Impacts of the DPP-4 Inhibitor Saxagliptin and SGLT-2 Inhibitor Dapagliflozin on the Gonads of Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Induction of Experimental Diabetes
2.3. Treatment
2.4. Sperm Comet Test
2.5. Spermatocyte Chromosome Examination
2.6. Spermiogram Examination
2.7. Evaluation of Oxidative Stress
2.8. Statistics
3. Results
3.1. General Characteristics of the Mice
3.2. Effects of Saxagliptin and Dapagliflozin on Diabetes-Caused Sperm DNA Damage
3.3. Effects of Saxagliptin and Dapagliflozin on Diabetes-Caused Spermatocyte Chromosome Abnormalities
3.4. Effects of Saxagliptin and Dapagliflozin on Diabetes-Caused Changes in Sperm Motility, Counts, and Abnormalities
3.5. Effects of Saxagliptin and Dapagliflozin on Diabetes-Induced Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhindsa, S.; Ghanim, H.; Batra, M.; Dandona, P. Hypogonadotropic Hypogonadism in Men with Diabesity. Diabetes Care 2018, 41, 1516–1525. [Google Scholar] [CrossRef]
- Maresch, C.C.; Stute, D.C.; Alves, M.G.; Oliveira, P.F.; de Kretser, D.M.; Linn, T. Diabetes-induced hyperglycemia impairs male reproductive function: A systematic review. Hum. Reprod. Update 2018, 24, 86–105. [Google Scholar] [CrossRef]
- O’Donnell, L.; Stanton, P.; de Kretser, D.M. Endocrinology of the Male Reproductive System and Spermatogenesis. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Condorelli, R.A.; La Vignera, S.; Mongioi, L.M.; Alamo, A.; Calogero, A.E. Diabetes Mellitus and Infertility: Different Pathophysiological Effects in Type 1 and Type 2 on Sperm Function. Front. Endocrinol. 2018, 9, 268. [Google Scholar] [CrossRef]
- He, Z.; Yin, G.; Li, Q.Q.; Zeng, Q.; Duan, J. Diabetes Mellitus Causes Male Reproductive Dysfunction: A Review of the Evidence and Mechanisms. In Vivo 2021, 35, 2503–2511. [Google Scholar] [CrossRef]
- Agbaje, I.M.; Rogers, D.A.; McVicar, C.M.; McClure, N.; Atkinson, A.B.; Mallidis, C.; Lewis, S.E. Insulin dependant diabetes mellitus: Implications for male reproductive function. Hum. Reprod. 2007, 22, 1871–1877. [Google Scholar] [CrossRef]
- Schoeller, E.L.; Albanna, G.; Frolova, A.I.; Moley, K.H. Insulin rescues impaired spermatogenesis via the hypothalamic-pituitary-gonadal axis in Akita diabetic mice and restores male fertility. Diabetes 2012, 61, 1869–1878. [Google Scholar] [CrossRef]
- Pelusi, C. The Effects of the New Therapeutic Treatments for Diabetes Mellitus on the Male Reproductive Axis. Front. Endocrinol. 2022, 13, 821113. [Google Scholar] [CrossRef]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef]
- Padda, I.S.; Mahtani, A.U.; Parmar, M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. In StatPearls; Treasure Island (FL) ineligible companies. Disclosure: Arun Mahtani declares no relevant financial relationships with ineligible companies. Disclosure: Mayur Parmar declares no relevant financial relationships with ineligible companies; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Ballester, J.; Munoz, M.C.; Dominguez, J.; Rigau, T.; Guinovart, J.J.; Rodriguez-Gil, J.E. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J. Androl. 2004, 25, 706–719. [Google Scholar] [CrossRef]
- Eleazu, C.O.; Eleazu, K.C.; Chukwuma, S.; Essien, U.N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diabetes Metab. Disord. 2013, 12, 60. [Google Scholar] [CrossRef]
- Like, A.A.; Rossini, A.A. Streptozotocin-induced pancreatic insulitis: New model of diabetes mellitus. Science 1976, 193, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Gangadharan Komala, M.; Gross, S.; Zaky, A.; Pollock, C.; Panchapakesan, U. Saxagliptin reduces renal tubulointerstitial inflammation, hypertrophy and fibrosis in diabetes. Nephrology 2016, 21, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Zhao, Y.; Wang, Q.; Hillebrands, J.L.; van den Born, J.; Ji, L.; An, T.; Qin, G. Dapagliflozin Attenuates Renal Tubulointerstitial Fibrosis Associated with Type 1 Diabetes by Regulating STAT1/TGFbeta1 Signaling. Front. Endocrinol. 2019, 10, 441. [Google Scholar] [CrossRef] [PubMed]
- Oakberg, E.F. Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am. J. Anat. 1956, 99, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.M.; Bakheet, S.A. Citalopram at the recommended human doses after long-term treatment is genotoxic for male germ cell. Food Chem. Toxicol. 2013, 53, 281–285. [Google Scholar] [CrossRef]
- Al-Hamamah, M.A.; Alotaibi, M.R.; Ahmad, S.F.; Nadeem, A.; Attia, M.S.M.; Ansari, M.A.; Bakheet, S.A.; Alanazi, M.M.; Attia, S.M. Treatment with the anti-CD20 monoclonal antibody rituximab mitigates gonadal disruptions in the collagen-induced arthritis in male DBA/1 J mouse model. Mutat. Res. 2022, 825, 111799. [Google Scholar] [CrossRef]
- Attia, S.M.; Badary, O.A.; Hamada, F.M.; de Angelis, M.H.; Adler, I.D. Orthovanadate increased the frequency of aneuploid mouse sperm without micronucleus induction in mouse bone marrow erythrocytes at the same dose level. Mutat. Res. 2005, 583, 158–167. [Google Scholar] [CrossRef]
- Attia, S.M.; Schmid, T.E.; Badary, O.A.; Hamada, F.M.; Adler, I.D. Molecular cytogenetic analysis in mouse sperm of chemically induced aneuploidy: Studies with topoisomerase II inhibitors. Mutat. Res. 2002, 520, 1–13. [Google Scholar] [CrossRef]
- Attia, S.M.; Ahmad, S.F.; Okash, R.M.; Bakheet, S.A. Aroclor 1254-induced genotoxicity in male gonads through oxidatively damaged DNA and inhibition of DNA repair gene expression. Mutagenesis 2014, 29, 379–384. [Google Scholar] [CrossRef]
- Bakheet, S.A.; Attia, S.M.; Al-Rasheed, N.M.; Al-Harbi, M.M.; Ashour, A.E.; Korashy, H.M.; Abd-Allah, A.R.; Saquib, Q.; Al-Khedhairy, A.A.; Musarrat, J. Salubrious effects of dexrazoxane against teniposide-induced DNA damage and programmed cell death in murine marrow cells. Mutagenesis 2011, 26, 533–543. [Google Scholar] [CrossRef]
- Adler, I.-D. Cytogenetic tests in mammals. In Mutagenicity Testing-A Practical Approach; Venitt, S., Parry, J.M., Eds.; IRL Press: Oxford, UK, 1984; pp. 275–306. [Google Scholar]
- Attia, S.M.; Al-Bakheet, S.A.; Al-Rasheed, N.M. Proanthocyanidins produce significant attenuation of doxorubicin-induced mutagenicity via suppression of oxidative stress. Oxid. Med. Cell. Longev. 2010, 3, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.M. Dominant lethal mutations of topoisomerase II inhibitors etoposide and merbarone in male mice: A mechanistic study. Arch. Toxicol. 2012, 86, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Wyrobek, A.J.; Bruce, W.R. Chemical induction of sperm abnormalities in mice. Proc. Natl. Acad. Sci. USA 1975, 72, 4425–4429. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.M.; Ahmad, S.F.; Ansaria, M.A.; Nadeem, A.; Al-Shabanah, O.A.; Al-Harbi, M.M.; Bakheet, S.A. Utility of Dexrazoxane for the Attenuation of Epirubicin-Induced Genetic Alterations in Mouse Germ Cells. PLoS ONE 2016, 11, e0163703. [Google Scholar] [CrossRef] [PubMed]
- Attia, S.M. The impact of quercetin on cisplatin-induced clastogenesis and apoptosis in murine marrow cells. Mutagenesis 2010, 25, 281–288. [Google Scholar] [CrossRef]
- Bakheet, S.A.; Attia, S.M. Evaluation of chromosomal instability in diabetic rats treated with naringin. Oxid. Med. Cell. Longev. 2011, 2011, 365292. [Google Scholar] [CrossRef]
- Luo, Z.C.; Jin, Z.R.; Jiang, Y.F.; Wei, T.J.; Cao, Y.L.; Zhang, Z.; Wei, R.; Jiang, H. The protective effects and underlying mechanisms of dapagliflozin on diabetes-induced testicular dysfunction. Asian J. Androl. 2023, 25, 331–338. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.; Vicari, E.; D’Agata, R.; Calogero, A.E. Diabetes mellitus and sperm parameters. J. Androl. 2012, 33, 145–153. [Google Scholar] [CrossRef]
- Ding, G.L.; Liu, Y.; Liu, M.E.; Pan, J.X.; Guo, M.X.; Sheng, J.Z.; Huang, H.F. The effects of diabetes on male fertility and epigenetic regulation during spermatogenesis. Asian J. Androl. 2015, 17, 948–953. [Google Scholar] [CrossRef]
- Pal, A.K.; Ambulkar, P.S.; Waghmare, J.E.; Wankhede, V.; Shende, M.R.; Tarnekar, A.M. Chromosomal Aberrations in Couples with Pregnancy Loss: A Retrospective Study. J. Hum. Reprod. Sci. 2018, 11, 247–253. [Google Scholar] [CrossRef]
- Attia, S.M.; Helal, G.K.; Alhaider, A.A. Assessment of genomic instability in normal and diabetic rats treated with metformin. Chem. Biol. Interact 2009, 180, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Tavares, R.S.; Escada-Rebelo, S.; Silva, A.F.; Sousa, M.I.; Ramalho-Santos, J.; Amaral, S. Antidiabetic therapies and male reproductive function: Where do we stand? Reproduction 2018, 155, R13–R37. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Van Ginneken, C.; De Cock, H.; Lambeir, A.M.; Van der Veken, P.; Augustyns, K.; Chen, X.; Scharpe, S.; De Meester, I. Enzyme activity and immunohistochemical localization of dipeptidyl peptidase 8 and 9 in male reproductive tissues. J. Histochem. Cytochem. 2009, 57, 531–541. [Google Scholar] [CrossRef] [PubMed]
- An, T.; Wang, Y.F.; Liu, J.X.; Pan, Y.Y.; Liu, Y.F.; He, Z.C.; Mo, F.F.; Li, J.; Kang, L.H.; Gu, Y.J.; et al. Comparative analysis of proteomes between diabetic and normal human sperm: Insights into the effects of diabetes on male reproduction based on the regulation of mitochondria-related proteins. Mol. Reprod. Dev. 2018, 85, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Hibi, H.; Ohori, T.; Yamada, Y. DPP-IV inhibitor may affect spermatogenesis. Diabetes Res. Clin. Pract. 2011, 93, e74–e75. [Google Scholar] [CrossRef]
- Ayuob, N.N.; Murad, H.A.; Ali, S.S. Impaired expression of sex hormone receptors in male reproductive organs of diabetic rat in response to oral antidiabetic drugs. Folia Histochem. Cytobiol. 2015, 53, 35–48. [Google Scholar] [CrossRef]
- Khalid, M.; Petroianu, G.; Adem, A. Advanced Glycation End Products and Diabetes Mellitus: Mechanisms and Perspectives. Biomolecules 2022, 12, 542. [Google Scholar] [CrossRef]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Zhang, P.; Li, T.; Wu, X.; Nice, E.C.; Huang, C.; Zhang, Y. Oxidative stress and diabetes: Antioxidative strategies. Front. Med. 2020, 14, 583–600. [Google Scholar] [CrossRef]
- Rahimi-Madiseh, M.; Malekpour-Tehrani, A.; Bahmani, M.; Rafieian-Kopaei, M. The research and development on the antioxidants in prevention of diabetic complications. Asian Pac. J. Trop. Med. 2016, 9, 825–831. [Google Scholar] [CrossRef]
- Tekin, S.; Beytur, A.; Cakir, M.; Taslidere, A.; Erden, Y.; Tekin, C.; Sandal, S. Protective effect of saxagliptin against renal ischaemia reperfusion injury in rats. Arch. Physiol. Biochem. 2022, 128, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Rossi, C.; Duranti, E.; Taddei, S.; Natali, A.; Virdis, A. Saxagliptin prevents vascular remodeling and oxidative stress in db/db mice. Role of endothelial nitric oxide synthase uncoupling and cyclooxygenase. Vasc. Pharmacol. 2016, 76, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Dede, E.; Liapis, D.; Davos, C.; Katsimpoulas, M.; Varela, A.; Mpotis, I.; Kostomitsopoulos, N.; Kadoglou, N.P.E. The effects of exercise training on cardiac matrix metalloproteinases activity and cardiac function in mice with diabetic cardiomyopathy. Biochem. Biophys. Res. Commun. 2022, 586, 8–13. [Google Scholar] [CrossRef]
- Sikalidis, A.K.; Maykish, A. The Gut Microbiome and Type 2 Diabetes Mellitus: Discussing a Complex Relationship. Biomedicines 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
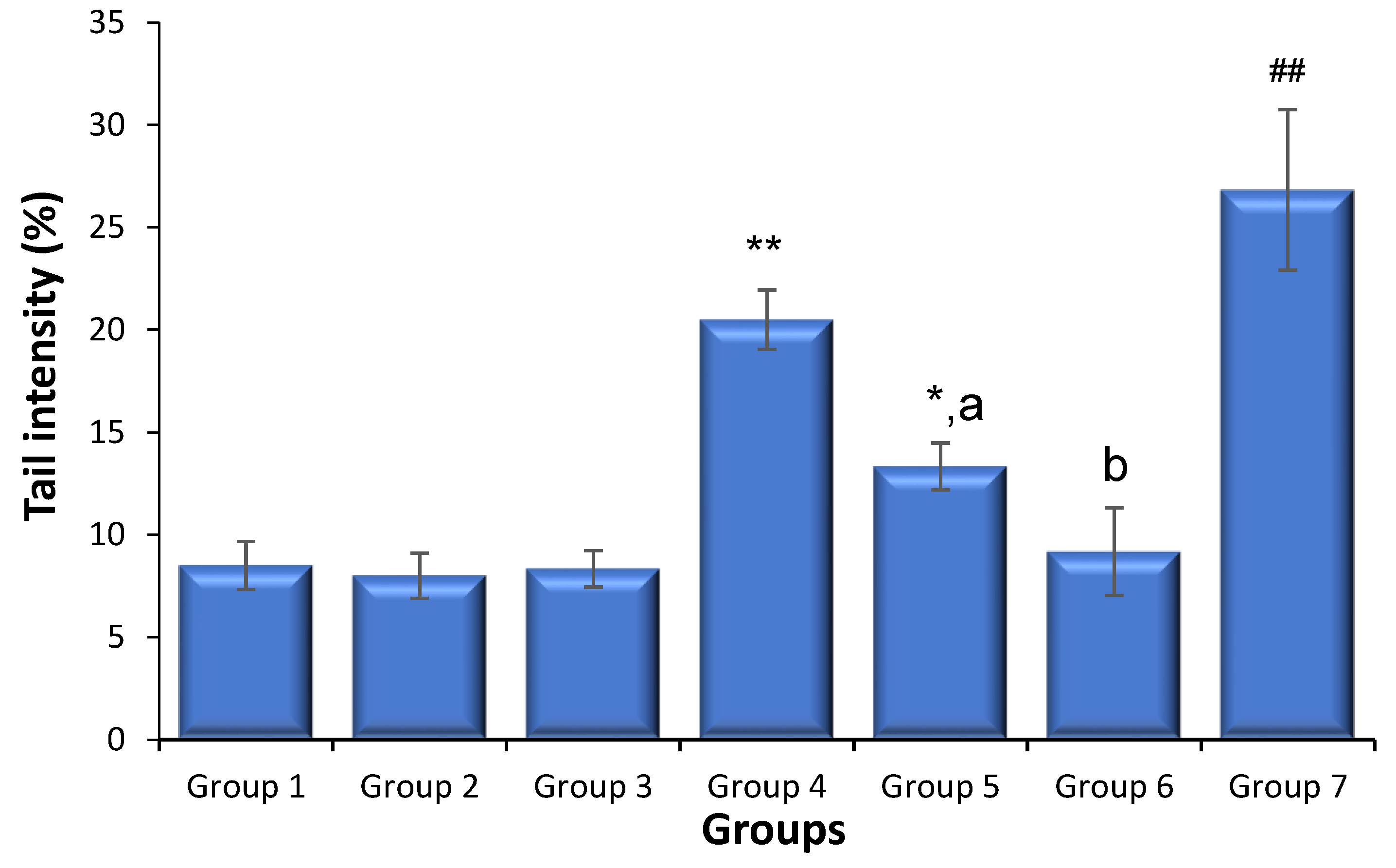

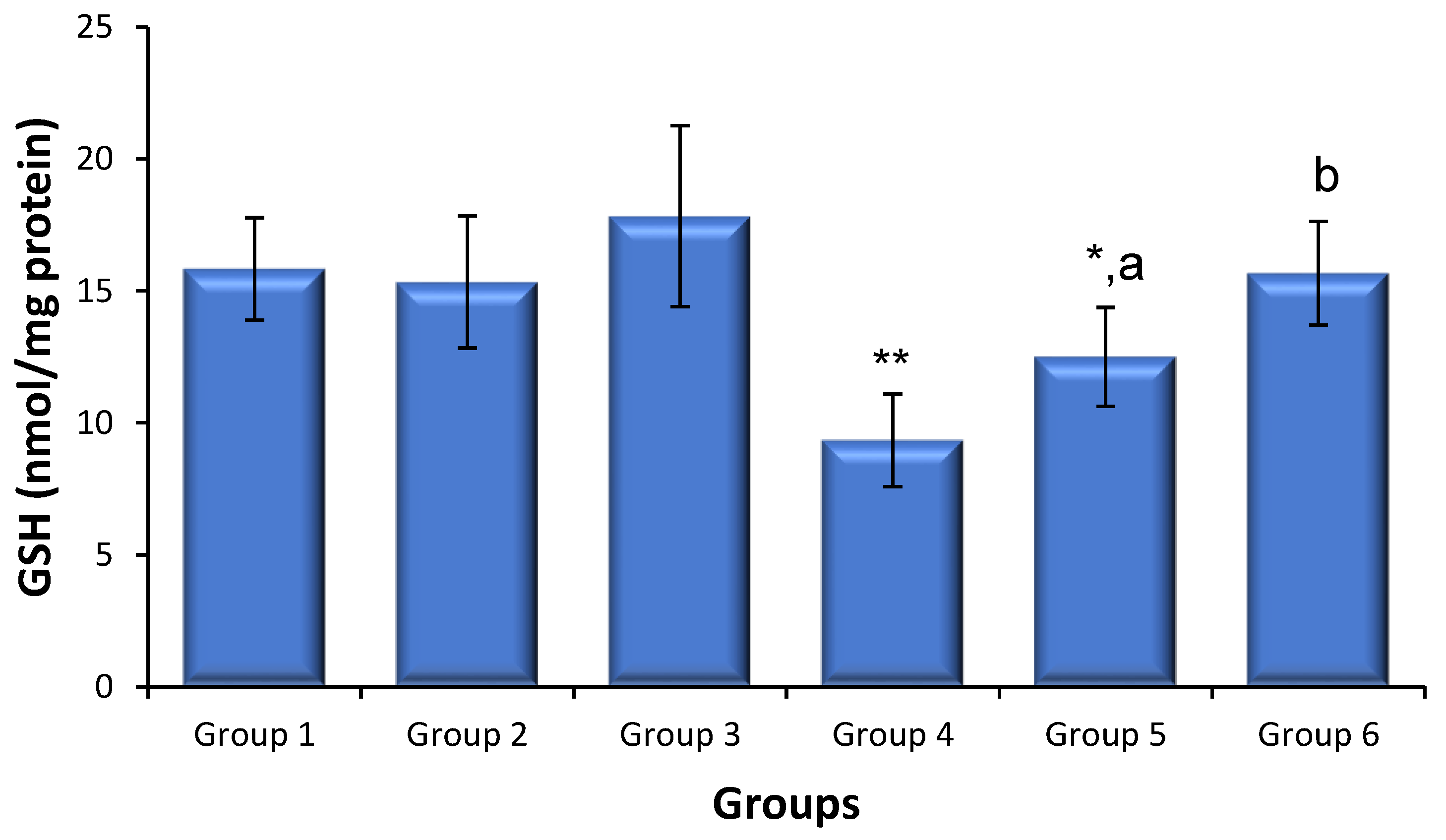
| Groups | Body Weight (g) | Testis Weight (g) | Testis Coefficient (%) | Blood Glucose(mg/dL) | ||
|---|---|---|---|---|---|---|
| Initial Weight | After Two Weeks | Final Weight | ||||
| Group 1 | 19.4 ± 1.5 | 23.3 ± 1.8 | 28.2 ± 1.4 | 0.198 ± 0.014 | 0.70 ± 0.02 | 145.6 ± 22.4 |
| Group 2 | 20.3 ± 1.6 | 23.8 ± 1.4 | 27.3 ± 1.9 | 0.188 ± 0.011 | 0.70 ± 0.03 | 152.5 ± 24.7 |
| Group 3 | 21.3 ± 1.7 | 22.8 ± 1.1 | 26.6 ± 1.8 | 0.190 ± 0.016 | 0.71 ± 0.04 | 149.1 ± 18.4 |
| Group 4 | 20.8 ± 1.4 | 24.1 ± 1.2 | 21.5 ± 1.4 ** | 0.128 ± 0.011 | 0.59 ± 0.02 ** | 467.5 ± 39.3 ** |
| Group 5 | 20.6 ± 1.5 | 24.2 ± 2.1 | 25.8 ± 1.7 | 0.168 ± 0.013 | 0.66 ± 0.01 *,a | 231.8 ± 25.6 b |
| Group 6 | 20.1 ± 1.8 | 22.5 ± 1.0 | 25.1 ± 1.4 | 0.176 ± 0.012 | 0.70 ± 0.03 b | 221.1 ± 22.5 b |
| Treatment Groups | Different Structural Chromosomal Aberrations Screened | Total Chromosomal Aberrations (%) | ||||
|---|---|---|---|---|---|---|
| X-Y Univalents | Autosomal Univalents | F/B | Polyploidy | MV | ||
| Group 1 | 8 | 6 | 2 | 2 | 1 | 3.16 ± 0.98 |
| Group 2 | 9 | 7 | 2 | 2 | 2 | 3.66 ± 1.21 |
| Group 3 | 8 | 5 | 3 | 1 | 2 | 3.16 ± 0.98 |
| Group 4 | 29 | 14 | 6 | 5 | 4 | 9.67 ± 1.94 ** |
| Group 5 | 16 | 8 | 4 | 1 | 2 | 5.16 ± 0.98 a |
| Group 6 | 13 | 4 | 4 | 1 | 1 | 3.83 ± 1.16 b |
| Group 7 | 32 | 16 | 6 | 3 | 5 | 10.33 ± 2.58 ## |
| Treatment Groups | Mobility (%) | Morphology (%) | Sperm Counts (106/mL) | ||||
|---|---|---|---|---|---|---|---|
| Fast | Slow | Immobile | Normal | Abnormal Heads | Abnormal Tails | ||
| Group 1 | 58.5 ± 2.9 | 21.1 ± 1.2 | 20.3 ± 2.4 | 89.6 ± 1.5 | 5.5 ± 1.1 | 4.8 ± 0.5 | 57.1 ± 4.3 |
| Group 2 | 48.5 ± 2.0 * | 23.3 ± 1.8 | 28.1 ± 0.4 * | 86.6 ± 1.2 | 6.8 ± 0.8 | 6.5 ± 0.8 | 51.3 ± 2.8 * |
| Group 3 | 56.1 ± 1.9 | 20.0 ± 2.5 | 23.8 ± 1.1 | 90.1 ± 1.3 | 5.3 ± 0.8 | 4.5 ± 0.5 | 54.8 ± 2.8 |
| Group 4 | 40.3 ± 3.5 ** | 24.3 ± 2.1 | 35.3 ± 2.2 ** | 74.6 ± 1.7 ** | 10.8 ± 1.1 ** | 14.5 ± 1.1 ** | 40.3 ± 2.1 ** |
| Group 5 | 37.5 ± 2.8 ** | 26.0 ± 2.1 | 36.5 ± 2.3 ** | 72.1 ± 2.7 ** | 13.1 ± 1.6 ** | 14.6 ± 1.3 ** | 33.3 ± 1.7 ** |
| Group 6 | 54.0 ± 1.5 b | 21.8 ± 0.8 | 24.1 ± 1.4 b | 89.0 ± 1.0 a | 5.4 ± 0.5 b | 5.6 ± 0.6 b | 56.3 ± 2.2 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshamrani, A.A.; Al-Hamamah, M.A.; Albekairi, N.A.; Attia, M.S.M.; Ahmad, S.F.; Assiri, M.A.; Ansari, M.A.; Nadeem, A.; Bakheet, S.A.; Alanazi, W.A.; et al. Impacts of the DPP-4 Inhibitor Saxagliptin and SGLT-2 Inhibitor Dapagliflozin on the Gonads of Diabetic Mice. Biomedicines 2023, 11, 2674. https://doi.org/10.3390/biomedicines11102674
Alshamrani AA, Al-Hamamah MA, Albekairi NA, Attia MSM, Ahmad SF, Assiri MA, Ansari MA, Nadeem A, Bakheet SA, Alanazi WA, et al. Impacts of the DPP-4 Inhibitor Saxagliptin and SGLT-2 Inhibitor Dapagliflozin on the Gonads of Diabetic Mice. Biomedicines. 2023; 11(10):2674. https://doi.org/10.3390/biomedicines11102674
Chicago/Turabian StyleAlshamrani, Ali A., Mohammed A. Al-Hamamah, Norah A. Albekairi, Mohamed S. M. Attia, Sheikh F. Ahmad, Mohammed A. Assiri, Mushtaq A. Ansari, Ahmed Nadeem, Saleh A. Bakheet, Wael A. Alanazi, and et al. 2023. "Impacts of the DPP-4 Inhibitor Saxagliptin and SGLT-2 Inhibitor Dapagliflozin on the Gonads of Diabetic Mice" Biomedicines 11, no. 10: 2674. https://doi.org/10.3390/biomedicines11102674
APA StyleAlshamrani, A. A., Al-Hamamah, M. A., Albekairi, N. A., Attia, M. S. M., Ahmad, S. F., Assiri, M. A., Ansari, M. A., Nadeem, A., Bakheet, S. A., Alanazi, W. A., & Attia, S. M. (2023). Impacts of the DPP-4 Inhibitor Saxagliptin and SGLT-2 Inhibitor Dapagliflozin on the Gonads of Diabetic Mice. Biomedicines, 11(10), 2674. https://doi.org/10.3390/biomedicines11102674






