CrossRef]
- Zeng, T.; Xu, M.; Zhang, W.; Gu, X.; Zhao, F.; Liu, X.; Zhang, X. Autophagy Inhibition and MicroRNA-199a-5p Upregulation in Paclitaxel-resistant A549/T Lung Cancer Cells. Oncol. Rep. 2021, 46, 149. [Google Scholar] [CrossRef]
- Vykoukal, J.; Fahrmann, J.F.; Patel, N.; Shimizu, M.; Ostrin, E.J.; Dennison, J.B.; Ivan, C.; Goodman, G.E.; Thornquist, M.D.; Barnett, M.J.; et al. Contributions of Circulating MicroRNAs for Early Detection of Lung Cancer. Cancers 2022, 14, 4221. [Google Scholar] [CrossRef]
- Li, J.; Che, L.; Xu, C.; Lu, D.; Xu, Y.; Liu, M.; Chai, W. XIST/miR-34a-5p/PDL1 axis regulated the development of lung cancer cells and the immune function of CD8+ T cells. Recept. Signal Transduct. Res. 2022, 42, 469–478. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, Y.; Yang, Q.; Liu, B.; Wu, J.; Zhang, Y.; Yang, C.; Jiang, Y. MiR-497 and MiR-34a Retard Lung Cancer Growth by Co-Inhibiting Cyclin E1 (CCNE1). Oncotarget 2015, 6, 13149–13163. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-L.; Liu, X.-M.; Zhang, C.-Y.; Zhou, J.-B.; Shao, Y.; Liang, C.; Wang, H.-M.; Hua, Z.-Y.; Lu, S.-D.; Ma, Z.-L. MicroRNA-34a/EGFR Axis Plays Pivotal Roles in Lung Tumorigenesis. Oncogenesis 2017, 6, e372. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Wang, L.; Bajinka, O.; Wu, G.; Tan, Y. Abnormal Gene Expression Regulation Mechanism of Myeloid Cell Nuclear Differentiation Antigen in Lung Adenocarcinoma. Biology 2022, 11, 1047. [Google Scholar] [CrossRef]
- Yang, T.; Li, H.; Chen, T.; Ren, H.; Shi, P.; Chen, M. LncRNA MALAT1 Depressed Chemo-Sensitivity of NSCLC Cells through Directly Functioning on MiR-197-3p/P120 Catenin Axis. Mol. Cells 2019, 42, 270–283. [Google Scholar] [CrossRef]
- Fathinavid, A.; Ghobadi, M.Z.; Najafi, A.; Masoudi-Nejad, A. Identification of Common MicroRNA between COPD and Non-Small Cell Lung Cancer through Pathway Enrichment Analysis. BMC Genom. Data 2021, 22, 41. [Google Scholar] [CrossRef]
- Sokolowski, J.W., Jr.; Burgher, L.W.; Jones, F.L., Jr.; Patterson, J.R.; Selecky, P.A. Guidelines for fiberoptic bronchoscopy in adults. American Thoracic Society guidelines. Medical Section of the American Lung Association. Am. Rev. Respir. Dis. 1987, 136, 1066. [Google Scholar] [CrossRef]
- Howe, K. Extraction of MiRNAs from Formalin-Fixed Paraffin-Embedded (FFPE) Tissues. In MicroRNA Profiling; Rani, S., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1509, pp. 17–24. [Google Scholar] [CrossRef]
- Perri, M.; Caroleo, M.C.; Liu, N.; Gallelli, L.; De Sarro, G.; Kagechika, H.; Cione, E. 9-cis Retinoic acid modulates myotrophin expression and its miR in physiological and pathophysiological cell models. Exp. Cell Res. 2017, 354, 25–30. [Google Scholar] [CrossRef]
- Gallelli, L.; Cione, E.; Peltrone, F.; Siviglia, S.; Verano, A.; Chirchiglia, D.; Zampogna, S.; Guidetti, V.; Sammartino, L.; Montana, A.; et al. Hsa-miR-34a-5p and hsa-miR-375 as Biomarkers for Monitoring the Effects of Drug Treatment for Migraine Pain in Children and Adolescents: A Pilot Study. J. Clin. Med. 2019, 8, 928. [Google Scholar] [CrossRef] [Green Version]
- Kakimoto, Y.; Tanaka, M.; Kamiguchi, H.; Ochiai, E.; Osawa, M. MicroRNA Stability in FFPE Tissue Samples: Dependence on GC Content. PLoS ONE 2016, 11, e0163125. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, D.; Li, X.; Shao, Y.; He, Y.; Yu, H.; Ma, Z. MiR-199a-5p Suppresses Non-Small Cell Lung Cancer via Targeting MAP3K11. J. Cancer 2019, 10, 2472–2479. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.S.; Qing, H.; Gui, H.; Luo, J.; Dai, L.J.; Wang, B. Role of caprin-1 in carcinogenesis. Oncol. Lett. 2019, 18, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Ye, B.; Wang, P.; Yao, F.; Zhang, C.; Yu, G. Overview of MicroRNA-199a Regulation in Cancer. CMAR 2019, 11, 10327–10335. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Patel, D.; Jia, Y.; Yu, Z.; Liu, X.; Shi, H.; Liu, H. MARCH8 Suppresses Tumor Metastasis and Mediates Degradation of STAT3 and CD44 in Breast Cancer Cells. Cancers 2021, 13, 2550. [Google Scholar] [CrossRef]
- Fan, J.; Tian, L.; Li, M.; Huang, S.-H.; Zhang, J.; Zhao, B. MARCH8 Is Associated with Poor Prognosis in Non-Small Cell Lung Cancers Patients. Oncotarget 2017, 8, 108238–108248. [Google Scholar] [CrossRef] [Green Version]
- Mizuno, S.; Bogaard, H.J.; Gomez-Arroyo, J.; Alhussaini, A.; Kraskauskas, D.; Cool, C.D.; Voelkel, N.F. MicroRNA-199a-5p Is Associated With Hypoxia-Inducible Factor-1α Expression in Lungs From Patients With COPD. Chest 2012, 142, 663–672. [Google Scholar] [CrossRef] [Green Version]
- Roberts, T.L.; Idris, A.; Dunn, J.A.; Kelly, G.M.; Burnton, C.M.; Hodgson, S.; Hardy, L.L.; Garceau, V.; Sweet, M.J.; Ross, I.L.; et al. HIN-200 Proteins Regulate Caspase Activation in Response to Foreign Cytoplasmic DNA. Science 2009, 323, 1057–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-M.; Chen, S.-W.; Chen, G.; Zhou, H.-F.; Gan, T.-Q.; Zeng, J.-J.; Li, Z.-Y. Clinical Value and Potential Mechanism of MiRNA-33a-5p in Lung Squamous Cell Carcinoma. Anal. Cell. Pathol. 2021, 2021, 6614331. [Google Scholar] [CrossRef]
- Lai, L.; Azzam, K.M.; Lin, W.-C.; Rai, P.; Lowe, J.M.; Gabor, K.A.; Madenspacher, J.H.; Aloor, J.J.; Parks, J.S.; Näär, A.M.; et al. MicroRNA-33 Regulates the Innate Immune Response via ATP Binding Cassette Transporter-Mediated Remodeling of Membrane. Microdomains. J. Biol. Chem. 2016, 291, 19651–19660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, S.; Zhou, G.; Hu, W.; Pei, H. SMAD-6, -7 and -9 Are Potential Molecular Biomarkers for the Prognosis in Human Lung Cancer. Oncol. Lett. 2020, 20, 2633–2644. [Google Scholar] [CrossRef]
- Tong, L.; Shen, S.; Huang, Q.; Fu, J.; Wang, T.; Pan, L.; Zhang, P.; Chen, G.; Huang, T.; Li, K.; et al. Proteasome-Dependent Degradation of Smad7 Is Critical for Lung Cancer Metastasis. Cell Death Differ. 2020, 27, 1795–1806. [Google Scholar] [CrossRef]
- Lu, X.; Yin, B.; Wang, X.; Wang, F.; Li, Y.; Wang, N.; Yang, X.; Jiang, W. Long Non-coding RNA-ZNF281 Upregulates PTEN Expression via Downregulation of MicroRNA-221 in Non-small Cell Lung Cancer. Oncol. Lett. 2020, 20, 2962–2968. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Fortunato, O.; Borzi, C.; Milione, M.; Centonze, G.; Conte, D.; Boeri, M.; Verri, C.; Moro, M.; Facchinetti, F.; Andriani, F.; et al. Circulating Mir-320a Promotes Immunosuppressive Macrophages M2 Phenotype Associated with Lung Cancer Risk. Int. J. Cancer 2019, 144, 2746–2761. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.-Y.; Zhang, X.-P.; Wu, J.-H.; Qiu, X.-S.; Wang, E.-H. Expression of LATS1 Contributes to Good Prognosis and Can Negatively Regulate YAP Oncoprotein in Non-Small-Cell Lung Cancer. Tumor Biol. 2014, 35, 6435–6443. [Google Scholar] [CrossRef]
- Xie, Y.; Lv, Y.; Zhang, Y.; Liang, Z.; Han, L.; Xie, Y. LATS2 Promotes Apoptosis in Non-Small Cell Lung Cancer A549 Cells via Triggering Mff-Dependent Mitochondrial Fission and Activating the JNK Signaling Pathway. Biomed. Pharmacother. 2019, 109, 679–689. [Google Scholar] [CrossRef]
- Matamala, N.; Lara, B.; Gómez-Mariano, G.; Martínez, S.; Vázquez-Domínguez, I.; Otero-Sobrino, Á.; Muñoz-Callejas, A.; Sánchez, E.; Esquinas, C.; Bustamante, A.; et al. MiR-320c Regulates SERPINA1 Expression and Is Induced in Patients With Pulmonary Disease. Arch. Bronconeumol. 2021, 57, 457–463. [Google Scholar] [CrossRef]
- Muth, M.; Theophile, K.; Hussein, K.; Jacobi, C.; Kreipe, H.; Bock, O. Hypoxia-Induced down-Regulation of MicroRNA-449a/b Impairs Control over Targeted SERPINE1 (PAI-1) MRNA—A Mechanism Involved in SERPINE1 (PAI-1) Overexpression. J. Transl. Med. 2010, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wang, H.; Wang, Z.; Xiao, W. Plasminogen activator inhibitor-1 promotes inflammatory process induced by cigarette smoke extraction or lipopolysaccharides in alveolar epithelial cells. Exp. Lung Res. 2009, 35, 795–805. [Google Scholar] [CrossRef]
- To, M.; Takagi, D.; Akashi, K.; Kano, I.; Haruki, K.; Barnes, P.J.; Ito, K. Sputum Plasminogen Activator Inhibitor-1 Elevation by Oxidative Stress-Dependent Nuclear Factor-ΚB Activation in COPD. Chest 2013, 144, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Savarimuthu Francis, S.M.; Davidson, M.R.; Tan, M.E.; Wright, C.M.; Clarke, B.E.; Duhig, E.E.; Bowman, R.V.; Hayward, N.K.; Fong, K.M.; Yang, I.A. MicroRNA-34c Is Associated with Emphysema Severity and Modulates SERPINE1 Expression. BMC Genom. 2014, 15, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.; Liu, J.; Zhao, G.; Fan, M.; Song, G.; Zhang, Y.; Weng, Z.; Zhang, Y. Repression of Toll-like Receptor-4 by MicroRNA-149-3p Is Associated with Smoking-Related COPD. COPD 2017, 12, 705–715. [Google Scholar] [CrossRef] [Green Version]
- Tian, W.; Yang, H.; Zhou, B. Integrative Analysis of Exosomal MicroRNA-149-5p in Lung Adenocarcinoma. Aging 2021, 13, 7382–7396. [Google Scholar] [CrossRef]
- He, S.; Ma, X.; Ye, Y.; Zhang, M.; Zhuang, J.; Song, Y.; Xia, W. HEATR1 modulates cell survival in non-small cell lung cancer via activation of the p53/PUMA signaling pathway. OncoTargets Ther. 2019, 12, 4001–4011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, L.H.; Spieker, T.; Koschmieder, S.; Humberg, J.; Jungen, D.; Bulk, E.; Hascher, A.; Wittmer, D.; Marra, A.; Hillejan, L.; et al. The Long Noncoding MALAT-1 RNA Indicates a Poor Prognosis in Non-Small Cell Lung Cancer and Induces Migration and Tumor Growth. J. Thorac. Oncol. 2011, 6, 1984–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Q.; Wang, H.; Xu, S. MARCH9 Suppresses Lung Adenocarcinoma Progression by Downregulating ICAM-1. Cell Physiol. Biochem. 2018, 50, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Peinado, V.I.; Ramírez, J.; Melgosa, T.; Roca, J.; Rodriguez-Roisin, R.; Barberà, J.A. Characterization of Pulmonary Vascular Remodelling in Smokers and Patients with Mild COPD. Eur. Respir. J. 2002, 19, 632–638. [Google Scholar] [CrossRef] [Green Version]
- Musri, M.M.; Coll-Bonfill, N.; Maron, B.A.; Peinado, V.I.; Wang, R.-S.; Altirriba, J.; Blanco, I.; Oldham, W.M.; Tura-Ceide, O.; García-Lucio, J.; et al. MicroRNA Dysregulation in Pulmonary Arteries from Chronic Obstructive Pulmonary Disease. Relationships with Vascular Remodeling. Am. J. Respir. Cell Mol. Biol. 2018, 59, 490–499. [Google Scholar] [CrossRef]
- Wang, L.; Yu, J.; Xu, J.; Zheng, C.; Li, X.; Du, J. The Analysis of MicroRNA-34 Family Expression in Human Cancer Studies Comparing Cancer Tissues with Corresponding Pericarcinous Tissues. Gene 2015, 554, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Slabáková, E.; Culig, Z.; Remšík, J.; Souček, K. Alternative Mechanisms of MiR-34a Regulation in Cancer. Cell Death Dis. 2017, 8, e3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortez, M.A.; Ivan, C.; Valdecanas, D.; Wang, X.; Peltier, H.J.; Ye, Y.; Araujo, L.; Carbone, D.P.; Shilo, K.; Giri, D.K.; et al. PDL1 Regulation by P53 via MiR-34. JNCI J. Natl. Cancer Inst. 2016, 108, djv303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Silveira, D.A.; Barbé-Tuana, F.M.; Mombach, J.C.M. Integrative data modeling from lung and lymphatic cancer predicts functional roles for miR-34a and miR-16 in cell fate regulation. Sci. Rep. 2020, 10, 2511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, J.R.; Vuppusetty, C.; Colley, T.; Papaioannou, A.I.; Fenwick, P.; Donnelly, L.; Ito, K.; Barnes, P.J. Oxidative Stress Dependent MicroRNA-34a Activation via PI3Kα Reduces the Expression of Sirtuin-1 and Sirtuin-6 in Epithelial Cells. Sci. Rep. 2016, 6, 35871. [Google Scholar] [CrossRef] [Green Version]
- Swaffer, M.P.; Jones, A.W.; Flynn, H.R.; Snijders, A.P.; Nurse, P. CDK substrate phosphorylation and ordering the cell cycle. Cell 2016, 167, 1750–1761.E16. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Wang, L.; Zheng, Z.; Chen, W.; Du, P.; Zhao, H. Cyclin-Dependent Kinase 6 (CDK6) Is a Candidate Diagnostic Biomarker for Early Non-Small Cell Lung Cancer. Transl. Cancer Res. TCR 2020, 9, 95–103. [Google Scholar] [CrossRef]
- Reiss, K.; Ludwig, A.; Saftig, P. Breaking up the Tie: Disintegrin-like Metalloproteinases as Regulators of Cell Migration in Inflammation and Invasion. Pharmacol. Ther. 2006, 111, 985–1006. [Google Scholar] [CrossRef]
- Guo, J.; He, L.; Yuan, P.; Wang, P.; Lu, Y.; Tong, F.; Wang, Y.; Yin, Y.; Tian, J.; Sun, J. ADAM10 Overexpression in Human Non-Small Cell Lung Cancer Correlates with Cell Migration and Invasion through the Activation of the Notch1 Signaling Pathway. Oncol. Rep. 2012, 28, 1709–1718. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Shi, W.; Dou, B.; Wang, J.; Peng, W.; Liu, X.; Zhao, D.; Tang, F.; Wu, Y.; Li, X.; et al. XBP1- IGFBP3 Signaling Pathway Promotes NSCLC Invasion and Metastasis. Front. Oncol. 2021, 11, 654995. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wan, S.; Li, J.; Xu, Y.; Lou, X.; Sun, M.; Wang, S. Expression and Prognostic Value of E2F3 Transcription Factor in Non-small Cell Lung Cancer. Oncol. Lett. 2021, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Willinger, C.M.; Rong, J.; Tanriverdi, K.; Courchesne, P.L.; Huan, T.; Wasserman, G.A.; Lin, H.; Dupuis, J.; Joehanes, R.; Jones, M.R.; et al. MicroRNA Signature of Cigarette Smoking and Evidence for a Putative Causal Role of MicroRNAs in Smoking-Related Inflammation and Target Organ Damage. Circ. Cardiovasc. Genet. 2017, 10, e001678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, S.T.; Kurti, A.N.; Redner, R.; White, T.J.; Gaalema, D.E.; Roberts, M.E.; Doogan, N.J.; Tidey, J.W.; Miller, M.E.; Stanton, C.A.; et al. A literature review on prevalence of gender differences and intersections with other vulnerabilities to tobacco use in the United States, 2004–2014. Prev. Med. 2015, 80, 89–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Healthy Control (HC) | NSCLC | COPD | p-Value | |
|---|---|---|---|---|
| Total Participants (N) | 6 | 9 | 6 | |
| Age (SD) | 70.3 (7.27) | 76.7 (3.82) | 65.0 (11.31) | 0.0001 |
| Gender (M/F) | 5/1 | 7/2 | 3/3 | 0.0097 |
| Smoking history (pack years) | 5.0 | 21.5 | 17 | <0.0001 |
| Current smoker | 1 | 3 | 4 | 0.0481 |
| Former smoker | 2 | 5 | 0 | <0.0001 |
| FEV1 % predicted (SD) | 91% (16) | 94% (19) | 67% (16) | <00001 |
| FEV/FVC (SD) | 88 (5) | 78 (4) | 62 (3) | <0.0001 |
| Comorbidities | ||||
| Arterial Hypertension (%) | 3 (50.0%) | 4 (44.4%) | 2 (33.3%) | 0.0001 |
| Other cardiovascular diseases (%) | 1 (16.6%) | 4 (44.4%) | 1 (16.6%) | <0.0001 |
| Diabetes Mellitus (%) | 2 (33.3%) | 5 (55.5%) | 1 (16.6%) | <0.0001 |
| Medications | ||||
| Inhaled corticosteroids (N, %) | 0 | 1 (11.11%) | 1 (16.6%) | <0.001 |
| LABA/SABA/LAMA (N, %) | 0 | 0 (0 | 3 (50%) | <0.0001 |
| Number of Target Genes | ||
|---|---|---|
| miRNA | miR Target Link Human | DIANA Tools |
| hsa-miR-33a-5p | 191 | 713 |
| hsa-miR-149-3p | 1274 | 1469 |
| hsa-miR-197-3p | 559 | 1004 |
| hsa-miR-199a-5p | 280 | 666 |
| hsa-miR-320a-3p | 841 | 1946 |
| hsa-miR-34a-5p | 615 | 1108 |
| Abbreviation | Gene Name | Methods | Tissue |
|---|---|---|---|
| ADAM10 | A disintegrin and metalloproteinase 10 | Bi | Intestine |
| XBP1 | X-Box Binding Protein 1 | Bi, IP, MA | Intestine, Bone Marrow, Kidney |
| ZNF281 | Zinc Finger Protein 281 | IP | Kidney, Bone Marrow |
| CDK6 | Cyclin-Dependent Kinase 6 | Bi, MA, IP, qP, RA, O, WB | Intestine, Bone Marrow, Kidney, Liver |
| LATS1 | Large Tumor Suppressor Kinase 1 | IP | Pancreas, Cervix |
| LATS2 | Large Tumor Suppressor Kinase 2 | IP | Cervix |
| MARCH8 | Membrane Associated Ring-CH-Type Finger 8 | IP | Pancreas |
| MARCH9 | Membrane Associated Ring-CH-Type Finger 9 | IP | Mammary Gland |
| SMAD7 | SMAD Family Member 7 | IP | Cervix |
| E2F3 | E2F Transcription Factor 3 | Bi, MA, IP, QP, WB | Kidney, Embryo, Cervix, Intestine, Bone Marrow |
| CAPRIN 1 | Cytoplasmic activation/proliferation-associated protein-1 | IP | Pancreas |
| HEATR1 | HEAT repeat-containing protein 1 | IP | Bone Marrow |
| Biochemical Pathways | ||
|---|---|---|
| miRNA | Validated Target Genes | |
| NOTCH1 pathway; cell migration and invasion | hsa-miR-34a-5p | ADAM10 |
| XBP1 | ||
| NF-kappa B pathway; inhibition of cell apoptosis and promotion of cell proliferation; poor overall survival | hsa-miR-33a-5p hsa-miR-320a-3p | ZNF281 |
| hsa-miR-149-3p | HEATR1 | |
| Cell cycle deregulation and uncontrolled cell proliferation | hsa-miR-34a-5p hsa-miR-199a-5p | CDK6 CAPRIN1 |
| JNK, GPCR, and Hippo pathways; loss of tumor suppressor capability | hsa-miR-320a-3p | LATS1 LATS2 |
| PI3K, mTOR, and ICAM-1 signaling pathways; poor clinical outcomes | hsa-miR-199a-5p | MARCH8 |
| hsa-miR-197-3p | MARCH9 | |
| TGFβ signaling pathway; promotion of epithelial–mesenchymal transitions and metastasis | hsa-miR-33a-5p | SMAD7 |
| Bcl-2 signaling pathway; uncontrolled tumor growth | hsa-miR-34a-5p | E2F3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirra, D.; Esposito, R.; Spaziano, G.; La Torre, C.; Vocca, C.; Tallarico, M.; Cione, E.; Gallelli, L.; D’Agostino, B. Lung microRNAs Expression in Lung Cancer and COPD: A Preliminary Study. Biomedicines 2023, 11, 736. https://doi.org/10.3390/biomedicines11030736
Mirra D, Esposito R, Spaziano G, La Torre C, Vocca C, Tallarico M, Cione E, Gallelli L, D’Agostino B. Lung microRNAs Expression in Lung Cancer and COPD: A Preliminary Study. Biomedicines. 2023; 11(3):736. https://doi.org/10.3390/biomedicines11030736
Chicago/Turabian StyleMirra, Davida, Renata Esposito, Giuseppe Spaziano, Chiara La Torre, Cristina Vocca, Martina Tallarico, Erika Cione, Luca Gallelli, and Bruno D’Agostino. 2023. "Lung microRNAs Expression in Lung Cancer and COPD: A Preliminary Study" Biomedicines 11, no. 3: 736. https://doi.org/10.3390/biomedicines11030736
APA StyleMirra, D., Esposito, R., Spaziano, G., La Torre, C., Vocca, C., Tallarico, M., Cione, E., Gallelli, L., & D’Agostino, B. (2023). Lung microRNAs Expression in Lung Cancer and COPD: A Preliminary Study. Biomedicines, 11(3), 736. https://doi.org/10.3390/biomedicines11030736








