Prognostic Significance of β-Catenin in Relation to the Tumor Immune Microenvironment in Oral Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Specimens
2.2. Immunohistochemistry (IHC)
2.3. Statistical Analysis
3. Results
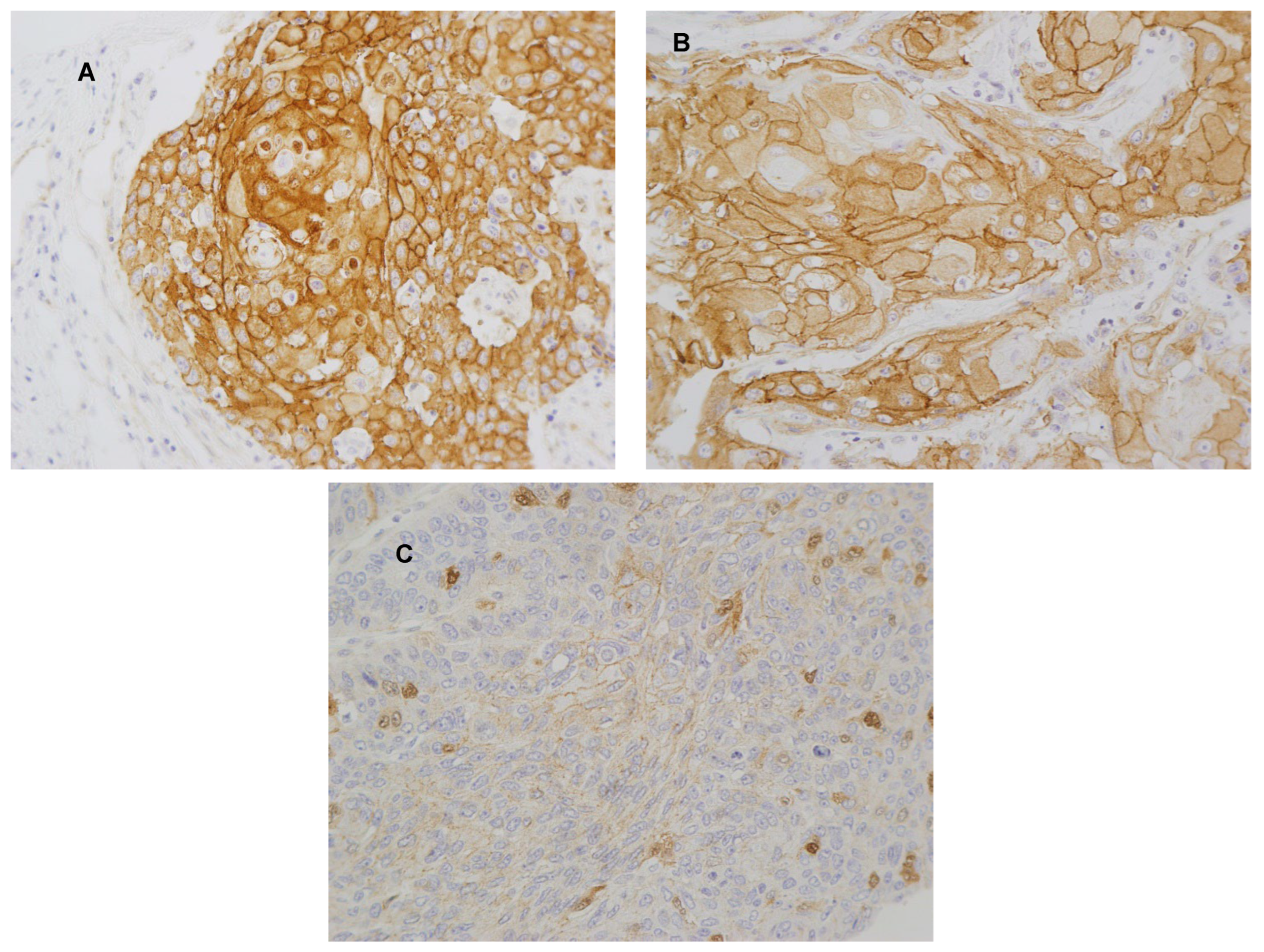
3.1. Immunohistochemical Analysis of β-Catenin, PD-L1, and CD8+ TIL Density in OSCC Patient Samples
3.2. Associations between the Expression of β-Catenin, PD-L1, and CD8+ TIL Density in OSCC
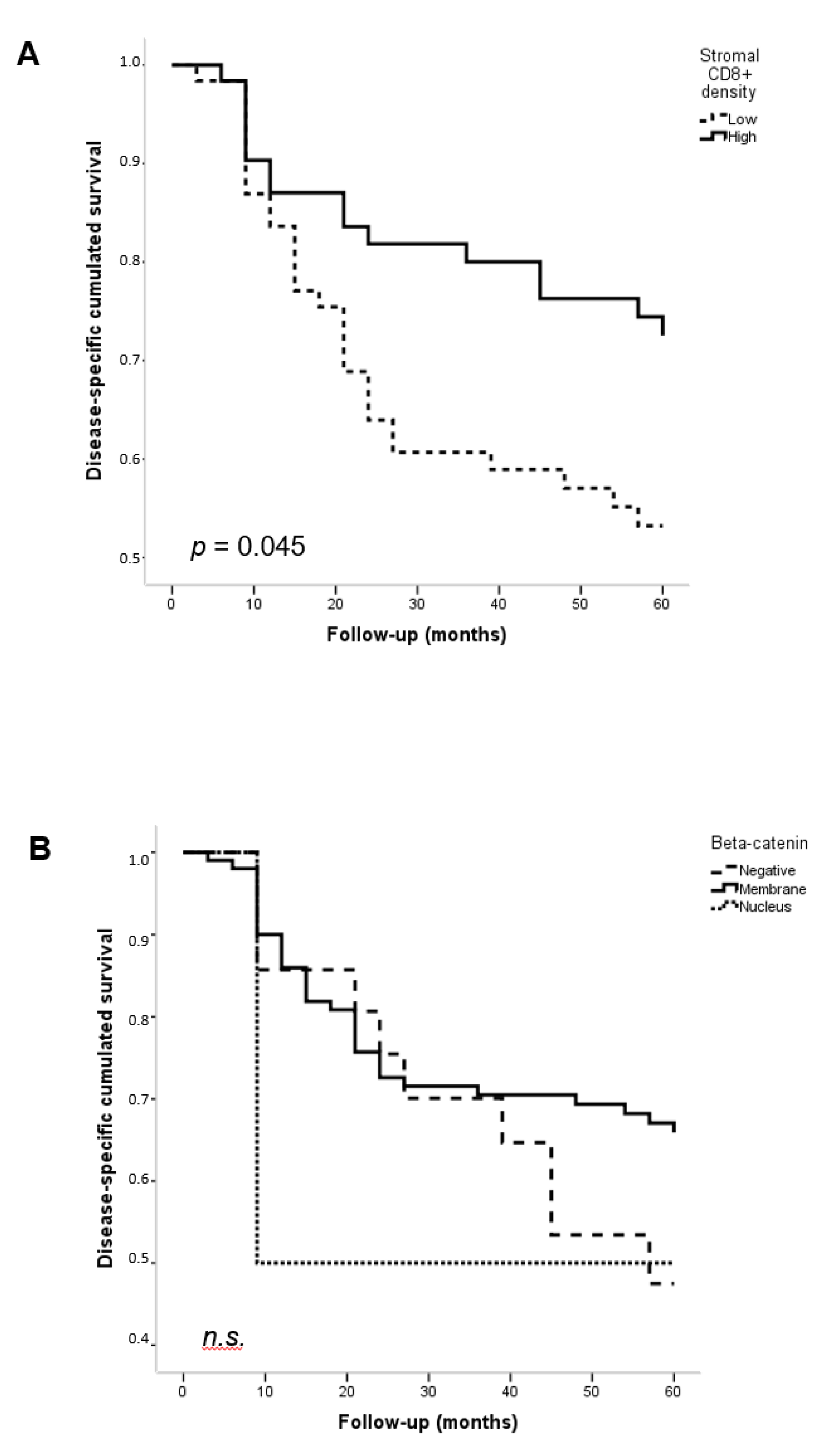
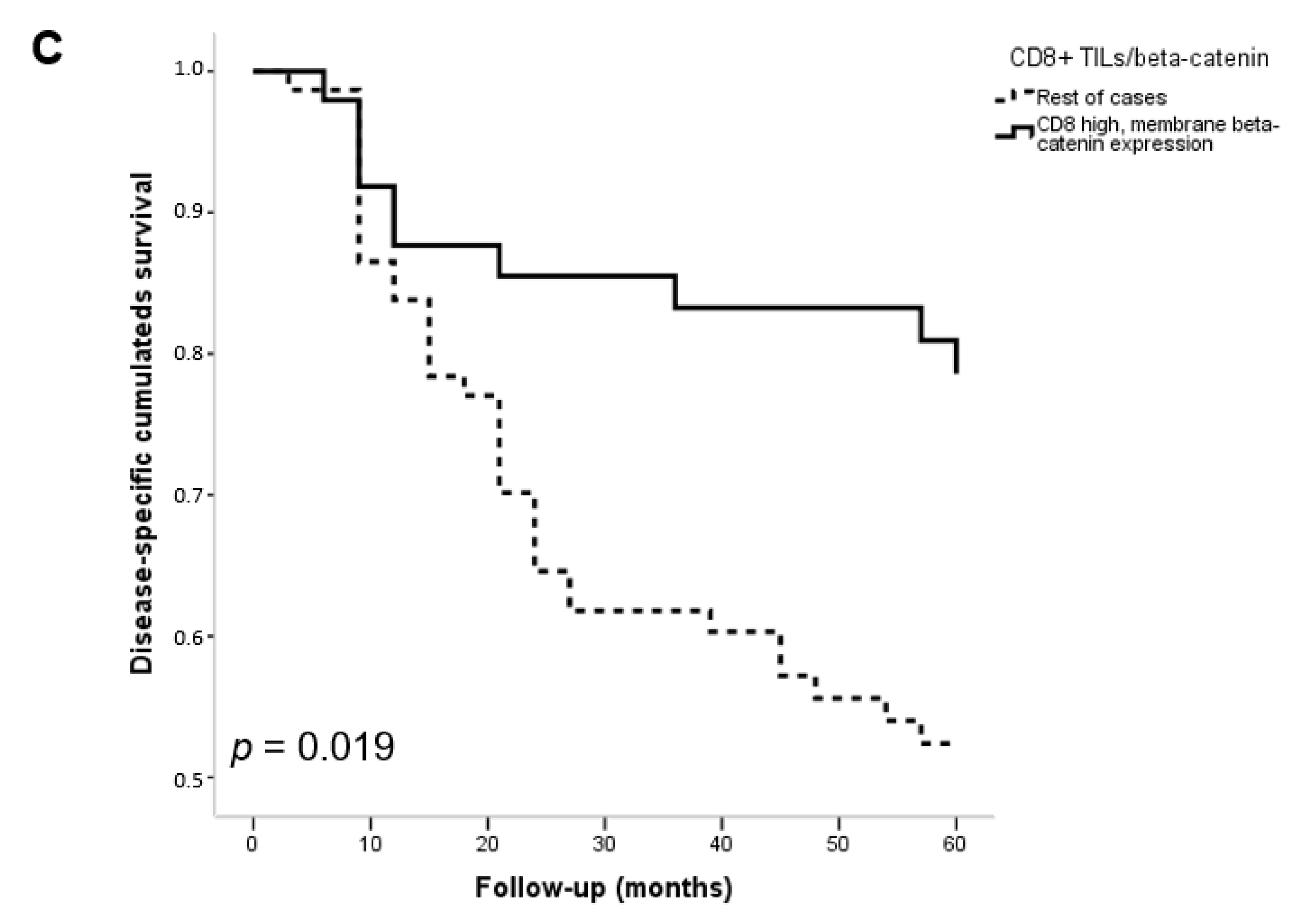
3.3. Associations with Clinicopathological Features and Patient Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Ethics Approval and Consent to Participate
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Freddie, B. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xiang, Y.; Li, F.; Yin, C.; Li, B.; Ke, V. WNT/β-Catenin signaling pathway regulating T cell-inflammation in the tumor microenvironment. Front. Immunol. 2019, 10, 2293. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, C.C.; Jin, L.; Zhang, X.D. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann. Oncol. 2016, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Luo, L.; Xu, C.; Yang, X.; Liu, T.; Luo, J.; Shi, W.; Yang, L.; Zheng, Y.; Yang, J. Tumor specificity of WNT ligands and receptors reveals universal squamous cell carcinoma oncogenes. BMC Cancer 2022, 22, 790. [Google Scholar] [CrossRef] [PubMed]
- Thommen, D.D.; Schumacher, T.N. T cell dysfunction in cancer. Cancer Cell 2018, 33, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Naito, Y.; Saito, K.; Shiiba, K.; Ohuchi, A.; Saigenji, K.; Nagura, H.; Ohtani, H. CD8+ T cells infiltrated within cancer cell nests as a prognostic factor in human colorectal cancer. Cancer Res. 1998, 58, 3491–3494. [Google Scholar]
- Du, L.; Lee, J.H.; Jiang, H.; Wang, C.; Wang, V.; Zheng, Z.; Shao, F.; Xu, D.; Xia, V.; Li, J.; et al. β-Catenin induces transcriptional expression of PD-L1 to promote glioblastoma immune evasion. J. Exp. Med. 2020, 217, e20191115. [Google Scholar] [CrossRef]
- Luke, J.J.; Bao, R.; Sweis, R.F.; Spranger, S.; Gajewski, T.F. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin. Cancer Res. 2019, 25, 3074–3083. [Google Scholar] [CrossRef]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell. 2009, 17, 9–26. [Google Scholar] [CrossRef]
- Xie, J.; Huang, L.; Lu, Y.G.; Zheng, D.L. Roles of the Wnt signaling pathway in head and neck squamous cell carcinoma. Front. Mol. Biosci. 2021, 7, 590912. [Google Scholar] [CrossRef] [PubMed]
- Patni, A.P.; Harishankar, M.K.; Joseph, J.P.; Sreeshma, B.; Jayaraj, R.; Devi, A. Comprehending the crosstalk between Notch, Wnt and Hedgehog signaling pathways in oral squamous cell carcinoma—Clinical implications. Cell Oncol. 2021, 44, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Cadigan, K.M.; Waterman, M.L. TCF/LEFs and Wnt signaling in the nucleus. Cold Spring Harb. Perspect. Biol. 2012, 4, a007906. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, C.; Liu, F.; Ou, Y. Clinical significance of activated Wnt/β-catenin signaling in apoptosis inhibition of oral cancer. Open Life Sci. 2021, 16, 1045–1052. [Google Scholar] [CrossRef]
- Iwai, S.; Yonekawa, A.; Harada, C.; Hamada, M.; Katagiri, W.; Nakazawa, M.; Yura, Y. Involvement of the Wnt-β-catenin pathway in invasion and migration of oral squamous carcinoma cells. Int. J. Oncol. 2010, 37, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Noguti, J.D.E.; Moura, C.F.; Hossaka, T.A.; Franco, M.; Oshima, C.T.; Dedivitis, R.A.; Ribeiro, D.A. The role of canonical WNT signaling pathway in oral carcinogenesis: A comprehensive review. Anticancer Res. 2012, 32, 873–878. [Google Scholar]
- Ramos-García, P.; González-Moles, M.Á. Prognostic and clinicopathological significance of the aberrant expression of β-catenin in oral squamous cell carcinoma: A systematic review and meta-analysis. Cancers 2022, 14, 479. [Google Scholar] [CrossRef]
- Ridge, J.A.; Lydiatt, W.M.; Patel, S.G.; Glastonbury, C.M.; Brandwein-Gensler, M.; Gossein, R.A.; Shah, J.P. Oral Cavity. In AJCC Cancer Staging Manual, 8th ed.; Editor Amin, M.B., Ed.; Springer: Chicago, IL, USA, 2017; pp. 79–94. [Google Scholar]
- Müller, S. Update from the 4th edition of the World Health Organization of Head and Neck Tumours: Tumours of the oral cavity and mobile tongue. Head Neck Pathol. 2017, 11, 33–40. [Google Scholar] [CrossRef]
- Lequerica-Fernández, P.; Suárez-Canto, J.; Rodríguez-Santamarta, T.; Rodrigo, J.P.; Suárez-Sánchez, F.J.; Blanco-Lorenzo, V.; Domínguez-Iglesias, F.; García-Pedrero, J.M.; de Vicente, J.C. Prognostic relevance of CD4+, CD8+ and FOXP3+ TILs in oral squamous cell carcinoma and correlations with PD-L1 and cancer stem cell markers. Biomedicines 2021, 9, 653. [Google Scholar] [CrossRef]
- Suárez-Sánchez, F.J.; Lequerica-Fernández, P.; Suárez-Canto, J.; Rodrigo, J.P.; Rodríguez-Santamarta, T.; Domínguez-Iglesias, F.; García-Pedrero, J.; de Vicente, J.C. Macrophages in oral carcinomas: Relationship with cancer stem cell markers and PD-L1 expression. Cancers 2020, 12, 1764. [Google Scholar] [CrossRef]
- Suárez-Sánchez, F.J.; Lequerica-Fernández, P.; Rodrigo, J.P.; Hermida-Prado, F.; Suárez-Canto, J.; Rodríguez-Santamarta, T.; Domínguez-Iglesias, F.; García-Pedrero, J.; de Vicente, J.C. Tumor-infiltrating CD20+ B lymphocytes: Significance and prognostic implications in oral cancer microenvironment. Cancers 2021, 13, 395. [Google Scholar] [CrossRef] [PubMed]
- de Vicente, J.C.; Rodríguez-Santamarta, T.; Rodrigo, J.P.; Blanco-Lorenzo, V.; Allonca, E.; García-Pedrero, J.M. PD-L1 expression in tumor cells is an independent unfavorable prognostic factor in oral squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2019, 28, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.W.; Ngiow, S.F.; Ribas, A.; Smyth, M.J. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015, 75, 2139–2145. [Google Scholar] [CrossRef] [PubMed]
- Steinhart, Z.; Angers, S. Wnt signaling in development and tissue homeostasis. Development 2018, 145, dev146589. [Google Scholar] [CrossRef]
- Shang, S.; Hua, F.; Hu, Z.W. The regulation of beta-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef]
- Huber, A.H.; Weis, W.I. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell 2001, 105, 391–402. [Google Scholar] [CrossRef]
- Akinyamoju, A.O.; Lawal, A.O.; Adisa, A.O.; Adeyemi, B.F.; Kolude, B.J. Immunohistochemical expression of E-cadherin and β-catenin in oral squamous cell carcinoma. West Afr. Coll. Surg. 2023, 13, 43–47. [Google Scholar] [CrossRef]
- Laxmidevi, L.B.; Angadi, P.V.; Pillai, R.K.; Chandreshekar, C. Aberrant β-catenin expression in the histologic differentiation of oral squamous cell carcinoma and verrucous carcinoma: An immunohistochemical study. J. Oral Sci. 2010, 52, 633–640. [Google Scholar] [CrossRef][Green Version]
- Zaid, K.W. Immunohistochemical assessment of E-cadherin and β-catenin in the histological differentiations of oral squamous cell carcinoma. Asian Pac. J. Cancer Prev. 2014, 15, 8847–8853. [Google Scholar] [CrossRef]
- Al-Rawi, N.; Al Ani, M.; Quadri, A.; Hamdoon, Z.; Awwad, A.; Al Kawas, S.; Al Nuaimi, A. Prognostic significance of E-Cadherin, β-Catenin and cyclin D1 in oral squamous cell carcinoma: A tissue microarray study. Histol. Histopathol. 2021, 36, 1073–1083. [Google Scholar] [CrossRef]
- Mahomed, F.; Altini, M.; Meer, S. Altered E-cadherin/beta-catenin expression in oral squamous carcinoma with and without nodal metastasis. Oral Dis. 2007, 13, 386–392. [Google Scholar] [CrossRef]
- Tanaka, N.; Odajima, T.; Ogi, K.; Ikeda, T.; Satoh, M. Expression of E-cadherin, alpha-catenin, and beta-catenin in the process of lymph node metastasis in oral squamous cell carcinoma. Br. J. Cancer 2003, 89, 557–563. [Google Scholar] [CrossRef]
- Zhao, X.J.; Li, H.; Chen, H.; Liu, Y.X.; Zhang, L.H.; Liu, S.X.; Feng, Q.L. Expression of e-cadherin and beta-catenin in human esophageal squamous cell carcinoma: Relationships with prognosis. World J. Gastroenterol. 2003, 9, 225–232. [Google Scholar] [CrossRef]
- Pirinen, R.T.; Hirvikoski, P.; Johansson, R.T.; Hollmén, S.; Kosma, V.M. Reduced expression of alpha-catenin, beta-catenin, and gamma-catenin is associated with high cell proliferative activity and poor differentiation in non-small cell lung cancer. J. Clin. Pathol. 2001, 54, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Pukkila, M.J.; Virtaniemi, J.A.; Kumpulainen, E.J.; Pirinen, R.T.; Johansson, R.T.; Valtonen, H.J.; Juhola, M.T.; Kosma, V.M. Nuclear beta catenin expression is related to unfavourable outcome in oropharyngeal and hypopharyngeal squamous cell carcinoma. J. Clin. Pathol. 2001, 54, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Marinkova, R.; Nenkov, M.; Jin, L.; Huber, O.; Sonnemann, J.; Peca, N.; Gabler, N.; Chen, Y. Tumor-intrinsic PD-L1 exerts an oncogenic function through the activation of the Wnt/β-catenin pathway in human non-small cell lung cancer. Int. J. Mol. Sci. 2022, 23, 11031. [Google Scholar] [CrossRef]
- Fu, L.; Fan, J.; Maity, S.; McFadden, G.; Shi, Y.; Kong, W. PD-L1 interacts with Frizzled 6 to activate β-catenin and form a positive feedback loop to promote cancer stem cell expansion. Oncogene 2022, 41, 1100–1113. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Canteli, M.; Juesas, L.; Garmendia, V.; Otero-Rosales, M.; Calvo, A.; Alvarez-Fernández, M.; Astudillo, A.; Montuenga, V.; García-Pedrero, J.M.; Rodrigo, J.P. Tumor-intrinsic nuclear β-catenin associates with an immune ignorance phenotype and a poorer prognosis in head and neck squamous cell carcinomas. Int. J. Mol. Sci. 2022, 23, 11559. [Google Scholar] [CrossRef]
- Anastas, J.N.; Moon, R.T. WNT signalling pathways as therapeutic targets in cancer. Nat. Rev. Cancer 2013, 13, 11–26. [Google Scholar] [CrossRef]
- Han, C.; Fu, Y.X. β-Catenin regulates tumor-derived PD-L1. J. Exp. Med. 2020, 217, e20200684. [Google Scholar] [CrossRef]
- Fu, C.; Liang, X.; Cui, W.; Ober-Blöbaum, J.L.; Vazzana, J.; Shrikant, P.A.; Lee, K.P.; Clausen, B.E.; Mellman, I.; Jiang, A. β-catenin in dendritic cells exerts opposite functions in cross-priming and maintenance of CD8+ T cells through regulation of IL-10. Proc. Natl. Acad. Sci. USA 2015, 112, 2823–2828. [Google Scholar] [CrossRef] [PubMed]
- van Loosdregt, J.; Fleskens, V.; Tiemessen, M.M.; Mokry, M.; van Boxtel, R.; Meerding, J.; Pals, C.E.G.M.; Kurek, D.; Baert, M.R.M.; Delemarre, E.M.; et al. Canonical Wnt signaling negatively modulates regulatory T cell function. Immunity 2013, 39, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Kaler, P.; Augenlicht, V.; Klampfer, L. Activating mutations in β-catenin in colon cancer cells alter their interaction with macrophages; the role of snail. PLoS ONE 2012, 7, e45462. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Liang, Y.; Anders, R.A.; Taube, J.M.; Qiu, X.; Mulgaonkar, A.; Liu, X.; Harrington, S.M.; Guo, J.; Xin, Y.; et al. PD-L1 on host cells is essential for PD-L1 blockade-mediated tumor regression. J. Clin. Investig. 2018, 128, 580–588. [Google Scholar] [CrossRef]
- Rhee, C.S.; Sen, M.; Lu, D.; Wu, C.; Leoni, L.; Rubin, J.; Corr, M.; Carson, D.A. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene 2002, 21, 6598–6605. [Google Scholar] [CrossRef] [PubMed]
- Prgomet, V.; Andersson, V.; Lindberg, P. Higher expression of WNT5A protein in oral squamous cell carcinoma compared with dysplasia and oral mucosa with a normal appearance. Eur. J. Oral Sci. 2017, 125, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yan, Y.; Gu, M.; Wang, X.; Zhu, H.; Zhang, S.; Wang, W. High expression levels of Wnt5a and Ror2 in laryngeal squamous cell carcinoma are associated with poor prognosis. Oncol. Lett. 2017, 14, 2232–2238. [Google Scholar] [CrossRef][Green Version]
- Sakamoto, T.; Kawano, S.; Matsubara, R.; Goto, Y.; Jinno, T.; Maruse, Y.; Kaneko, N.; Hashiguchi, Y.; Hattori, T.; Tanaka, S.; et al. Critical roles of Wnt5a-Ror2 signaling in aggressiveness of tongue squamous cell carcinoma and production of matrix metalloproteinase-2 via ΔNp63β-mediated epithelial-mesenchymal transition. Oral Oncol. 2017, 69, 15–25. [Google Scholar] [CrossRef]
- Xie, H.; Ma, Y.; Li, J.; Chen, H.; Xie, V.; Chen, M.; Zhao, X.; Tang, S.; Zhao, S.; Zhang, Y.; et al. WNT7A Promotes EGF-induced migration of oral squamous cell carcinoma cells by activating β-catenin/MMP9-mediated signaling. Front. Pharmacol. 2020, 11, 98. [Google Scholar] [CrossRef]
- Trujillo, J.A.; Sweis, R.F.; Bao, R.; Luke, J.J. T cell-inflamed versus non-T cell-inflamed tumors: A conceptual framework for cancer immunotherapy drug development and combination therapy selection. Cancer Immunol. Res. 2018, 6, 990–1000. [Google Scholar] [CrossRef]
| Variable | Number (%) |
| Age (year) (mean ± SD; median; range) | 58.69 ± 14.34; 57; 28–91 |
| Gender | |
| Men | 82 (66) |
| Women | 43 (34) |
| Tobacco use | |
| Smoker | 84 (67) |
| Non-smoker | 41 (33) |
| Alcohol use | |
| Drinker | 69 (55) |
| Non-drinker | 56 (45) |
| Location of oral squamous oral cell carcinoma | |
| Tongue | 51 (41) |
| Floor of the mouth | 37 (30) |
| Gum | 22 (18) |
| Buccal | 7 (5) |
| Retromolar | 6 (5) |
| Palate | 2 (1) |
| Tumor status | |
| pT1 | 27 (22) |
| pT2 | 54 (43) |
| pT3 | 16 (13) |
| pT4 | 28 (22) |
| Nodal status | |
| pN0 | 76 (61) |
| pN1 | 25 (20) |
| pN2 | 24 (19) |
| Clinical stage | |
| Stage I | 20 (16) |
| Stage II | 32 (26) |
| Stage III | 26 (21) |
| Stage IV | 47 (37) |
| G status | |
| G1 | 80 (64) |
| G2 | 41 (33) |
| G3 | 4 (3) |
| CD8+ Compartment | β-Catenin Expression | No. Cases | Mean CD8+ TIL Density (SD) | p |
| Stromal | Negative Membrane Nucleus | 21 102 2 | 280.15 (279.15) 160.43 (179.83) 30.00 (41.48) | 0.020 |
| Tumoral | Negative Membrane Nucleus | 21 102 2 | 67.41 (54.74) 44.63 (57.40) 6.83 (3.50) | 0.029 |
| Type of Immune TME | β-Catenin Expression | p | ||
|---|---|---|---|---|
| Negative | Membrane | Nucleus | ||
| Type I (PD-L1+/CD8+ high) | 3 (16%) | 10 (10%) | 0 (0%) | |
| Type II (PD-L1−/CD8+ low) | 1 (5%) | 37 (37%) | 2 (100%) | 0.012 |
| Type III (PD-L1+/CD8+ low) | 2 (11%) | 3 (3%) | 0 (0%) | |
| Type IV (PD-L1−/CD8+ high) | 13 (68%) | 51 (50%) | 0 (0%) | |
| Mean (SD) | TILs | p | ||
|---|---|---|---|---|
| Negative | Mild-Moderate | Intense | ||
| Stromal CD68+ | 110.55 (73.53) | 140.56 (94.67) | 118.72 (54.29) | 0.20 |
| Tumoral CD68+ | 37.86 (36.65) | 61.39 (47.64) | 115.66 (36.22) | <0.0001 |
| Stromal CD163+ | 165.35 (90.93) | 172.52 (109.10) | 149.66 (81.73) | 0.92 |
| Tumoral CD163+ | 25.79 (27.58) | 36.19 (28.62) | 54.33 (32.04) | 0.006 |
| Stromal CD8+ | 168.86 (203.92) | 202.73 (211.62) | 83.66 (100.49) | 0.11 |
| Tumoral CD8+ | 34.65 (46.44) | 62.46 (64.67) | 76.27 (70.72) | 0.02 |
| Stromal CD20+ | 36.15 (73.23) | 54.48 (88.84) | 17.50 (35.96) | 0.06 |
| Tumoral CD20+ | 1.21 (2.98) | 2.34 (3.87) | 0.50 (0.91) | 0.26 |
| Stromal CD4+ | 52.81 (78.38) | 61.48 (56.03) | 19.27 (8.58) | 0.08 |
| Tumoral CD4+ | 4.66 (10.40) | 8.43 (14.83) | 1.38 (1.28) | 0.05 |
| Stromal FOXP3+ | 12.62 (18.38) | 19.65 (31.70) | 17.16 (15.30) | 0.51 |
| Tumoral FOXP3+ | 2.10 (3.66) | 4.30 (9.07) | 4.83 (4.54) | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lequerica-Fernández, P.; Rodríguez-Santamarta, T.; García-García, E.; Blanco-Lorenzo, V.; Torres-Rivas, H.E.; Rodrigo, J.P.; Suárez-Sánchez, F.J.; García-Pedrero, J.M.; De Vicente, J.C. Prognostic Significance of β-Catenin in Relation to the Tumor Immune Microenvironment in Oral Cancer. Biomedicines 2023, 11, 2675. https://doi.org/10.3390/biomedicines11102675
Lequerica-Fernández P, Rodríguez-Santamarta T, García-García E, Blanco-Lorenzo V, Torres-Rivas HE, Rodrigo JP, Suárez-Sánchez FJ, García-Pedrero JM, De Vicente JC. Prognostic Significance of β-Catenin in Relation to the Tumor Immune Microenvironment in Oral Cancer. Biomedicines. 2023; 11(10):2675. https://doi.org/10.3390/biomedicines11102675
Chicago/Turabian StyleLequerica-Fernández, Paloma, Tania Rodríguez-Santamarta, Eduardo García-García, Verónica Blanco-Lorenzo, Héctor E. Torres-Rivas, Juan P. Rodrigo, Faustino J. Suárez-Sánchez, Juana M. García-Pedrero, and Juan Carlos De Vicente. 2023. "Prognostic Significance of β-Catenin in Relation to the Tumor Immune Microenvironment in Oral Cancer" Biomedicines 11, no. 10: 2675. https://doi.org/10.3390/biomedicines11102675
APA StyleLequerica-Fernández, P., Rodríguez-Santamarta, T., García-García, E., Blanco-Lorenzo, V., Torres-Rivas, H. E., Rodrigo, J. P., Suárez-Sánchez, F. J., García-Pedrero, J. M., & De Vicente, J. C. (2023). Prognostic Significance of β-Catenin in Relation to the Tumor Immune Microenvironment in Oral Cancer. Biomedicines, 11(10), 2675. https://doi.org/10.3390/biomedicines11102675






