Long-Term Tamoxifen Effects in the Cyclic Interaction of the Endocannabinoid and Endocrine System in the Rat Central Nervous System
Abstract
1. Introduction
2. Materials and Methods
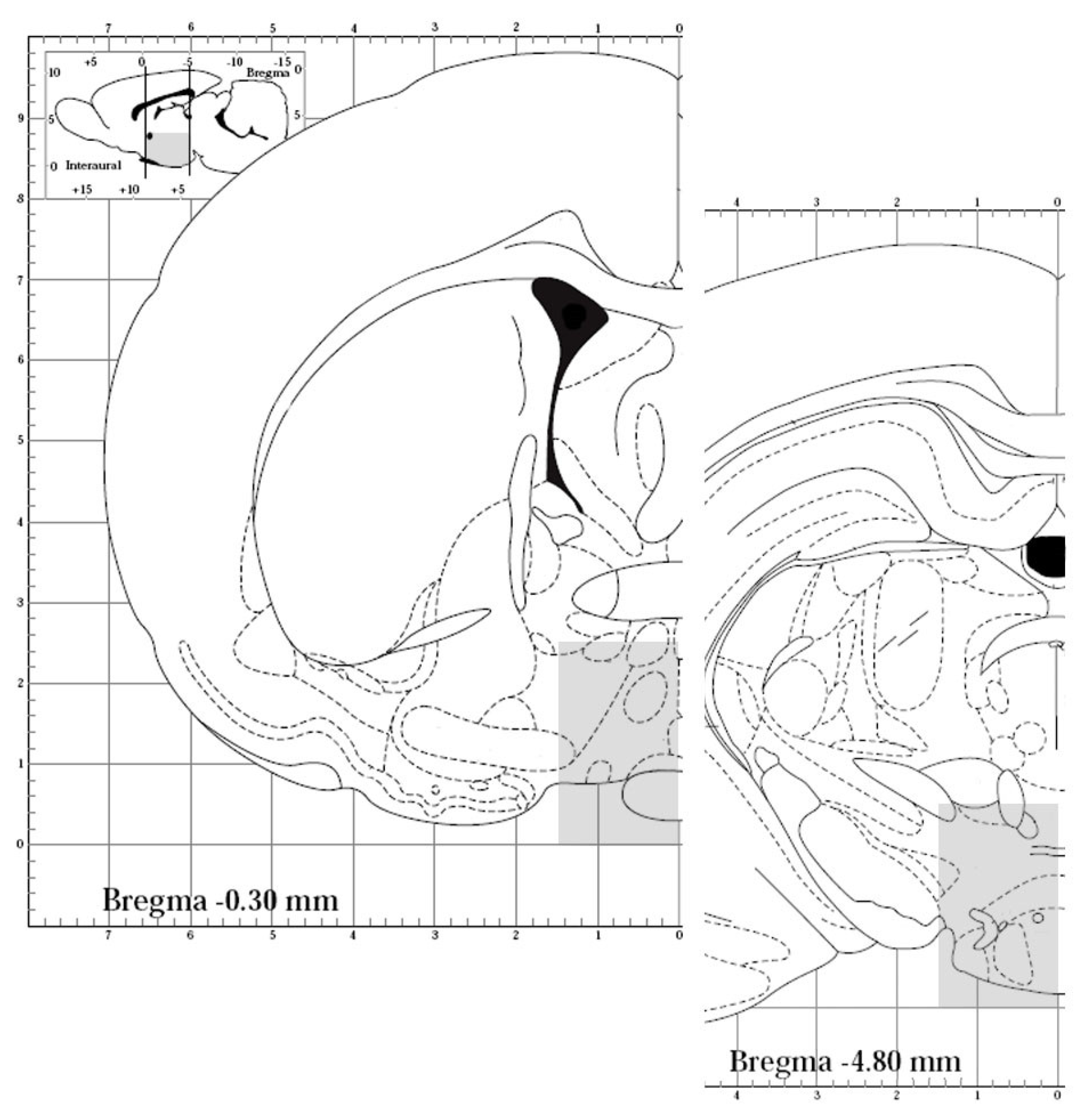
2.1. Animal Care and Use
2.2. Tissue Processing
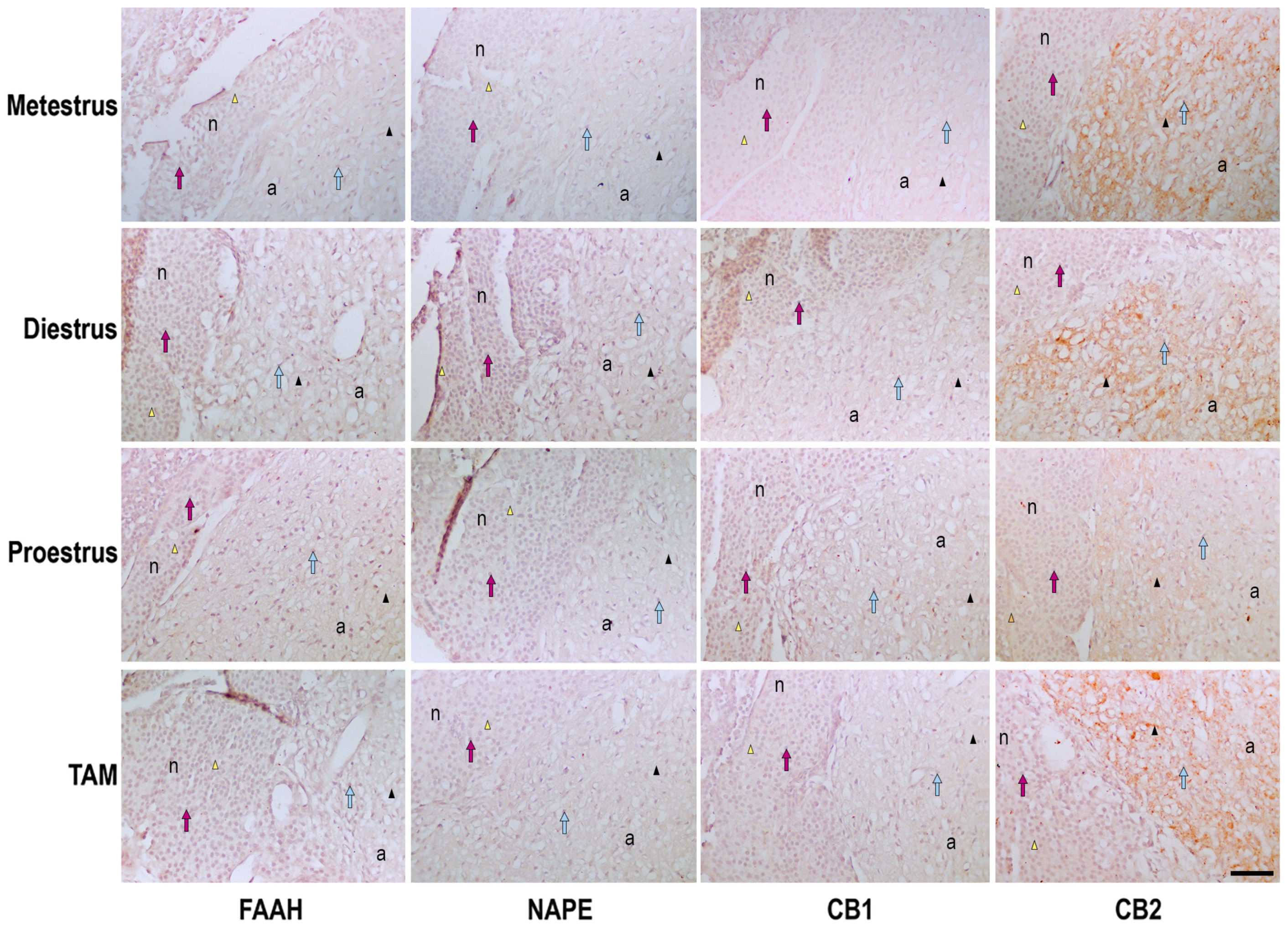
2.3. Immunohistochemistry Processing and Qualitative Analysis of Pituitary
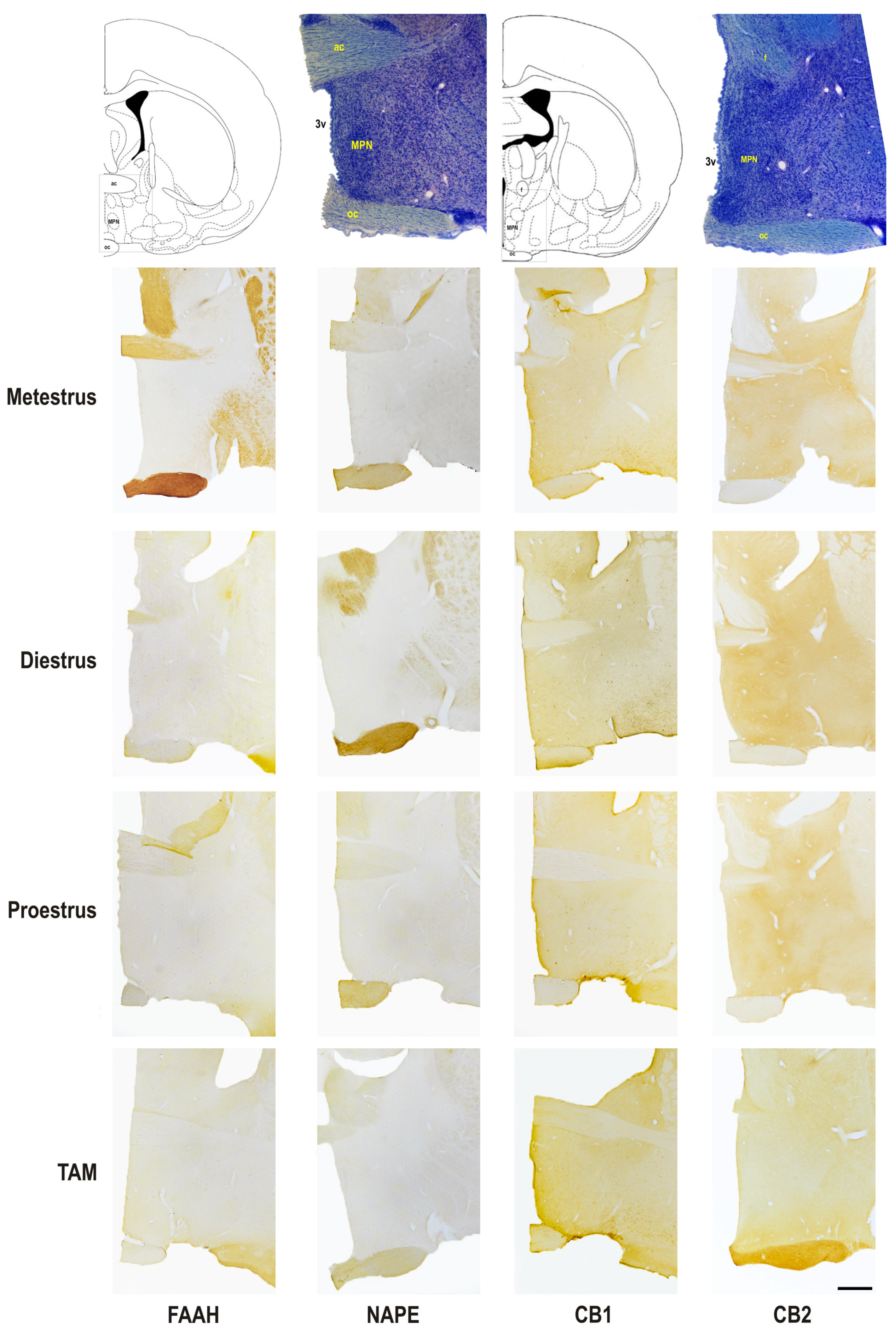
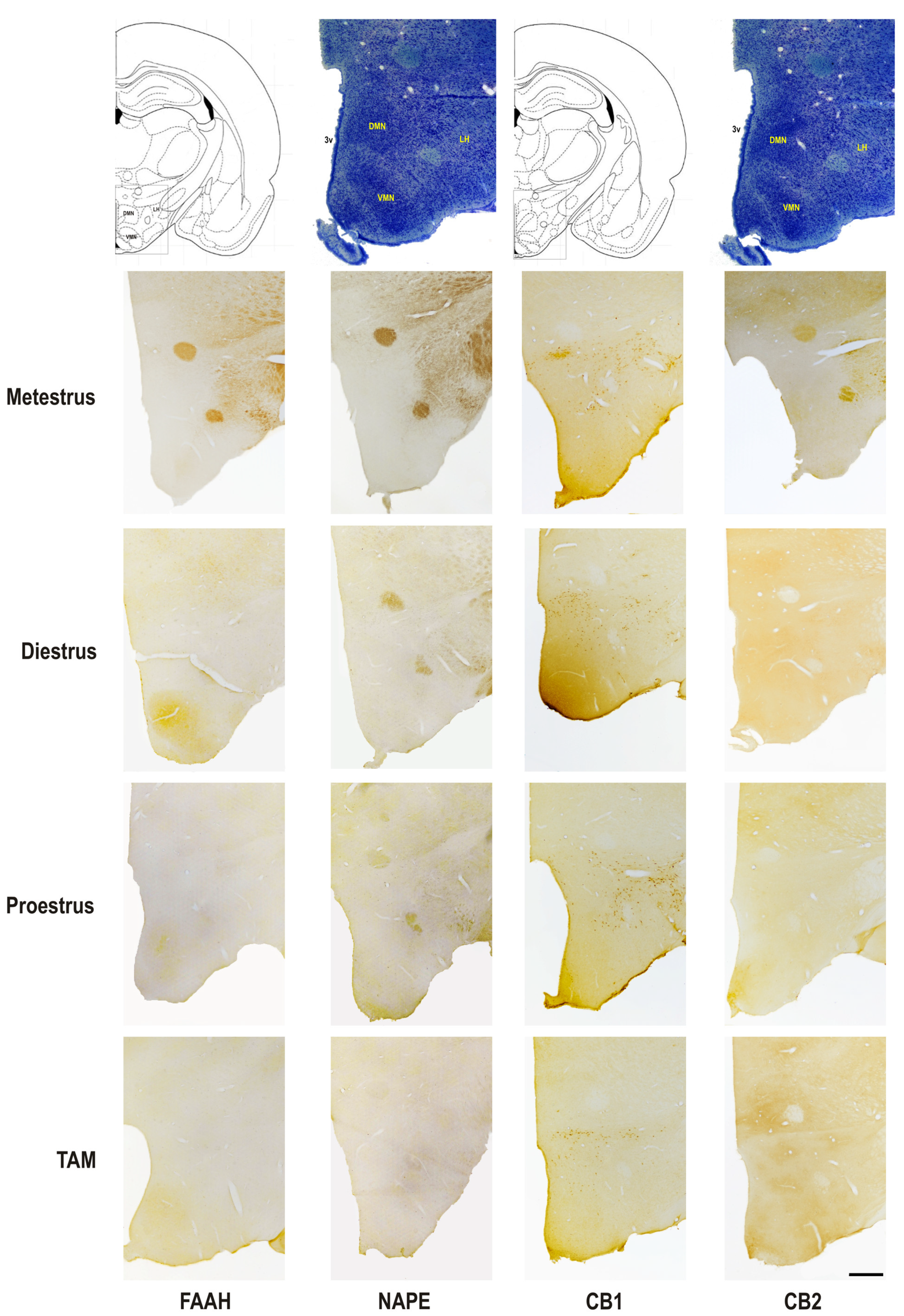
2.4. Immunohistochemistry Processing and Qualitative Analysis of Hypothalamus
2.5. Western Blotting
2.6. UPLC-MS/MS Method for AEA Quantification
2.7. Statistical Analysis
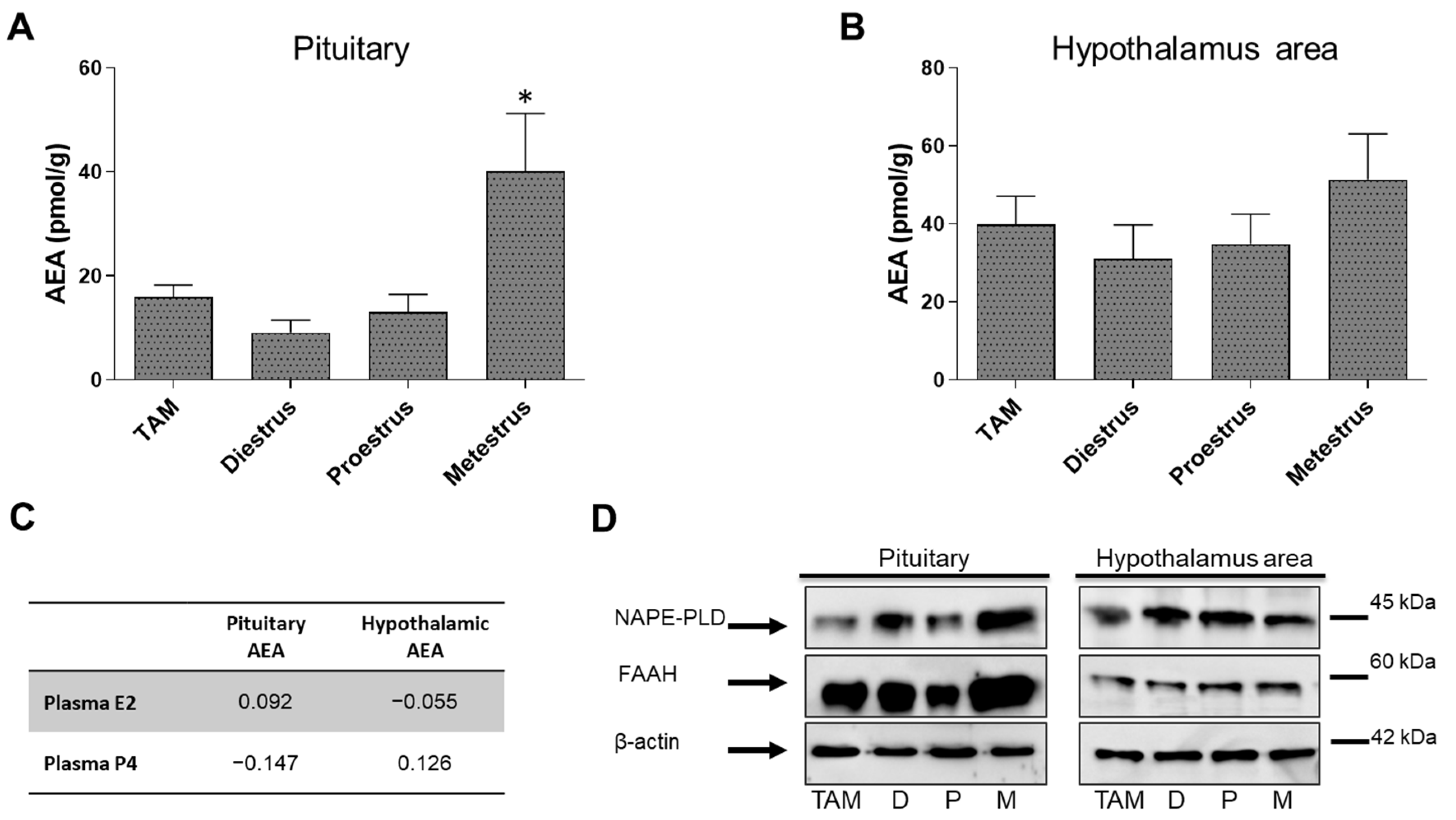
3. Results
3.1. Fluctuations of AEA Levels and AEA-Metabolic Enzymes on Pituitary and POA-Hyp
3.2. Semi-Quantitative Analysis of Protein Expression in the Pituitary
3.3. Semi-Quantitative Analysis of Protein Expression in the Pre-Optic Area and Hypothalamus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toyoda, H. CB1 cannabinoid receptor-mediated plasticity of GABAergic synapses in the mouse insular cortex. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Pacheco-Colón, I.; Limia, J.M.; Gonzalez, R. Nonacute effects of cannabis use on motivation and reward sensitivity in humans: A systematic review. Psychol. Addict. Behav. 2018, 32, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Akirav, I. The Role of Cannabinoids in Modulating Emotional and Non-Emotional Memory Processes in the Hippocampus. Front. Behav. Neurosci. 2011, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Prenderville, J.A.; Kelly, Á.M.; Downer, E.J. The role of cannabinoids in adult neurogenesis. Br. J. Pharmacol. 2015, 172, 3950–3963. [Google Scholar] [CrossRef] [PubMed]
- Giuffrida, A.; Leweke, F.M.; Gerth, C.W.; Schreiber, D.; Koethe, D.; Faulhaber, J.; Klosterkötter, J.; Piomelli, D. Cerebrospinal Anandamide Levels are Elevated in Acute Schizophrenia and are Inversely Correlated with Psychotic Symptoms. Neuropsychopharmacology 2004, 29, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Romero-Sanchiz, P.; Nogueira-Arjona, R.; Pastor, A.; Araos, P.; Serrano, A.; Boronat, A.; Garcia-Marchena, N.; Mayoral-Cleries, F.; Bordallo, A.; Alen, F.; et al. Plasma concentrations of oleoylethanolamide in a primary care sample of depressed patients are increased in those treated with selective serotonin reuptake inhibitor-type antidepressants. Neuropharmacology 2019, 149, 212–220. [Google Scholar] [CrossRef]
- Koethe, D.; Pahlisch, F.; Hellmich, M.; Rohleder, C.; Mueller, J.K.; Meyer-Lindenberg, A.; Torrey, E.F.; Piomelli, D.; Leweke, F.M. Familial abnormalities of endocannabinoid signaling in schizophrenia. World J. Biol. Psychiatry 2018, 20, 117–125. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Costa, M.; Almada, M.; Correia-Da-Silva, G.; Teixeira, N. Endogenous cannabinoids revisited: A biochemistry perspective. Prostaglandins Other Lipid Mediat. 2013, 102–103, 13–30. [Google Scholar] [CrossRef]
- Muccioli, G.G. Endocannabinoid biosynthesis and inactivation, from simple to complex. Drug Discov. Today 2010, 15, 474–483. [Google Scholar] [CrossRef]
- Maia, J.; Almada, M.; Silva, A.; Correia-Da-Silva, G.; Teixeira, N.; Sá, S.; Fonseca, B.M. The endocannabinoid system expression in the female reproductive tract is modulated by estrogen. J. Steroid Biochem. Mol. Biol. 2017, 174, 40–47. [Google Scholar] [CrossRef]
- Wenger, T.; Gerendai, I.; Fezza, F.; González, S.; Bisogno, T.; Fernandez-Ruiz, J.; Di Marzo, V. The hypothalamic levels of the endocannabinoid, anandamide, peak immediately before the onset of puberty in female rats. Life Sci. 2002, 70, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Goparaju, S.K.; Wang, L.; Liu, J.; Bátkai, S.; Járai, Z.; Fezza, F.; Miura, G.I.; Palmiter, R.D.; Sugiura, T.; et al. Leptin-regulated endocannabinoids are involved in maintaining food intake. Nature 2001, 410, 822–825. [Google Scholar] [CrossRef] [PubMed]
- Scorticati, C.; Fernández-Solari, J.; De Laurentiis, A.; Mohn, C.; Prestifilippo, J.P.; Lasaga, M.; Seilicovich, A.; Billi, S.; Franchi, A.; McCann, S.M.; et al. The inhibitory effect of anandamide on luteinizing hormone-releasing hormone secretion is reversed by estrogen. Proc. Natl. Acad. Sci. USA 2004, 101, 11891–11896. [Google Scholar] [CrossRef]
- El-Talatini, M.R.; Taylor, A.H.; Konje, J.C. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil. Steril. 2010, 93, 1989–1996. [Google Scholar] [CrossRef]
- Scorticati, C.; Mohn, C.; De Laurentiis, A.; Vissio, P.; Fernández Solari, J.; Seilicovich, A.; McCann, S.M.; Rettori, V. The effect of anandamide on prolactin secretion is modulated by estrogen. Proc. Natl. Acad. Sci. USA 2003, 100, 2134–2139. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Temin, S.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Rowden, D.; Solky, A.J.; et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor–Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2014, 32, 2255–2269. [Google Scholar] [CrossRef]
- Komm, B.S.; Mirkin, S. An overview of current and emerging SERMs. J. Steroid Biochem. Mol. Biol. 2014, 143, 207–222. [Google Scholar] [CrossRef]
- Ribnikar, D.; Sousa, B.; Cufer, T.; Cardoso, F. Extended adjuvant endocrine therapy—A standard to all or some? Breast 2017, 32, 112–118. [Google Scholar] [CrossRef]
- Rosenberg, S.M.; Stanton, A.L.; Petrie, K.J.; Partridge, A.H. Symptoms and Symptom Attribution Among Women on Endocrine Therapy for Breast Cancer. Oncologist 2015, 20, 598–604. [Google Scholar] [CrossRef]
- Moon, Z.; Moss-Morris, R.; Hunter, M.S.; Hughes, L.D. Measuring illness representations in breast cancer survivors (BCS) prescribed tamoxifen: Modification and validation of the Revised Illness Perceptions Questionnaire (IPQ-BCS). Psychol. Health 2017, 32, 439–458. [Google Scholar] [CrossRef]
- Chen, X.; Li, J.; Chen, J.; Li, D.; Ye, R.; Zhang, J.; Zhu, C.; Tian, Y.; Wang, K. Decision-making impairments in breast cancer patients treated with tamoxifen. Horm. Behav. 2014, 66, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Holsboer, F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J. Psychiatr. Res. 1999, 33, 181–214. [Google Scholar] [CrossRef] [PubMed]
- Maric, N.; Adzic, M. Pharmacological modulation of HPA axis in depression - new avenues for potential therapeutic benefits. Psychiatr. Danub. 2013, 25, 299–305. [Google Scholar] [PubMed]
- Stoffel, E.; Craft, R. Ovarian hormone withdrawal-induced “depression” in female rats. Physiol. Behav. 2004, 83, 505–513. [Google Scholar] [CrossRef]
- Sá, S.I.; Fonseca, B.M.; Teixeira, N.; Madeira, M.D. Induction and subcellular redistribution of progesterone receptor A and B by tamoxifen in the hypothalamic ventromedial neurons of young adult female Wistar rats. Mol. Cell. Endocrinol. 2016, 420, 1–10. [Google Scholar] [CrossRef]
- Resende, A.D.; Leal, S.; Batista-Pinto, C.; Garcez, F.; Sá, S.I. Hepatic effects of long-term tamoxifen administration to cycling female rats. J. Biochem. Mol. Toxicol. 2019, 33, e22293. [Google Scholar] [CrossRef]
- Leal, S.; Rocha, L.; Silva, A.; Faria, J.; Dinis-Oliveira, R.J.; Sá, S.I. Evaluation of progressive hepatic histopathology in long-term tamoxifen therapy. Pathol. Res. Pr. 2018, 214, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- Pinto, C.A.; Fonseca, B.M.; Sá, S.I. Effects of chronic tamoxifen treatment in female rat sexual behaviour. Heliyon 2022, 8, e12362. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Madeira, M.; Sá, S. Effects of gonadal steroids and of estrogen receptor agonists on the expression of estrogen receptor alpha in the medial preoptic nucleus of female rats. Neuroscience 2015, 310, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.; Madeira, M.D.; Sá, S.I. Effects of sex steroids and estrogen receptor agonists on the expression of estrogen receptor alpha in the principal division of the bed nucleus of the stria terminalis of female rats. Brain Res. 2014, 1582, 99–106. [Google Scholar] [CrossRef]
- Chen, D.J.; Gao, M.; Gao, F.F.; Su, Q.X.; Wu, J. Brain cannabinoid receptor 2: Expression, function and modulation. Acta Pharmacol. Sin. 2017, 38, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, A.H.; Shelton, C.C.; Advani, T.; Cravatt, B.F. Mice lacking fatty acid amide hydrolase exhibit a cannabinoid receptor-mediated phenotypic hypoalgesia. Pain 2004, 109, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Kathuria, S.; Gaetani, S.; Fegley, D.; Valiño, F.; Duranti, A.; Tontini, A.; Mor, M.; Tarzia, G.; La Rana, G.; Calignano, A.; et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003, 9, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Waleh, N.S.; Cravatt, B.F.; Apte-Deshpande, A.; Terao, A.; Kilduff, T.S. Transcriptional regulation of the mouse fatty acid amide hydrolase gene. Gene 2002, 291, 203–210. [Google Scholar] [CrossRef]
- Wenger, T.; Moldrich, G. The role of endocannabinoids in the hypothalamic regulation of visceral function. Prostaglandins Leukot Essent Fatty Acids 2002, 66, 301–307. [Google Scholar] [CrossRef]
- Smith, M.S.; Freeman, M.E.; Neill, J.D. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: Prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology 1975, 96, 219–226. [Google Scholar] [CrossRef]
- Micevych, P.; Sinchak, K. Estradiol regulation of progesterone synthesis in the brain. Mol. Cell Endocrinol. 2008, 290, 44–50. [Google Scholar] [CrossRef]
- Micevych, P.; Soma, K.K.; Sinchak, K. Neuroprogesterone: Key to estrogen positive feedback? Brain Res. Rev. 2008, 57, 470–480. [Google Scholar] [CrossRef]
- Mani, S.K.; Blaustein, J.D. Neural progestin receptors and female sexual behavior. Neuroendocrinology 2012, 96, 152–161. [Google Scholar] [CrossRef]
- Becker, J.B. Sex differences in addiction. Dialogues Clin. Neurosci. 2016, 18, 395–402. [Google Scholar] [CrossRef]
- Ikemoto, S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci. Biobehav. Rev. 2010, 35, 129–150. [Google Scholar] [CrossRef]
- Parsons, L.H.; Hurd, Y.L. Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 2015, 16, 579–594. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S.; Woolley, C.S. Estradiol and progesterone regulate neuronal structure and synaptic connectivity in adult as well as developing brain. Exp. Gerontol. 1994, 29, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Prange-Kiel, J.; Fester, L.; Zhou, L.; Jarry, H.; Rune, G.M. Estrus cyclicity of spinogenesis: Underlying mechanisms. J. Neural Transm. 2009, 116, 1417–1425. [Google Scholar] [CrossRef] [PubMed]
- Gorzalka, B.B.; Dang, S.S. Minireview: Endocannabinoids and gonadal hormones: Bidirectional interactions in physiology and behavior. Endocrinology 2012, 153, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.N.; Karacabeyli, E.S.; Gorzalka, B.B. Estrogen recruits the endocannabinoid system to modulate emotionality. Psychoneuroendocrinology 2007, 32, 350–357. [Google Scholar] [CrossRef]
- López, H.H.; Zappia, K.; Cushman, C.L.; Chadwick, B. Acute cannabinoid administration attenuates female socio-sexual motivation. Pharmacol. Biochem. Behav. 2010, 94, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Micale, V.; Drago, F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018, 834, 230–239. [Google Scholar] [CrossRef]
| Antibody Name | Antibody ID (RRID) | Host | Catalog Number | Used Dilution (Brain) | Used Dilution (Pituitary) | Used Dilution (WB) |
|---|---|---|---|---|---|---|
| CB1 (N-15) | AB_637711 | Goat | sc-10066 | 1:1500 | 1:200 | 1:200 |
| CB2 (H-60) | AB_2082784 | Rabbit | sc-25494 | 1:1000 | 1:200 | 1:200 |
| FAAH (V-17) | AB_2231531 | Goat | sc-26427 | 1:1000 | 1:100 | 1:200 |
| NAPE-PLD (G-13) | AB_10841204 | Goat | sc-163117 | 1:1000 | 1:100 | 1:200 |
| β-actin (C4) | AB_2714189 | Mouse | sc-47778 | -- | -- | 1:500 |
| Anti-Goat IgG (H+L) | AB_2336126 | Rabbit | BA-5000 | 1:400 | 1:150 | 1:2000 |
| Anti-Rabbit IgG | AB_2313606 | Goat | BA-1000 | 1:400 | 1:150 | 1:2000 |
| Neurohypophysis | Adenohypophysis | ||
|---|---|---|---|
| FAAH | Metestrus | ++ | ++ |
| Diestrus | +++ | +++ | |
| Proestrus | ++ | ++ | |
| TAM | ++ | ++ | |
| NAPE | Metestrus | + | + |
| Diestrus | ++ | ++ | |
| Proestrus | + | +/− | |
| TAM | +/− | + | |
| CB1 | Metestrus | + | + |
| Diestrus | +/− | +/− | |
| Proestrus | ++ | ++ | |
| TAM | + | + | |
| CB2 | Metestrus | + | +++ |
| Diestrus | +/− | +++ | |
| Proestrus | + | ++ | |
| TAM | + | +++ | |
| MPN | DMN | VMN | LH | ||
|---|---|---|---|---|---|
| FAAH | Metestrus | +/− | +/− | ++ | + |
| Diestrus | + | +/− | ++ | +/− | |
| Proestrus | + | +/− | + | +/− | |
| TAM | ++ | +/− | ++ | +/− | |
| NAPE | Metestrus | + | + | + | + |
| Diestrus | +/− | +/− | +/− | +/− | |
| Proestrus | +/− | +/− | + | + | |
| TAM | +/− | + | +/− | +/− | |
| CB1 | Metestrus | ++ | + | + | ++ |
| Diestrus | + | + | ++ | ++ | |
| Proestrus | ++ | + | + | + | |
| TAM | ++ | ++ | + | +++ | |
| CB2 | Metestrus | ++ | + | + | ++ |
| Diestrus | ++ | ++ | ++ | + | |
| Proestrus | + | + | ++ | ++ | |
| TAM | ++ | +/− | +/− | +/− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, B.M.; Bhowmick, N.; Cunha, S.; Maia, J.; Correia-da-Silva, G.; Teixeira, N.; Sá, S.I. Long-Term Tamoxifen Effects in the Cyclic Interaction of the Endocannabinoid and Endocrine System in the Rat Central Nervous System. Biomedicines 2023, 11, 720. https://doi.org/10.3390/biomedicines11030720
Fonseca BM, Bhowmick N, Cunha S, Maia J, Correia-da-Silva G, Teixeira N, Sá SI. Long-Term Tamoxifen Effects in the Cyclic Interaction of the Endocannabinoid and Endocrine System in the Rat Central Nervous System. Biomedicines. 2023; 11(3):720. https://doi.org/10.3390/biomedicines11030720
Chicago/Turabian StyleFonseca, Bruno M., Niloy Bhowmick, Sara Cunha, João Maia, Georgina Correia-da-Silva, Natércia Teixeira, and Susana I. Sá. 2023. "Long-Term Tamoxifen Effects in the Cyclic Interaction of the Endocannabinoid and Endocrine System in the Rat Central Nervous System" Biomedicines 11, no. 3: 720. https://doi.org/10.3390/biomedicines11030720
APA StyleFonseca, B. M., Bhowmick, N., Cunha, S., Maia, J., Correia-da-Silva, G., Teixeira, N., & Sá, S. I. (2023). Long-Term Tamoxifen Effects in the Cyclic Interaction of the Endocannabinoid and Endocrine System in the Rat Central Nervous System. Biomedicines, 11(3), 720. https://doi.org/10.3390/biomedicines11030720






