2,4-Dichlorophenoxyacetic Acid Induces Degeneration of mDA Neurons In Vitro
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
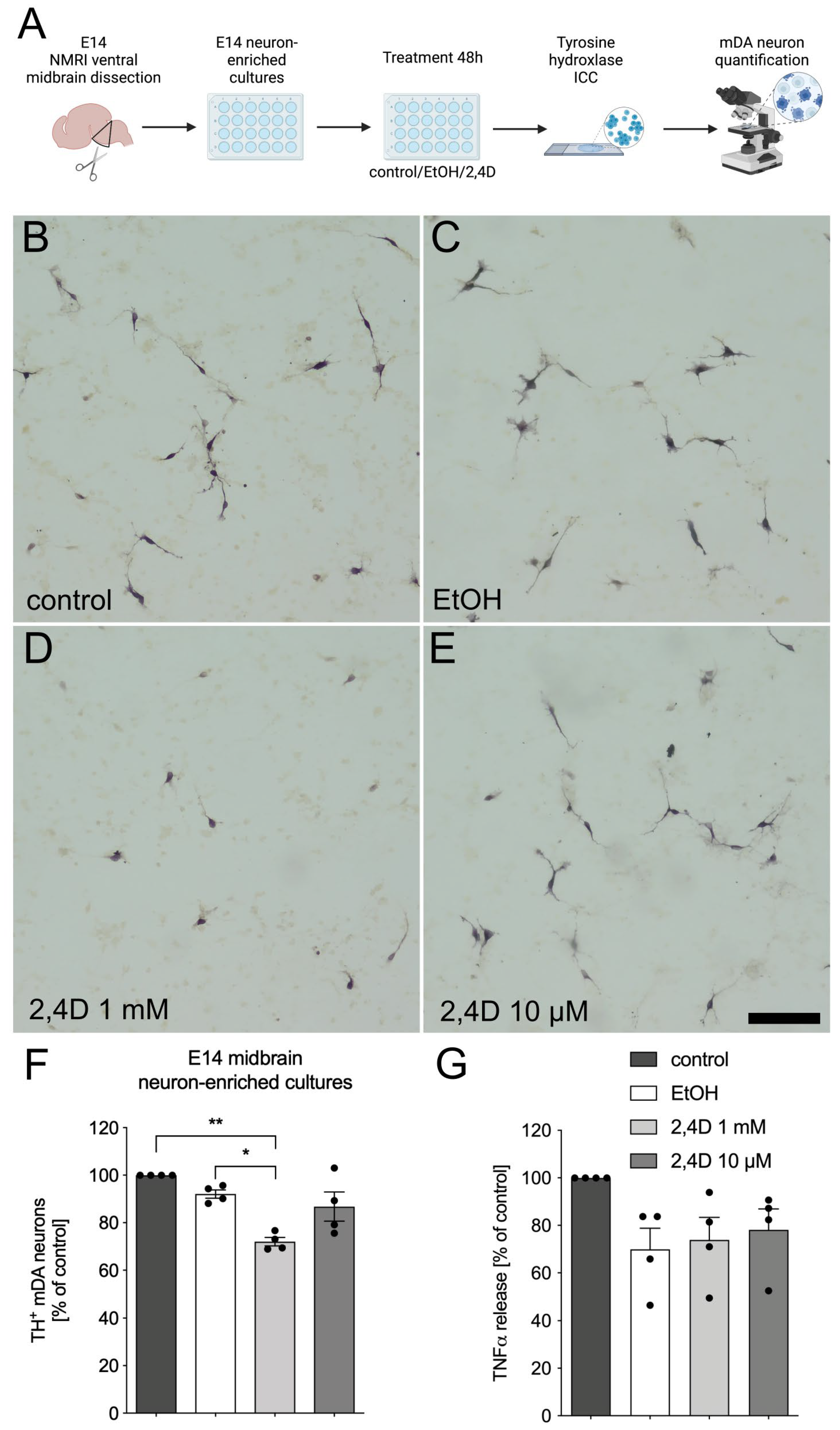
2.2. E14 Ventral Midbrain Dopaminergic Neuron-Enriched Cultures
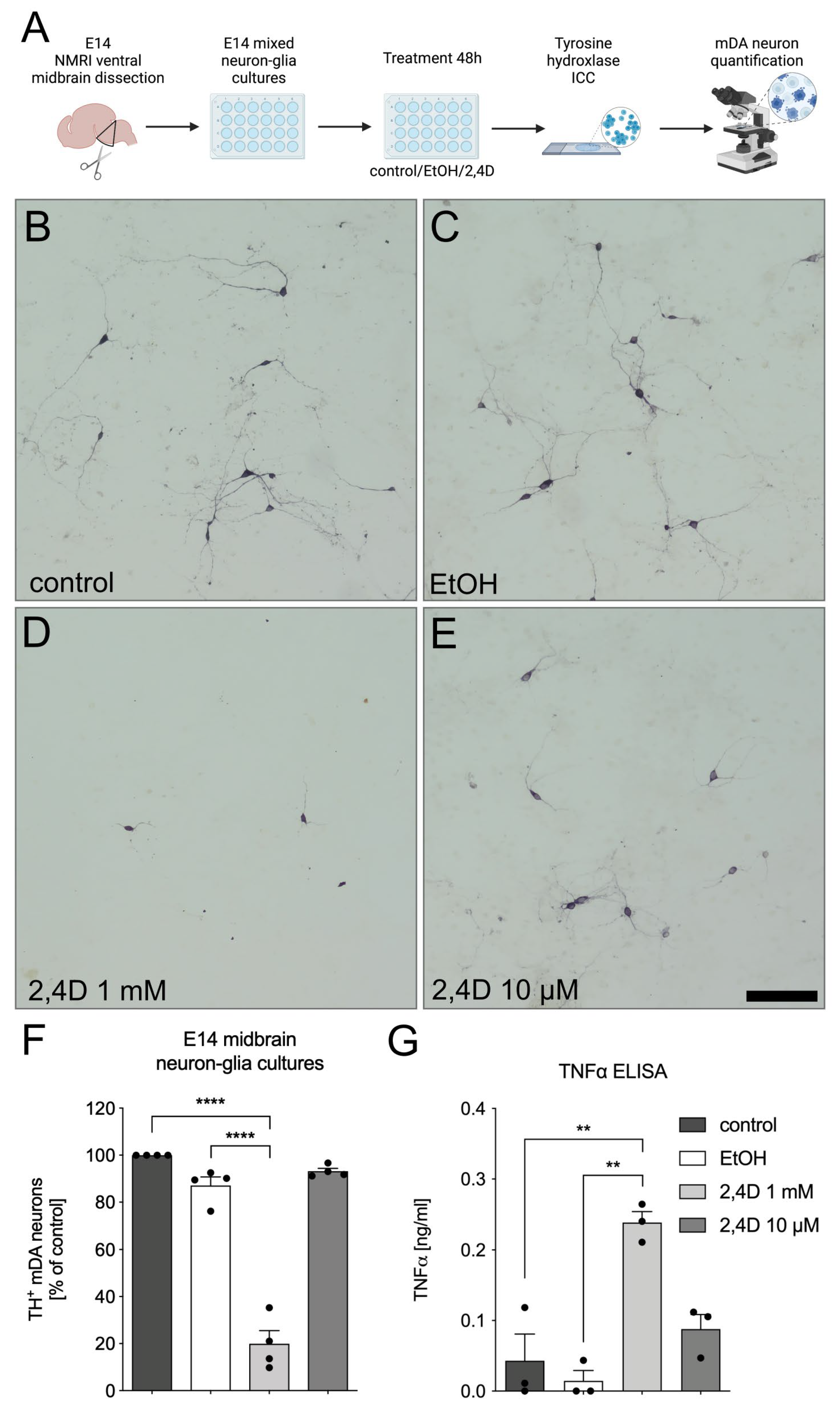
2.3. E14 Ventral Midbrain Dopaminergic Neuron-Glia Cultures
2.4. Primary Microglia Culture
2.5. Cytokines and Reagents
2.6. Immunocytochemistry
2.7. MTT Assay
2.8. TNFα and IL6 ELISA
2.9. Statistics
3. Results
3.1. Treatment with 2,4D Reduces Number of TH+ Dopaminergic Neurons in E14 Midbrain Neuron-Enriched Cultures
3.2. Increased Neurotoxicity of 2,4D in E14 Midbrain Neuron-Glia Cultures
3.3. Microglia Reactivity Is Not Directly Triggered by 2,4D Application
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jellinger, K.A. The Pathology of Parkinson’s Disease. Adv. Neurol. 2001, 86, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, R.; Basak, I.; Patil, K.S.; Alves, G.; Larsen, J.P.; Møller, S.G. Parkinson’s Disease and Age: The Obvious but Largely Unexplored Link. Exp. Gerontol. 2015, 68, 33–38. [Google Scholar] [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D.L. The Prevalence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Cherian, A.; Divya, K.P.; Vijayaraghavan, A. Parkinson’s Disease—Genetic Cause. Curr. Opin. Neurol. 2023, 36, 292. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s Disease: A Target for Neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-Mediated Neurotoxicity: Uncovering the Molecular Mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.; Zöller, T.; Attaai, A.; Spittau, B. Microglia-Mediated Neuroinflammation and Neurotrophic Factor-Induced Protection in the MPTP Mouse Model of Parkinson’s Disease-Lessons from Transgenic Mice. Int. J. Mol. Sci. 2016, 17, 151. [Google Scholar] [CrossRef]
- Tanner, C.M.; Ross, G.W.; Jewell, S.A.; Hauser, R.A.; Jankovic, J.; Factor, S.A.; Bressman, S.; Deligtisch, A.; Marras, C.; Lyons, K.E.; et al. Occupation and Risk of Parkinsonism: A Multicenter Case-Control Study. Arch. Neurol. 2009, 66, 1106–1113. [Google Scholar] [CrossRef]
- Berkley, M.C.; Magee, K.R. Neuropathy Following Exposure to a Dimethylamine Salt of 2, 4-D. Arch. Intern. Med. 1963, 111, 351–352. [Google Scholar] [CrossRef]
- Mori de Moro, G.; Duffard, R.; Evangelista de Duffard, A.M. Neurotoxicity of 2,4-Dichlorophenoxyacetic Butyl Ester in Chick Embryos. Neurochem. Res. 1993, 18, 353–359. [Google Scholar] [CrossRef]
- Duffard, R.O.; Mori de Moro, G.B.; Evangelista de Duffard, A.M. Vulnerability of Myelin Development of the Chick to the Herbicide 2,4-Dichlorophenoxyacetic Butyl Ester. Neurochem. Res. 1987, 12, 1077–1080. [Google Scholar] [CrossRef]
- De Moliner, K.L.; Evangelista de Duffard, A.M.; Soto, E.; Duffard, R.; Adamo, A.M. Induction of Apoptosis in Cerebellar Granule Cells by 2,4-Dichlorophenoxyacetic Acid. Neurochem. Res. 2002, 27, 1439–1446. [Google Scholar] [CrossRef]
- Bongiovanni, B.; Ferri, A.; Brusco, A.; Rassetto, M.; Lopez, L.M.; Evangelista de Duffard, A.M.; Duffard, R. Adverse Effects of 2,4-Dichlorophenoxyacetic Acid on Rat Cerebellar Granule Cell Cultures Were Attenuated by Amphetamine. Neurotox. Res. 2011, 19, 544–555. [Google Scholar] [CrossRef]
- Rosso, S.B.; Cáceres, A.O.; de Duffard, A.M.; Duffard, R.O.; Quiroga, S. 2,4-Dichlorophenoxyacetic Acid Disrupts the Cytoskeleton and Disorganizes the Golgi Apparatus of Cultured Neurons. Toxicol. Sci. 2000, 56, 133–140. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bortolozzi, A.; Evangelista de Duffard, A.M.; Dajas, F.; Duffard, R.; Silveira, R. Intracerebral Administration of 2,4-Diclorophenoxyacetic Acid Induces Behavioral and Neurochemical Alterations in the Rat Brain. Neurotoxicology 2001, 22, 221–232. [Google Scholar] [CrossRef]
- Oliveira, G.H.; Palermo-Neto, J. Effects of 2,4-Dichlorophenoxyacetic Acid (2,4-D) on Open-Field Behaviour and Neurochemical Parameters of Rats. Pharmacol. Toxicol. 1993, 73, 79–85. [Google Scholar] [CrossRef]
- Bortolozzi, A.; Duffard, R.O.; Rubio, M.; Sturtz, N.; Evangelista de Duffard, A.M. Regionally Specific Changes in Central Brain Monoamine Levels by 2,4-Dichlorophenoxyacetic Acid in Acute Treated Rats. Neurotoxicology 1998, 19, 839–851. [Google Scholar]
- Spittau, B.; Zhou, X.; Ming, M.; Krieglstein, K. IL6 Protects MN9D Cells and Midbrain Dopaminergic Neurons from MPP+-Induced Neurodegeneration. Neuromolecular Med. 2012, 14, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zöller, T.; Krieglstein, K.; Spittau, B. TGFβ1 Inhibits IFNγ-Mediated Microglia Activation and Protects mDA Neurons from IFNγ-Driven Neurotoxicity. J. Neurochem. 2015, 134, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Spittau, B.; Wullkopf, L.; Zhou, X.; Rilka, J.; Pfeifer, D.; Krieglstein, K. Endogenous Transforming Growth Factor-Beta Promotes Quiescence of Primary Microglia in Vitro. Glia 2013, 61, 287–300. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.C. Heavy Metal Intensification of DAB-Based HRP Reaction Product. J. Histochem. Cytochem. 1981, 29, 775. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Zhou, X.; Spittau, B. Lipopolysaccharide-Induced Microglia Activation Promotes the Survival of Midbrain Dopaminergic Neurons In Vitro. Neurotox. Res. 2018, 33, 856–867. [Google Scholar] [CrossRef]
- Nimmervoll, B.; White, R.; Yang, J.-W.; An, S.; Henn, C.; Sun, J.-J.; Luhmann, H.J. LPS-Induced Microglial Secretion of TNFα Increases Activity-Dependent Neuronal Apoptosis in the Neonatal Cerebral Cortex. Cerebral Cortex 2013, 23, 1742–1755. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Schneble-Löhnert, N.; Lajqi, T.; Wetzker, R.; Müller, J.P.; Bauer, R. PI3Kγ Mediates Microglial Proliferation and Cell Viability via ROS. Cells 2021, 10, 2534. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Keizer, R.F.; Pritchard, J.B. 2,4-Dichlorophenoxyacetic Acid Intoxication Increases Its Accumulation within the Brain. Brain Res. 1988, 440, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Bortolozzi, A.; Duffard, R.; de Duffard, A.M.E. Asymmetrical Development of the Monoamine Systems in 2,4-Dichlorophenoxyacetic Acid Treated Rats. Neurotoxicology 2003, 24, 149–157. [Google Scholar] [CrossRef]
- Hühner, L.; Rilka, J.; Gilsbach, R.; Zhou, X.; Machado, V.; Spittau, B. Interleukin-4 Protects Dopaminergic Neurons In Vitro but Is Dispensable for MPTP-Induced Neurodegeneration In Vivo. Front. Mol. Neurosci. 2017, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Machado, V.; Haas, S.J.-P.; von Bohlen Und Halbach, O.; Wree, A.; Krieglstein, K.; Unsicker, K.; Spittau, B. Growth/Differentiation Factor-15 Deficiency Compromises Dopaminergic Neuron Survival and Microglial Response in the 6-Hydroxydopamine Mouse Model of Parkinson’s Disease. Neurobiol. Dis. 2016, 88, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, A.; Krieglstein, K.; Spittau, B. Distribution of Microglia in the Postnatal Murine Nigrostriatal System. Cell Tissue Res. 2013, 351, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Spittau, B. Aging Microglia-Phenotypes, Functions and Implications for Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 194. [Google Scholar] [CrossRef]
- Holtman, I.R.; Raj, D.D.; Miller, J.A.; Schaafsma, W.; Yin, Z.; Brouwer, N.; Wes, P.D.; Möller, T.; Orre, M.; Kamphuis, W.; et al. Induction of a Common Microglia Gene Expression Signature by Aging and Neurodegenerative Conditions: A Co-Expression Meta-Analysis. Acta Neuropathol. Commun. 2015, 3, 31. [Google Scholar] [CrossRef]
- Block, M.L.; Hong, J.-S. Microglia and Inflammation-Mediated Neurodegeneration: Multiple Triggers with a Common Mechanism. Prog. Neurobiol. 2005, 76, 77–98. [Google Scholar] [CrossRef]
- Wilms, H.; Zecca, L.; Rosenstiel, P.; Sievers, J.; Deuschl, G.; Lucius, R. Inflammation in Parkinson’s Diseases and Other Neurodegenerative Diseases: Cause and Therapeutic Implications. Curr. Pharm. Des. 2007, 13, 1925–1928. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.L.; James-Kracke, M.; Sun, G.Y.; Sun, A.Y. Oxidative and Inflammatory Pathways in Parkinson’s Disease. Neurochem. Res. 2009, 34, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Timmer, M.; Cesnulevicius, K.; Hitti, E.; Kotlyarov, A.; Gaestel, M. MAPKAP Kinase 2-Deficiency Prevents Neurons from Cell Death by Reducing Neuroinflammation--Relevance in a Mouse Model of Parkinson’s Disease. J. Neurochem. 2008, 105, 2039–2052. [Google Scholar] [CrossRef] [PubMed]
- Mangano, E.N.; Hayley, S. Inflammatory Priming of the Substantia Nigra Influences the Impact of Later Paraquat Exposure: Neuroimmune Sensitization of Neurodegeneration. Neurobiol. Aging 2009, 30, 1361–1378. [Google Scholar] [CrossRef]
- Gao, H.-M.; Liu, B.; Hong, J.-S. Critical Role for Microglial NADPH Oxidase in Rotenone-Induced Degeneration of Dopaminergic Neurons. J. Neurosci. 2003, 23, 6181–6187. [Google Scholar] [CrossRef]
- Gao, H.-M.; Kotzbauer, P.T.; Uryu, K.; Leight, S.; Trojanowski, J.Q.; Lee, V.M.-Y. Neuroinflammation and Oxidation/Nitration of Alpha-Synuclein Linked to Dopaminergic Neurodegeneration. J. Neurosci. 2008, 28, 7687–7698. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, F.; Jia, L.; Chai, R.; Wang, H.; Wang, X.; Li, J.; Wang, K.; Zhang, P.; Yang, H. 2,4-Dichlorophenoxyacetic Acid Induces ROS Activation in NLRP3 Inflammatory Body-Induced Autophagy Disorder in Microglia and the Protective Effect of Lycium Barbarum Polysaccharide. Environ. Toxicol. 2022, 37, 1136–1151. [Google Scholar] [CrossRef] [PubMed]
- Butovsky, O.; Jedrychowski, M.P.; Moore, C.S.; Cialic, R.; Lanser, A.J.; Gabriely, G.; Koeglsperger, T.; Dake, B.; Wu, P.M.; Doykan, C.E.; et al. Identification of a Unique TGF-β-Dependent Molecular and Functional Signature in Microglia. Nat. Neurosci. 2014, 17, 131–143. [Google Scholar] [CrossRef]
- Ferger, B.; Leng, A.; Mura, A.; Hengerer, B.; Feldon, J. Genetic Ablation of Tumor Necrosis Factor-Alpha (TNF-Alpha) and Pharmacological Inhibition of TNF-Synthesis Attenuates MPTP Toxicity in Mouse Striatum. J. Neurochem. 2004, 89, 822–833. [Google Scholar] [CrossRef]
- Sriram, K.; Matheson, J.M.; Benkovic, S.A.; Miller, D.B.; Luster, M.I.; O’Callaghan, J.P. Deficiency of TNF Receptors Suppresses Microglial Activation and Alters the Susceptibility of Brain Regions to MPTP-Induced Neurotoxicity: Role of TNF-Alpha. FASEB J. 2006, 20, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Sriram, K.; Matheson, J.M.; Benkovic, S.A.; Miller, D.B.; Luster, M.I.; O’Callaghan, J.P. Mice Deficient in TNF Receptors Are Protected against Dopaminergic Neurotoxicity: Implications for Parkinson’s Disease. FASEB J. 2002, 16, 1474–1476. [Google Scholar] [CrossRef] [PubMed]
- McCoy, M.K.; Martinez, T.N.; Ruhn, K.A.; Szymkowski, D.E.; Smith, C.G.; Botterman, B.R.; Tansey, K.E.; Tansey, M.G. Blocking Soluble Tumor Necrosis Factor Signaling with Dominant-Negative Tumor Necrosis Factor Inhibitor Attenuates Loss of Dopaminergic Neurons in Models of Parkinson’s Disease. J. Neurosci. 2006, 26, 9365–9375. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.Y.; Benveniste, E.N. Tumor Necrosis Factor-Alpha Production by Astrocytes. Induction by Lipopolysaccharide, IFN-Gamma, and IL-1 Beta. J. Immunol. 1990, 144, 2999–3007. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russ, T.; Enders, L.; Zbiegly, J.M.; Potru, P.S.; Wurm, J.; Spittau, B. 2,4-Dichlorophenoxyacetic Acid Induces Degeneration of mDA Neurons In Vitro. Biomedicines 2023, 11, 2882. https://doi.org/10.3390/biomedicines11112882
Russ T, Enders L, Zbiegly JM, Potru PS, Wurm J, Spittau B. 2,4-Dichlorophenoxyacetic Acid Induces Degeneration of mDA Neurons In Vitro. Biomedicines. 2023; 11(11):2882. https://doi.org/10.3390/biomedicines11112882
Chicago/Turabian StyleRuss, Tamara, Lennart Enders, Julia M. Zbiegly, Phani Sankar Potru, Johannes Wurm, and Björn Spittau. 2023. "2,4-Dichlorophenoxyacetic Acid Induces Degeneration of mDA Neurons In Vitro" Biomedicines 11, no. 11: 2882. https://doi.org/10.3390/biomedicines11112882
APA StyleRuss, T., Enders, L., Zbiegly, J. M., Potru, P. S., Wurm, J., & Spittau, B. (2023). 2,4-Dichlorophenoxyacetic Acid Induces Degeneration of mDA Neurons In Vitro. Biomedicines, 11(11), 2882. https://doi.org/10.3390/biomedicines11112882




