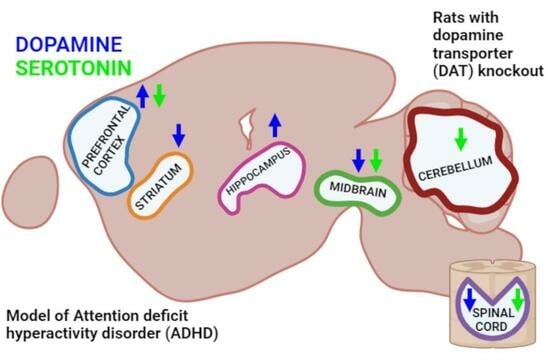
Alterations in Serotonin Neurotransmission in Hyperdopaminergic Rats Lacking the Dopamine Transporter
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
2.2. Sample Collection
2.3. Genotyping
2.4. Determination of Monoamines
2.5. RNA Isolation
2.6. cDNA Synthesis and Real-Time RT–PCR
2.7. Statistical Analysis
3. Results
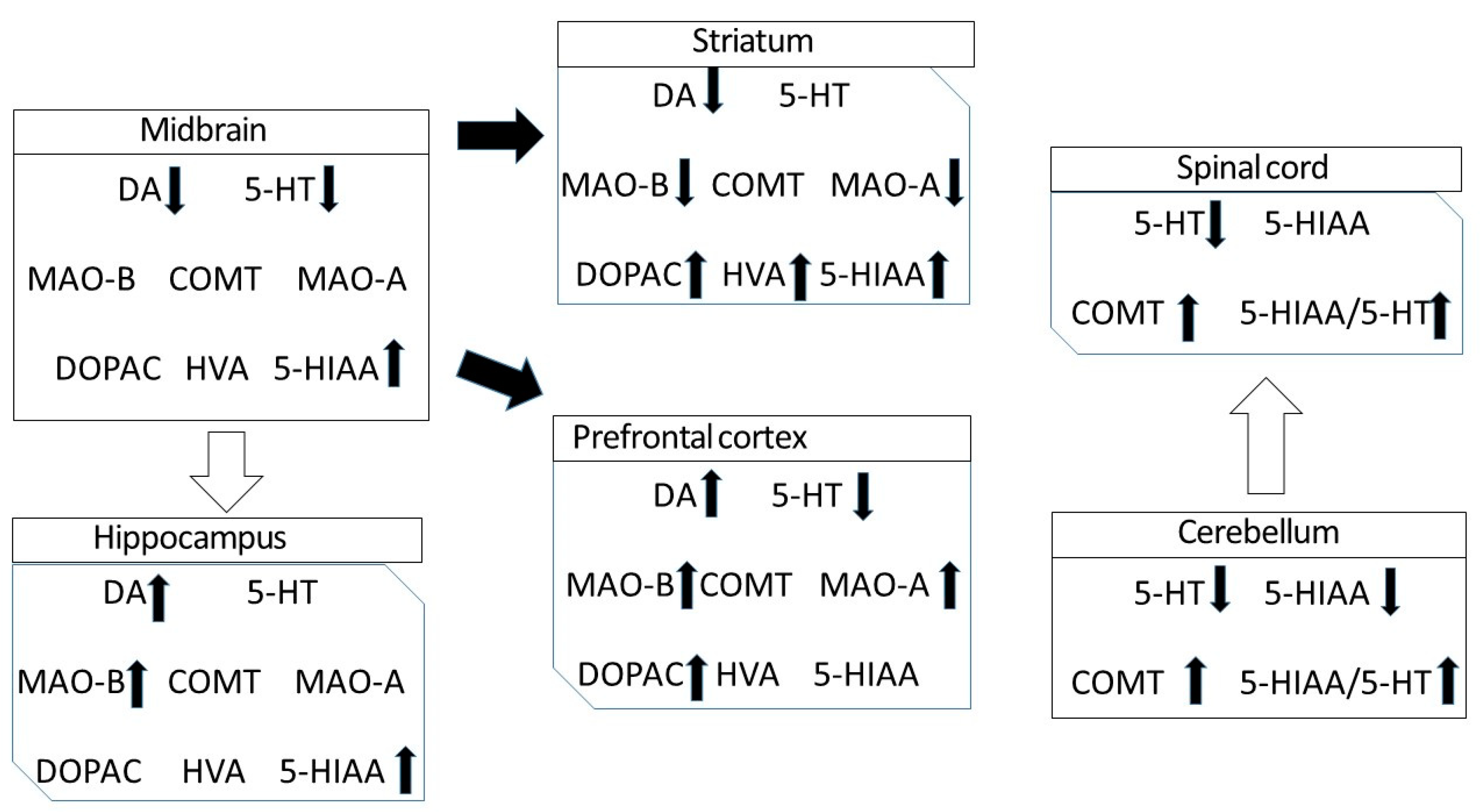
3.1. DA, 5-HT and Their Metabolites in the Different Regions of CNS of DAT-KO Rats
3.2. Turnover Rates of DA and 5-HT in the Different Regions of DAT-KO Rats
3.3. mRNA Expression of the Main Enzymes Involved in Monoamine Metabolism in Different Regions of DAT-KO Rats
4. Discussion
5. Highlights and Limitations
5.1. Highlights
- The knockout of the gene encoding DAT in rats leads not only to a significant redistribution of dopamine in various structures of the CNS but also to pronounced changes in the level of serotonin, which are most notable in the cerebellum and the spinal cord.
- The knockout of the gene encoding DAT leads also to alterations in the production of RNA enzymes involved in monoamine metabolism, MAO-A, MAO-B and COMT, in the most brain areas studied.
5.2. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lerner, T.N.; Holloway, A.L.; Seiler, J.L. Dopamine, Updated: Reward Prediction Error and Beyond. Curr. Opin. Neurobiol. 2020, 67, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, S.A.; Laudani, S.; Contarini, G.; De Luca, A.; Geraci, F.; Managò, F.; Papaleo, F.; Salomone, S.; Drago, F.; Leggio, G.M. Dopamine, Cognitive Impairments and Second-Generation Antipsychotics: From Mechanistic Advances to More Personalized Treatments. Pharmaceuticals 2020, 13, 365. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.; Reyes-Resina, I.; Navarro, G. Dopamine in Health and Disease: Much More Than a Neurotransmitter. Biomedicines 2021, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.-P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef]
- Bamalan, O.A.; Moore, M.J.; Khalili, Y.A.; Al Khalili, Y. Physiology, Serotonin; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Yagishita, S. Transient and sustained effects of dopamine and serotonin signaling in motivation-related behavior. Psychiatry Clin. Neurosci. 2019, 74, 91–98. [Google Scholar] [CrossRef]
- Weintraub, D.; Claassen, D.O. Impulse Control and Related Disorders in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 133, 679–717. [Google Scholar] [CrossRef]
- van Galen, K.A.; ter Horst, K.W.; Booij, J.; la Fleur, S.E.; Serlie, M.J. The role of central dopamine and serotonin in human obesity: Lessons learned from molecular neuroimaging studies. Metabolism 2018, 85, 325–339. [Google Scholar] [CrossRef]
- Devroye, C.; Cathala, A.; Piazza, P.V.; Spampinato, U. The central serotonin2B receptor as a new pharmacological target for the treatment of dopamine-related neuropsychiatric disorders: Rationale and current status of research. Pharmacol. Ther. 2018, 181, 143–155. [Google Scholar] [CrossRef]
- Momiyama, T.; Nishijo, T. Dopamine and Serotonin-Induced Modulation of GABAergic and Glutamatergic Transmission in the Striatum and Basal Forebrain. Front. Neuroanat. 2017, 11, 42. [Google Scholar] [CrossRef]
- Hikosaka, O.; Sesack, S.R.; Lecourtier, L.; Shepard, P.D. Habenula: Crossroad between the Basal Ganglia and the Limbic System. J. Neurosci. 2008, 28, 11825–11829. [Google Scholar] [CrossRef]
- Schrag, A.; Taddei, R.N. Depression and Anxiety in Parkinson’s Disease. Int. Rev. Neurobiol. 2017, 133, 623–655. [Google Scholar] [CrossRef] [PubMed]
- Vo, A.; Ganjavi, H.; MacDonald, P.A. Levodopa has mood-enhancing effects in healthy elderly adults. Int. J. Geriatr. Psychiatry 2018, 33, 674–675. [Google Scholar] [CrossRef] [PubMed]
- Baronti, F.; Davis, T.L.; Boldry, R.C.; Mouradian, M.M.; Chase, T.N. Deprenyl effects on levodopa pharmacodynamics, mood, and free radical scavenging. Neurology 1992, 42, 541. [Google Scholar] [CrossRef]
- Metzger, M.; Souza, R.; Lima, L.B.; Bueno, D.; Gonçalves, L.; Sego, C.; Donato, J.; Shammah-Lagnado, S.J. Habenular connections with the dopaminergic and serotonergic system and their role in stress-related psychiatric disorders. Eur. J. Neurosci. 2019, 53, 65–88. [Google Scholar] [CrossRef] [PubMed]
- Vanmechelen, I.; Dan, B.; Feys, H.; Monbaliu, E. Test–retest reliability of the Dyskinesia Impairment Scale: Measuring dystonia and choreoathetosis in dyskinetic cerebral palsy. Dev. Med. Child. Neurol. 2019, 62, 489–493. [Google Scholar] [CrossRef]
- Seo, D.; Patrick, C.J.; Kennealy, P.J. Role of Serotonin and Dopamine System Interactions in the Neurobiology of Impulsive Aggression and its Comorbidity with other Clinical Disorders. Aggress. Violent Behav. 2008, 13, 383–395. [Google Scholar] [CrossRef]
- Kapur, S.; Remington, G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am. J. Psychiatry 1996, 153, 466–476. [Google Scholar] [CrossRef]
- Daw, N.D.; Kakade, S.; Dayan, P. Opponent interactions between serotonin and dopamine. Neural Netw. 2002, 15, 603–616. [Google Scholar] [CrossRef]
- Zhou, F.M.; Liang, Y.; Salas, R.; Zhang, L.; De Biasi, M.; Dani, J.A. Corelease of dopamine and serotonin from striatal dopamine terminals. Neuron 2005, 46, 65–74. [Google Scholar] [CrossRef]
- Shi, W.X.; Nathaniel, P.; Bunney, B.S. Ritanserin, a 5-HT2A/2C antagonist, reverses direct dopamine agonist-induced inhibition of midbrain dopamine neurons. J. Pharmacol. Exp. Ther. 1995, 274, 735–740. [Google Scholar]
- Larsen, M.B.; Sonders, M.S.; Mortensen, O.V.; Larson, G.A.; Zahniser, N.R.; Amara, S.G. Dopamine transport by the serotonin transporter: A Mechanistically distinct mode of substrate translocation. J. Neurosci. 2011, 31, 6605–6615. [Google Scholar] [CrossRef]
- Jones, S.R.; Gainetdinov, R.R.; Jaber, M.; Giros, B.; Wightman, R.M.; Caron, M.G. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc. Natl. Acad. Sci. USA 1998, 95, 4029–4034. [Google Scholar] [CrossRef]
- Leo, D.; Sukhanov, I.; Zoratto, F.; Illiano, P.; Caffino, L.; Sanna, F.; Messa, G.; Emanuele, M.; Esposito, A.; Dorofeikova, M.; et al. Pronounced Hyperactivity, Cognitive Dysfunctions, and BDNF Dysregulation in Dopamine Transporter Knock-out Rats. J. Neurosci. 2018, 38, 1959–1972. [Google Scholar] [CrossRef]
- Giros, B.; Jaber, M.; Jones, S.R.; Wightman, R.M.; Caron, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379, 606–612. [Google Scholar] [CrossRef]
- Shen, H.W.; Hagino, Y.; Kobayashi, H.; Shinohara-Tanaka, K.; Ikeda, K.; Yamamoto, H.; Yamamoto, T.; Lesch, K.P.; Murphy, D.L.; Hall, F.S.; et al. Regional Differences in Extracellular Dopamine and Serotonin Assessed by In Vivo Microdialysis in Mice Lacking Dopamine and/or Serotonin Transporters. Neuropsychopharmacology 2004, 29, 1790–1799. [Google Scholar] [CrossRef]
- Yamashita, M.; Fukushima, S.; Shen, H.-w.; Hall, F.S.; Uhl, G.R.; Numachi, Y.; Kobayashi, H.; Sora, I. Norepinephrine Transporter Blockade can Normalize the Prepulse Inhibition Deficits Found in Dopamine Transporter Knockout Mice. Neuropsychopharmacology 2006, 31, 2132–2139. [Google Scholar] [CrossRef]
- Fox, M.A.; Panessiti, M.G.; Hall, F.S.; Uhl, G.R.; Murphy, D.L. An evaluation of the serotonin system and perseverative, compulsive, stereotypical, and hyperactive behaviors in dopamine transporter (DAT) knockout mice. Psychopharmacology 2013, 227, 685–695. [Google Scholar] [CrossRef]
- Kurzina, N.; Aristova, I.; Volnova, A.; Gainetdinov, R. Deficit in working memory and abnormal behavioral tactics in dopamine transporter knockout rats during training in the 8-arm maze. Behav. Brain Res. 2020, 390, 112642. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. Rat Brain in Stereotaxic Coordinates; Elsevier Science: Amsterdam, The Netherlands, 2013; 472p. [Google Scholar]
- Jamal, M.; Ito, A.; Miki, T.; Suzuki, S.; Ohta, K.-I.; Kinoshita, H. Ethanol concentration induces production of 3,4-dihydroxyphenylacetic acid and homovanillic acid in mouse brain through activation of monoamine oxidase pathway. Neurosci. Lett. 2022, 782, 136689. [Google Scholar] [CrossRef]
- Jaber, M.; Dumartin, B.; Sagné, C.; Haycock, J.W.; Roubert, C.; Giros, B.; Bloch, B.; Caron, M.G. Differential regulation of tyrosine hydroxylase in the basal ganglia of mice lacking the dopamine transporter. Eur. J. Neurosci. 1999, 11, 3499–3511. [Google Scholar] [CrossRef]
- Jones, S.R.; Bowman, B.P.; Kuhn, C.M.; Wightman, R.M. Development of Dopamine Neurotransmission and Uptake Inhibition in the Caudate Nucleus as Measured by Fast-Cyclic Voltammetry. Synapse 1996, 24, 305–307. [Google Scholar] [CrossRef]
- Gainetdinov, R.R.; Caron, M.G. Monoamine transporters: From genes to behavior. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 261–284. [Google Scholar] [CrossRef]
- Sotnikova, T.D.; Beaulieu, J.M.; Barak, L.S.; Wetsel, W.C.; Caron, M.G.; Gainetdinov, R.R. Dopamine-independent locomotor actions of amphetamines in a novel acute mouse model of Parkinson disease. PLoS Biol. 2005, 3, e271. [Google Scholar] [CrossRef]
- Sukhanov, I.; Dorotenko, A.; Fesenko, Z.; Savchenko, A.; Efimova, E.V.; Mor, M.S.; Belozertseva, I.V.; Sotnikova, T.D.; Gainetdinov, R.R. Inhibition of PDE10A in a New Rat Model of Severe Dopamine Depletion Suggests New Approach to Non-Dopamine Parkinson’s Disease Therapy. Biomolecules 2022, 13, 9. [Google Scholar] [CrossRef]
- Volavka, J.; Bilder, R.; Nolan, K. Catecholamines and aggression: The role of COMT and MAO polymorphisms. Ann. N. Y. Acad. Sci. 2004, 1036, 393–398. [Google Scholar] [CrossRef]
- Hao, H.; Shao, M.; An, J.; Chen, C.; Feng, X.; Xie, S.; Gu, Z.; Chan, P. Association of Catechol-O-Methyltransferase and monoamine oxidase B gene polymorphisms with motor complications in parkinson’s disease in a Chinese population. Park. Relat. Disord. 2014, 20, 1041–1045. [Google Scholar] [CrossRef]
- Salvatore, M.F.; Pruett, B.S.; Spann, S.L.; Dempsey, C. Aging Reveals a Role for Nigral Tyrosine Hydroxylase ser31 Phosphorylation in Locomotor Activity Generation. PLoS ONE 2009, 4, e8466. [Google Scholar] [CrossRef]
- Eshleman, A.J.; Carmolli, M.; Cumbay, M.; Martens, C.R.; Neve, K.A.; Janowsky, A. Characteristics of drug interactions with recombinant biogenic amine transporters expressed in the same cell type. J. Pharmacol. Exp. Ther. 1999, 289, 877–885. [Google Scholar]
- Sesack, S.R.; Hawrylak, V.A.; Matus, C.; Guido, M.A.; Levey, A.I. Dopamine Axon Varicosities in the Prelimbic Division of the Rat Prefrontal Cortex Exhibit Sparse Immunoreactivity for the Dopamine Transporter. J. Neurosci. 1998, 18, 2697. [Google Scholar] [CrossRef]
- Schroder, E.A.; Wang, L.; Wen, Y.; Callahan, L.A.P.; Supinski, G.S. Skeletal muscle-specific calpastatin overexpression mitigates muscle weakness in aging and extends life span. J. Appl. Physiol. 2021, 131, 630–642. [Google Scholar] [CrossRef]
- Madras, B.K.; Miller, G.M.; Fischman, A.J. The Dopamine Transporter and Attention-Deficit/Hyperactivity Disorder. Biol. Psychiatry 2005, 57, 1397–1409. [Google Scholar] [CrossRef]
- Xu, T.X.; Sotnikova, T.D.; Liang, C.; Zhang, J.; Jung, J.U.; Spealman, R.D.; Gainetdinov, R.R.; Yao, W.D. Hyperdopaminergic tone erodes prefrontal long-term potential via a D2 receptor-operated protein phosphatase gate. J. Neurosci. 2009, 29, 14086–14099. [Google Scholar] [CrossRef]
- Turi, G.F.; Li, W.K.; Chavlis, S.; Pandi, I.; O’Hare, J.; Priestley, J.B.; Grosmark, A.D.; Liao, Z.; Ladow, M.; Zhang, J.F.; et al. Vasoactive Intestinal Polypeptide-Expressing Interneurons in the Hippocampus Support Goal-Oriented Spatial Learning. Neuron 2019, 101, 1150–1165.e8. [Google Scholar] [CrossRef]
- Adinolfi, A.; Zelli, S.; Leo, D.; Carbone, C.; Mus, L.; Illiano, P.; Alleva, E.; Gainetdinov, R.R.; Adriani, W. Behavioral characterization of DAT-KO rats and evidence of asocial-like phenotypes in DAT-HET rats: The potential involvement of norepinephrine system. Behav. Brain Res. 2019, 359, 516–527. [Google Scholar] [CrossRef]
- Mialet-Perez, J.; Santin, Y.; Parini, A. Monoamine oxidase-A, serotonin and norepinephrine: Synergistic players in cardiac physiology and pathology. J. Neural Transm. 2018, 125, 1627–1634. [Google Scholar] [CrossRef]
- Ito, M. Error detection and representation in the olivo-cerebellar system. Front. Neural Circuits 2013, 7, 1. [Google Scholar] [CrossRef]
- Stoodley, C.J. The cerebellum and neurodevelopmental disorders. Cerebellum 2016, 15, 34. [Google Scholar] [CrossRef]
- Sinzig, J.; Walter, D.; Doepfner, M. Attention Deficit/Hyperactivity Disorder in Children and Adolescents with Autism Spectrum Disorder. J. Atten. Disord. 2009, 13, 117–126. [Google Scholar] [CrossRef]
- Ronald, A.; Simonoff, E.; Kuntsi, J.; Asherson, P.; Plomin, R. Evidence for overlapping genetic influences on autistic and ADHD behaviours in a community twin sample. J. Child. Psychol. Psychiatry 2008, 49, 535–542. [Google Scholar] [CrossRef]
- Bruchhage, M.M.K.; Bucci, M.P.; Becker, E.B.E. Cerebellar involvement in autism and ADHD. Handb. Clin. Neurol. 2018, 155, 61–72. [Google Scholar] [CrossRef]
- Nijmeijer, K.J.; Huijsman, R.; Fabbricotti, I.N. Exploring the Role of Ownership Structures in the Results of Professional Health Care Franchises from a Multi-Actor Perspective. J. Mark. Channels 2014, 21, 159–179. [Google Scholar] [CrossRef]
- Saitow, F.; Hirono, M.; Suzuki, H. Serotonin and synaptic transmission in the cerebellum. In Handbook of the Cerebellum and Cerebellar Disorders; Springer: Dordrecht, The Netherlands, 2013; pp. 915–926. [Google Scholar] [CrossRef]
- Chugani, D.C.; Muzik, O.; Behen, M.; Rothermel, R.; Janisse, J.J.; Lee, J.; Chugani, H.T. Developmental Changes in Brain Serotonin Synthesis Capacity in Autistic and Nonautistic Children. Ann. Neurol. 1999, 45, 287–295. [Google Scholar] [CrossRef]
| No. | Name | Primer Sequence | Annealing Temperature |
|---|---|---|---|
| 1 | MAO-A | Forward 5′-GCCAGGAACGGAAATTTGTA-3′; reverse 5′-TCTCAGGTGGAAGCTCTGGT-3′ | 65 °C |
| 2 | MAO-B | Forward 5′-TGGGCCAAGAGATTCCCAGTGATG-3′; reverse 5′-AGAGTGTGGCAATCTGCTTTGTAG-3′ | 60 °C |
| 3 | COMT130 | Forward 5′-CTGGAGGCCATCGACACCTA-3′; reverse 5′-AGTAAGCTCCCAGCTCCAGCA-3′ | 60 °C |
| 4 | 18S (housekeeping gene) | Forward 5′-ACGGACCAGAGCGAAAGCAT-3′; reverse 5′-TGTCAATCCTGTCCGTGTCC-3′ | 60 °C |
| 5 | PPI (housekeeping gene) | Forward 5′-GGATTTGGCTATAAGGGTTC-3′; reverse 5′-GTTGTCCACAGTCGGAGA-3′ | 60 °C |
| DA | DOPAC | HVA | 5-HT | 5-HIAA | |
|---|---|---|---|---|---|
| Midbrain | |||||
| DAT-WT | 5.1± 0.9 | No data | No data | 5.1 ± 0.0 | 0.1 ± 0.0 |
| DAT-KO | 2.8 ± 0.1 | No data | No data | 2.7 ± 0.1 | 0.3 ± 0.0 |
| t | 2.4 | 1.2 | −5.4 | ||
| p | 0.012 | 0.008 | 0.003 | ||
| Striatum | |||||
| DAT-WT | 64.3 ± 2.7 | 6.0 ± 0.7 | 3.8 ± 0.7 | 8.2 ± 0.8 | * excluded data |
| DAT-KO | 7.5 ± 1.7 | 13.8 ± 1.7 | 13.8 ± 1.7 | 5.4 ± 1.3 | * excluded data |
| t | 18.9 | −3.7 | −5.3 | 1.7 | |
| p | 0.0002 | 0.0140 | 0.0075 | 0.1326 | |
| Prefrontal cortex | |||||
| DAT-WT | 0.8 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.1 | 16.5 ± 2.7 | 13.1 ± 1.9 |
| DAT-KO | 1.8 ± 0.1 | 0.5 ± 0.1 | 0.3 ± 0.1 | 6.8 ± 0.7 | 9.7 ± 4.1 |
| t | −16.0 | −2.8 | 0.4 | 3.7 | 0.6 |
| p | <0.0001 | 0.0271 | 0.6730 | 0.0069 | 0.5264 |
| Hippocampus | |||||
| DAT-WT | 0.4 ± 0.0 | 0.2 ± 0.0 | No data | 13.1 ± 0.7 | 15.5 ± 0.2 |
| DAT-KO | 0.9 ± 0.1 | 0.2 ± 0.0 | No data | 13.6 ± 0.5 | 17.0 ± 0.2 |
| t | −5.0 | 0.1 | −0.6 | −4.3 | |
| p | 0.0043 | 0.9513 | 0.5432 | 0.0078 | |
| Medulla oblongata | |||||
| DAT-WT | 1.1 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.1 | 17.5 ± 3.6 | 13.4 ± 2.3 |
| DAT-KO | 0.3 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 | 3.9 ± 1.1 |
| t | 3.9 | 4.4 | 2.1 | 5.5 | 4.0 |
| p | 0.0057 | 0.0032 | 0.0742 | 0.0009 | 0.0052 |
| Cerebellum | |||||
| DAT-WT | 1.0 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 2.7 ± 0.9 | 1.6 ± 0.2 |
| DAT-KO | 0.7 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.8 ± 0.1 |
| t | 1.8 | 1.8 | 2.1 | 2.9 | 3.0 |
| p | 0.1229 | 0.1134 | 0.0742 | 0.0220 | 0.0191 |
| Spinal cord | |||||
| DAT-WT | 0.5 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.1 | 6.0 ± 0.6 | 4.4 ± 0.8 |
| DAT-KO | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.1 | 1.8 ± 0.1 | 5.3 ± 0.9 |
| t | 3.4 | 1.4 | 1.1 | 7.9 | −0.8 |
| p | 0.0120 | 0.1889 | 0.3025 | 0.0001 | 0.4747 |
| DOPAC/DA | HVA/DA | 5-HIAA/5-HT | |
|---|---|---|---|
| Striatum | |||
| DAT-WT | 0.1 ± 0.0 | 0.1 ± 0.0 | No data |
| DAT-KO | 2.0 ± 0.2 | 3.8 ± 0.4 | No data |
| t | −9.7 | −9.2 | |
| p | 0.0023 | 0.0027 | |
| Prefrontal cortex | |||
| DAT-WT | 0.3 ± 0.1 | 0.4 ± 0.1 | 0.9 ± 0.2 |
| DAT-KO | 0.3 ± 0.0 | 0.1 ± 0.1 | 1.5 ± 0.6 |
| t | 0.2 | 2.4 | −0.9 |
| p | 0.8200 | 0.0475 | 0.4201 |
| Hippocampus | |||
| DAT-WT | 0.6 ± 0.1 | No data | 1.2 ± 0.0 |
| DAT-KO | 0.3 ± 0.0 | No data | 1.3 ± 0.0 |
| t | 4.3 | −1.2 | |
| p | 0.0075 | 0.2918 | |
| Medulla oblongata | |||
| DAT-WT | 0.3 ± 0.0 | 0.4 ± 0.1 | 0.9 ± 0.3 |
| DAT-KO | 0.2 ± 0.0 | 0.5 ± 0.1 | 0.0 ± 0.0 |
| t | 3.5 | −1.3 | |
| p | 0.0094 | 0.2309 | |
| Cerebellum | |||
| DAT-WT | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.7 ± 0.2 |
| DAT-KO | 0.2 ± 0.1 | 0.2 ± 0.1 | 3.5 ± 0.3 |
| t | −0.6 | −0.9 | −6.9 |
| p | 0.5745 | 0.3592 | 0.0002 |
| Spinal cord | |||
| DAT-WT | 0.4 ± 0.2 | 0.7 ± 0.4 | 0.7 ± 0.1 |
| DAT-KO | 0.8 ± 0.1 | 0.6 ± 0.4 | 3.1 ± 0.7 |
| t | −1.7 | 0.1 | −2.8 |
| p | 0.1272 | 0.8975 | 0.0281 |
| MAO-A | MAO-B | COMT | |
|---|---|---|---|
| Striatum | |||
| DAT-WT | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| DAT-KO | 0.4 ± 0.1 | 0.2 ± 0.1 | 1.0 ± 0.2 |
| t | 7.4 | 6.4 | 0.2 |
| p | 0.0003 | 0.0002 | 0.8688 |
| Prefrontal Cortex | |||
| DAT-WT | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| DAT-KO | 2.1 ± 0.2 | 2.0 ± 0.4 | 1.2 ± 0.2 |
| t | −4.2 | −2.3 | −0.8 |
| p | 0.0031 | 0.0454 | 0.4283 |
| Hippocampus | |||
| DAT-WT | 1.0 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| DAT-KO | 0.6 ± 0.2 | 2.2 ± 0.5 | 2.1 ± 0.7 |
| t | 1.8 | −2.7 | −1.6 |
| p | 0.1104 | 0.029 | 0.1561 |
| Medulla oblongata | |||
| DAT-WT | 1.2 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.1 |
| DAT-KO | 0.1 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 |
| t | 5.8 | 3.9 | 4.5 |
| p | 0.0004 | 0.0048 | 0.0015 |
| Cerebellum | |||
| DAT-WT | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.7 ± 0.2 |
| DAT-KO | 0.2 ± 0.1 | 0.2 ± 0.1 | 3.5 ± 0.3 |
| t | −0.6 | −0.9 | −6.9 |
| p | 0.5745 | 0.3592 | 0.0002 |
| Spinal cord | |||
| DAT-WT | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 |
| DAT-KO | 0.9 ± 0.1 | 13.0 ± 2.0 | 10.8 ± 3.5 |
| t | 0.8 | −5.8 | −2.8 |
| p | 0.4301 | 0.0004 | 0.0240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Traktirov, D.S.; Nazarov, I.R.; Artemova, V.S.; Gainetdinov, R.R.; Pestereva, N.S.; Karpenko, M.N. Alterations in Serotonin Neurotransmission in Hyperdopaminergic Rats Lacking the Dopamine Transporter. Biomedicines 2023, 11, 2881. https://doi.org/10.3390/biomedicines11112881
Traktirov DS, Nazarov IR, Artemova VS, Gainetdinov RR, Pestereva NS, Karpenko MN. Alterations in Serotonin Neurotransmission in Hyperdopaminergic Rats Lacking the Dopamine Transporter. Biomedicines. 2023; 11(11):2881. https://doi.org/10.3390/biomedicines11112881
Chicago/Turabian StyleTraktirov, Dmitrii S., Ilya R. Nazarov, Valeria S. Artemova, Raul R. Gainetdinov, Nina S. Pestereva, and Marina N. Karpenko. 2023. "Alterations in Serotonin Neurotransmission in Hyperdopaminergic Rats Lacking the Dopamine Transporter" Biomedicines 11, no. 11: 2881. https://doi.org/10.3390/biomedicines11112881
APA StyleTraktirov, D. S., Nazarov, I. R., Artemova, V. S., Gainetdinov, R. R., Pestereva, N. S., & Karpenko, M. N. (2023). Alterations in Serotonin Neurotransmission in Hyperdopaminergic Rats Lacking the Dopamine Transporter. Biomedicines, 11(11), 2881. https://doi.org/10.3390/biomedicines11112881