Cardiovascular Complications of Viral Respiratory Infections and COVID-19
Abstract
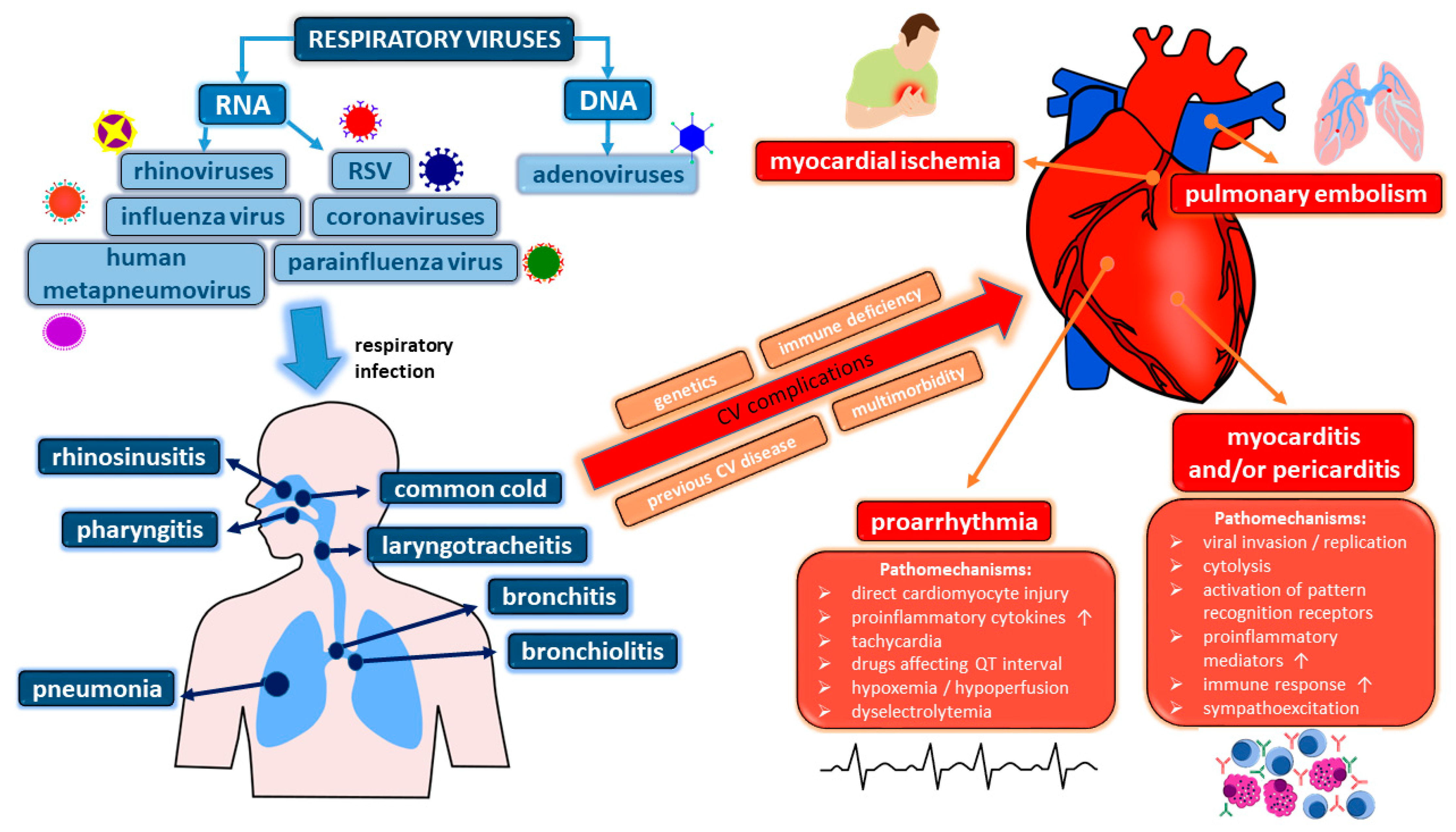
1. Introduction
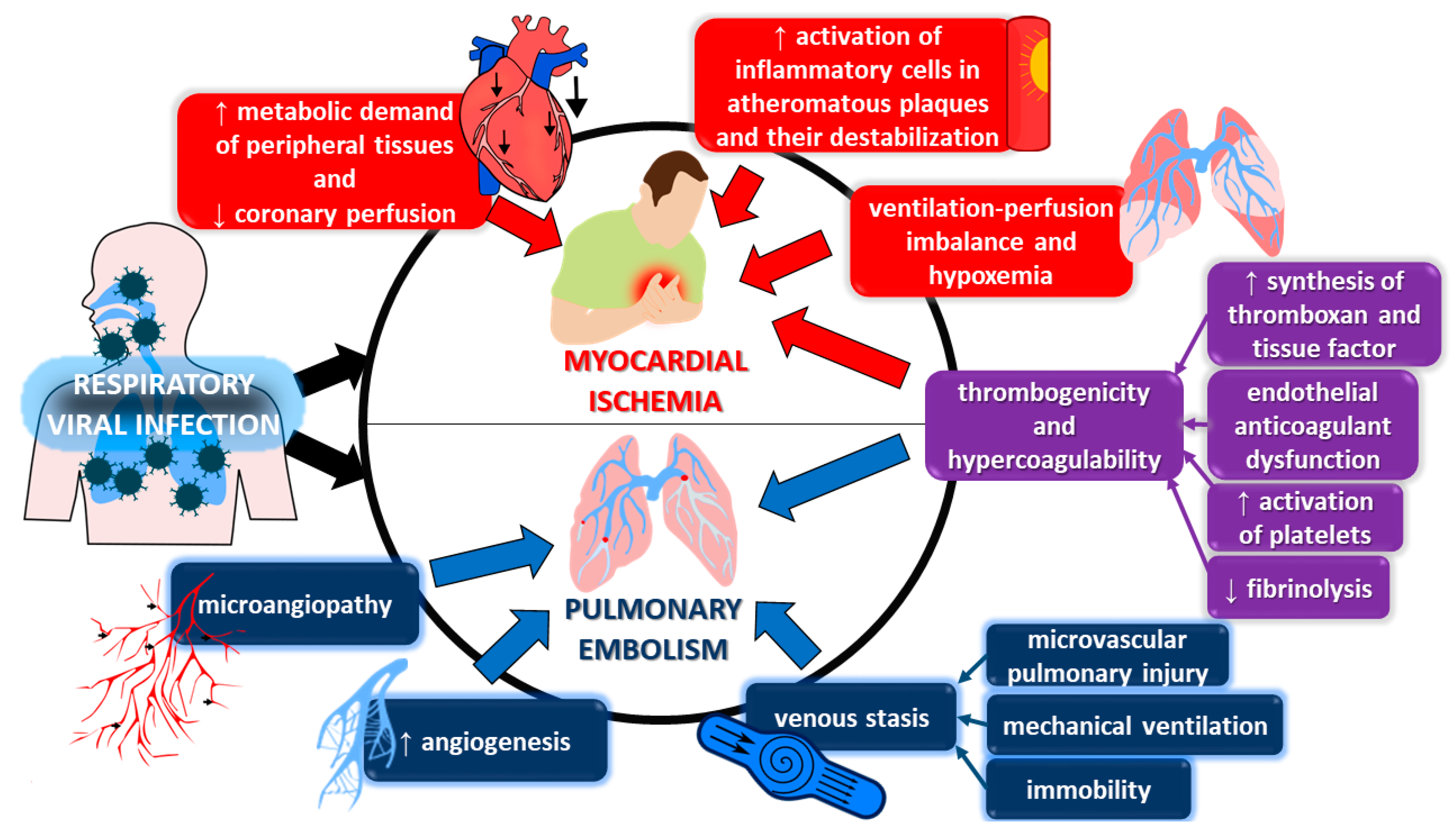
2. Ischemic Complications
3. Thromboembolism
4. Viral Myocarditis and (Post-)Inflammatory Cardiomyopathy
5. Pericardial Disease
6. Pro-Arrhythmia
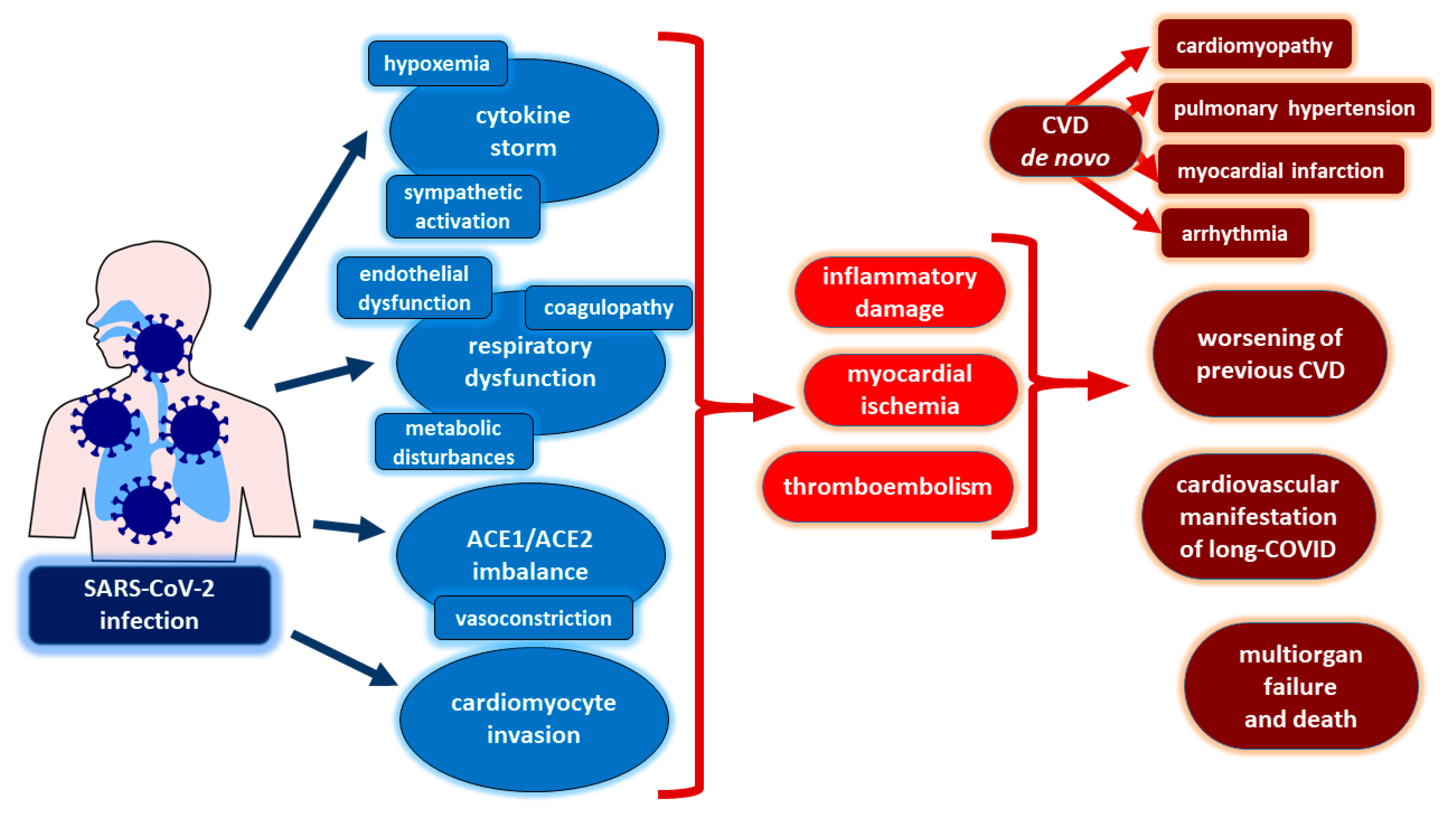
7. Coronaviruses and Severe Acute Respiratory Syndrome Coronavirus 2
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Charlton, C.L.; Babady, E.; Ginocchio, C.C.; Hatchette, T.F.; Jerris, R.C.; Li, Y.; Loeffelholz, M.; McCarter, Y.S.; Miller, M.B.; Novak-Weekley, S.; et al. Practical Guidance for Clinical Microbiology Laboratories: Viruses Causing Acute Respiratory Tract Infections. Clin. Microbiol. Rev. 2018, 32, e00042-18. [Google Scholar] [CrossRef]
- Varghese, B.M.; Dent, E.; Chilver, M.; Cameron, S.; Stocks, N.P. Epidemiology of viral respiratory infections in Australian working-age adults (20–64 years): 2010–2013. Epidemiol. Infect. 2018, 146, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Lam, T.T.; Zaraket, H.; Lipkin, W.I.; Drews, S.J.; Hatchette, T.F.; Heraud, J.M.; Koopmans, M.P.; INSPIRE Investigators. Global epidemiology of non-influenza RNA respiratory viruses: Data gaps and a growing need for surveillance. Lancet Infect. Dis. 2017, 17, e320–e326. [Google Scholar] [CrossRef]
- GBD 2013 DALYs and HALE Collaborators; Murray, C.J.; Barber, R.M.; Foreman, K.J.; Abbasoglu Ozgoren, A.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Abraham, J.P.; Abubakar, I.; et al. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: Quantifying the epidemiological transition. Lancet 2015, 386, 2145–2191. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Wunderink, R. Severe Respiratory Viral Infections. Infect. Dis. Clin. N. Am. 2017, 31, 455–474. [Google Scholar] [CrossRef]
- Jain, S.; Self, W.H.; Wunderink, R.G.; Fakhran, S.; Balk, R.; Bramley, A.M.; Reed, C.; Grijalva, C.G.; Anderson, E.J.; Courtney, D.M.; et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N. Engl. J. Med. 2015, 373, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Góes, L.G.; Zerbinati, R.M.; Tateno, A.F.; de Souza, A.V.; Ebach, F.; Corman, V.M.; Moreira-Filho, C.A.; Durigon, E.L.; da Silva Filho, L.V.; Drexler, J.F. Typical epidemiology of respiratory virus infections in a Brazilian slum. J. Med. Virol. 2020, 92, 1316–1321. [Google Scholar] [CrossRef]
- Eiros, J.M.; Ortiz de Lejarazu, R.; Tenorio, A.; Casas, I.; Pozo, F.; Ruiz, G.; Pérez-Breña, P. Microbiological diagnosis of viral respiratory infections. Enferm. Infecc. Microbiol. Clin. 2009, 27, 168–177. [Google Scholar] [CrossRef]
- Naz, R.; Gul, A.; Javed, U.; Urooj, A.; Amin, S.; Fatima, Z. Etiology of acute viral respiratory infections common in Pakistan: A review. Rev. Med. Virol. 2019, 29, e2024. [Google Scholar] [CrossRef]
- Ambrosioni, J.; Bridevaux, P.O.; Wagner, G.; Mamin, A.; Kaiser, L. Epidemiology of viral respiratory infections in a tertiary care centre in the era of molecular diagnosis, Geneva, Switzerland, 2011–2012. Clin. Microbiol. Infect. 2014, 20, O578–O584. [Google Scholar] [CrossRef]
- Subbarao, K.; Mahanty, S. Respiratory Virus Infections: Understanding COVID-19. Immunity 2020, 52, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction (2018). Circulation 2018, 138, e618–e651. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.J.; Pant, S.; Deshmukh, A.J.; Nalluri, N.; Badheka, A.O.; Shah, N.; Chothani, A.; Savani, G.T.; Schwartz, C.; Duvvuri, S.; et al. Seasonal variation of acute myocardial infarction related hospitalizations in the United States: Perspective over the last decade. Int. J. Cardiol. 2014, 172, e441–e442. [Google Scholar] [CrossRef]
- Davidson, J.; Warren-Gash, C. Cardiovascular complications of acute respiratory infections: Current research and future directions. Expert Rev. Anti-Infect. Ther. 2019, 17, 939–942. [Google Scholar] [CrossRef]
- Madjid, M.; Safavi-Naeini, P.; Solomon, S.D.; Vardeny, O. Potential effects of coronaviruses on the cardiovascular system. JAMA Cardiol. 2020, 5, 831–840. [Google Scholar] [CrossRef]
- Lippi, G.; Sanchis-Gomar, F. Cardiac troponin elevation in patients with influenza virus infections. Biomed. J. 2021, 44, 183–189. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811–818. [Google Scholar] [CrossRef]
- Warren-Gash, C.; Blackburn, R.; Whitaker, H.; McMenamin, J.; Hayward, A.C. Laboratory-Confirmed Respiratory Infections as Triggers for Acute Myocardial Infarction and Stroke: A Self-Controlled Case Series Analysis of National Linked Datasets from Scotland. Eur. Respir. J. 2018, 51, 1701–1794. [Google Scholar] [CrossRef]
- Davidson, J.A.; Banerjee, A.; Smeeth, L.; McDonald, H.I.; Grint, D.; Herrett, E.; Forbes, H.; Pebody, R.; Warren-Gash, C. Risk of acute respiratory infection and acute cardiovascular events following acute respiratory infection among adults with increased cardiovascular risk in England between 2008 and 2018: A retrospective, population-based cohort study. Lancet Digit. Health 2021, 3, e773–e783. [Google Scholar] [CrossRef]
- Kwong, J.C.; Schwartz, K.L.; Campitelli, M.A.; Chung, H.; Crowcroft, N.S.; Karnauchow, T.; Katz, K.; Ko, D.T.; McGeer, A.J.; McNally, D.; et al. Acute Myocardial Infarction after Laboratory-Confirmed Influenza Infection. N. Engl. J. Med. 2018, 378, 345–353. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Alvarez, K.N.; Weissfeld, L.A.; Angus, D.C.; Chirinos, J.A.; Chang, C.-C.H.; Newman, A.; Loehr, L.; Folsom, A.R.; Elkind, M.S.; et al. Association Between Hospitalization for Pneumonia and Subsequent Risk of Cardiovascular Disease. JAMA 2015, 313, 264–274. [Google Scholar] [CrossRef]
- Musher, D.M.; Abers, M.S.; Corrales-Medina, V.F. Acute Infection and Myocardial Infarction. N. Engl. J. Med. 2019, 380, 171–176. [Google Scholar] [CrossRef]
- Corrales-Medina, V.F.; Madjid, M.; Musher, D.M. Role of acute infection in triggering acute coronary syndromes. Lancet Infect. Dis. 2010, 10, 83–92. [Google Scholar] [CrossRef]
- Bazaz, R.; Marriott, H.M.; Francis, S.E.; Dockrell, D.H. Mechanistic links between acute respiratory tract infections and acute coronary syndromes. J. Infect. 2013, 66, 1–17. [Google Scholar] [CrossRef]
- Smeeth, L.; Cook, C.; Thomas, S.; Hall, A.J.; Hubbard, R.; Vallance, P. Risk of deep vein thrombosis and pulmonary embolism after acute infection in a community setting. Lancet 2006, 367, 1075–1079. [Google Scholar] [CrossRef]
- Beristain-Covarrubias, N.; Perez-Toledo, M.; Thomas, M.R.; Henderson, I.R.; Watson, S.P.; Cunningham, A.F. Understanding Infection-Induced Thrombosis: Lessons Learned From Animal Models. Front. Immunol. 2019, 10, 2569. [Google Scholar] [CrossRef]
- Di Minno, A.; Ambrosino, P.; Calcaterra, I.; Di Minno, M.N. COVID-19 and Venous Thromboembolism: A Meta-analysis of Literature Studies. Semin. Thromb. Hemost. 2020, 46, 763–771. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Voicu, S.; Ketfi, C.; Stépanian, A.; Chousterman, B.G.; Mohamedi, N.; Siguret, V.; Mebazaa, A.; Mégarbane, B.; Bonnin, P. Pathophysiological Processes Underlying the High Prevalence of Deep Vein Thrombosis in Critically Ill COVID-19 Patients. Front. Physiol. 2021, 11, 608–788. [Google Scholar] [CrossRef]
- Tschöpe, C.; Ammirati, E.; Bozkurt, B.; Caforio, A.L.; Cooper, L.T.; Felix, S.B.; Hare, J.M.; Heidecker, B.; Heymans, S.; Hübner, N.; et al. Myocarditis and inflammatory cardiomyopathy: Current evidence and future directions. Nat. Rev. Cardiol. 2021, 18, 169–193. [Google Scholar] [CrossRef]
- Gil-Cruz, C.; Perez-Shibayama, C.; De Martin, A.; Ronchi, F.; van der Borght, K.; Niederer, R.; Onder, L.; Lütge, M.; Novkovic, M.; Nindl, V.; et al. Microbiota- derived peptide mimics drive lethal inflammatory cardiomyopathy. Science 2019, 366, 881–886. [Google Scholar] [CrossRef]
- Pollack, A.; Kontorovich, A.R.; Fuster, V.; Dec, G.W. Viral myocarditis-diagnosis, treatment options, and current controversies. Nat. Rev. Cardiol. 2015, 12, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Kobak, K.A.; Franczuk, P.; Schubert, J.; Dzięgała, M.; Kasztura, M.; Tkaczyszyn, M.; Drozd, M.; Kosiorek, A.; Kiczak, L.; Bania, J.; et al. Primary Human Cardiomyocytes and Cardiofibroblasts Treated with Sera from Myocarditis Patients Exhibit an Increased Iron Demand and Complex Changes in the Gene Expression. Cells 2021, 10, 818. [Google Scholar] [CrossRef]
- Kühl, U.; Pauschinger, M.; Bock, T.; Klingel, K.; Schwimmbeck, C.P.; Seeberg, B.; Krautwurm, L.; Poller, W.; Schultheiss, H.-P.; Kandolf, R. Parvovirus B19 Infection Mimicking Acute Myocardial Infarction. Circulation 2003, 108, 945–950. [Google Scholar] [CrossRef]
- Kuhl, U.; Pauschinger, M.; Noutsias, M. High Prevalence of Viral Genomes and Multiple Viral Infections in the Myocardium of Adults with “Idiopathic” Left Ventricular Dysfunction. Circulation 2005, 111, 887–893. [Google Scholar] [CrossRef]
- Caforio, A.L.; Malipiero, G.; Marcolongo, R.; Iliceto, S. Myocarditis: A Clinical Overview. Curr. Cardiol. Rep. 2017, 19, 63. [Google Scholar] [CrossRef]
- He, Y.; Chipman, P.R.; Howitt, J.; Bator, C.M.; Whitt, M.A.; Baker, T.S.; Kuhn, R.J.; Anderson, C.W.; Freimuth, P.; Rossmann, M.G. Interaction of coxsackievirus B3 with the full length coxsackievirus-adenovirus receptor. Nat. Struct. Biol. 2001, 8, 874–878. [Google Scholar] [CrossRef]
- Badorff, C.; Lee, G.-H.; Lamphear, B.J.; Martone, M.E.; Campbell, K.P.; Rhoads, R.E.; Knowlton, K.U. Enteroviral protease 2A cleaves dystrophin: Evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat. Med. 1999, 5, 320–326. [Google Scholar] [CrossRef]
- Kühl, U.; Lassner, D.; von Schlippenbach, J.; Poller, W.; Schultheiss, H.-P. Interferon-Beta Improves Survival in Enterovirus-Associated Cardiomyopathy. J. Am. Coll. Cardiol. 2012, 60, 1295–1296. [Google Scholar] [CrossRef]
- Pauschinger, M.; Bowles, N.E.; Fuentes-Garcia, F.J.; Pham, V.; Kühl, U.; Schwimmbeck, P.L.; Schultheiss, H.-P.; Towbin, J.A. Detection of Adenoviral Genome in the Myocardium of Adult Patients with Idiopathic Left Ventricular Dysfunction. Circulation 1999, 99, 1348–1354. [Google Scholar] [CrossRef]
- Kumar, K.; Guirgis, M.; Zieroth, S.; Lo, E.; Menkis, A.H.; Arora, R.C.; Freed, D.H. Influenza Myocarditis and Myositis: Case Presentation and Review of the Literature. Can. J. Cardiol. 2011, 27, 514–522. [Google Scholar] [CrossRef]
- Adler, Y.; Charron, P.; Imazio, M.; Badano, L.; Barón-Esquivias, G.; Bogaert, J.; Brucato, A.; Gueret, P.; Klingel, K.; Lionis, C.; et al. The 2015 ESC Guidelines on the diagnosis and management of pericardial diseases. Eur. Heart J. 2015, 36, 2873–2874. [Google Scholar] [CrossRef]
- Imazio, M.; Cecchi, E.; Demichelis, B.; Chinaglia, A.; Ierna, S.; Demarie, D.; Ghisio, A.; Pomari, F.; Belli, R.; Trinchero, R. Myopericarditis versus viral or idiopathic acute pericarditis. Heart 2008, 94, 498–501. [Google Scholar] [CrossRef]
- Imazio, M. Contemporary management of pericardial diseases. Curr. Opin. Cardiol. 2012, 27, 308–317. [Google Scholar] [CrossRef]
- Imazio, M.; Cecchi, E.; Demichelis, B.; Ierna, S.; Demarie, D.; Ghisio, A.; Pomari, F.; Coda, L.; Belli, R.; Trinchero, R. Indicators of Poor Prognosis of Acute Pericarditis. Circulation 2007, 115, 2739–2744. [Google Scholar] [CrossRef]
- Rey, F.; Delhumeau-Cartier, C.; Meyer, P.; Genne, D. Is acute idiopathic pericarditis associated with recent upper respiratory tract infection or gastroenteritis? A case-control study. BMJ Open 2015, 5, e009141. [Google Scholar] [CrossRef] [PubMed]
- Imazio, M.; Brucato, A.; Barbieri, A.; Ferroni, F.; Maestroni, S.; Ligabue, G.; Chinaglia, A.; Cumetti, D.; Casa, G.D.; Bonomi, F.; et al. Good Prognosis for Pericarditis with and Without Myocardial Involvement. Circulation 2013, 128, 42–49. [Google Scholar] [CrossRef]
- Imazio, M.; Trinchero, R. Myopericarditis: Etiology, management, and prognosis. Int. J. Cardiol. 2008, 127, 17–26. [Google Scholar] [CrossRef]
- Peretto, G.; Sala, S.; Rizzo, S.; Palmisano, A.; Esposito, A.; De Cobelli, F.; Campochiaro, C.; De Luca, G.; Foppoli, L.; Dagna, L.; et al. Ventricular Arrhythmias in Myocarditis. J. Am. Coll. Cardiol. 2020, 75, 1046–1057. [Google Scholar] [CrossRef]
- Ivey, K.S.; Edwards, K.M.; Talbot, H.K. Respiratory Syncytial Virus and Associations with Cardiovascular Disease in Adults. J. Am. Coll. Cardiol. 2018, 71, 1574–1583. [Google Scholar] [CrossRef]
- Madjid, M.; Connolly, A.T.; Nabutovsky, Y.; Safavi-Naeini, P.; Razavi, M.; Miller, C.C. Effect of High Influenza Activity on Risk of Ventricular Arrhythmias Requiring Therapy in Patients with Implantable Cardiac Defibrillators and Cardiac Resynchronization Therapy Defibrillators. Am. J. Cardiol. 2019, 124, 44–50. [Google Scholar] [CrossRef]
- Volling, C.; Hassan, K.; Mazzulli, T.; Green, K.; Al-Den, A.; Hunter, P.; Mangat, R.; Ng, J.; McGeer, A. Respiratory syncytial virus infection-associated hospitalization in adults: A retrospective cohort study. BMC Infect. Dis. 2014, 14, 665. [Google Scholar] [CrossRef] [PubMed]
- Adegbala, O.; Olagoke, O.; Akintoye, E.; Adejumo, A.C.; Oluwole, A.; Jara, C.; Williams, K.; Briasoulis, A.; Afonso, L. Predictors, Burden, and the Impact of Arrhythmia on Patients Admitted for Acute Myocarditis. Am. J. Cardiol. 2019, 123, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Baksi, A.J.; Kanaganayagam, G.S.; Prasad, S.K. Arrhythmias in Viral Myocarditis and Pericarditis. Card. Electrophysiol. Clin. 2015, 7, 269–281. [Google Scholar] [CrossRef]
- Tse, G.; Yeo, J.M.; Chan, Y.W.; Lai, E.T.; Yan, B.P. What Is the Arrhythmic Substrate in Viral Myocarditis? Insights from Clinical and Animal Studies. Front. Physiol. 2016, 7, 308. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef]
- Yu, C. Cardiovascular complications of severe acute respiratory syndrome. Postgrad. Med. J. 2006, 82, 140–144. [Google Scholar] [CrossRef]
- Czubak, J.; Stolarczyk, K.; Orzeł, A.; Frączek, M.; Zatoński, T. Comparison of the clinical differences between COVID-19, SARS, influenza, and the common cold: A systematic literature review. Adv. Clin. Exp. Med. 2021, 30, 109–114. [Google Scholar] [CrossRef]
- Li, S.; Cheng, C.-W.; Fu, C.-L.; Chan, Y.-H.; Lee, M.-P.; Chan, J.; Yiu, S.-F. Left Ventricular Performance in Patients with Severe Acute Respiratory Syndrome. Circulation 2003, 108, 1798–1803. [Google Scholar] [CrossRef]
- Chong, P.Y.; Chui, P.; Ling, A.E.; Franks, T.J.; Tai, D.Y.; Leo, Y.S.; Kaw, G.J.; Wansaicheong, G.; Chan, K.P.; Ean Oon, L.L.; et al. Analysis of Deaths during the Severe Acute Respiratory Syndrome (SARS) Epidemic in Singapore: Challenges in Determining a SARS Diagnosis. Arch. Pathol. Lab. Med. 2004, 128, 95–204. [Google Scholar] [CrossRef]
- Alhogbani, T. Acute myocarditis associated with novel Middle East respiratory syndrome coronavirus. Ann. Saudi Med. 2016, 36, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Marshall, M. How COVID-19 can damage the brain. Nature 2020, 585, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Delli Muti, N.; Finocchi, F.; Tossetta, G.; Salvio, G.; Cutini, M.; Marzioni, D.; Balercia, G. Could SARS-CoV-2 infection affect male fertility and sexuality? APMIS 2022, 130, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Delli Muti, N.; Balercia, G.; Ciavattini, A.; Giannubilo, S.R.; Marzioni, D. Preeclampsia and severe acute respiratory syndrome coronavirus 2 infection: A systematic review. J. Hypertens. 2022, 40, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Windecker, S.; Andreini, D.; Arbelo, E.; Barbato, E.; Bartorelli, A.L.; Baumbach, A.; Behr, E.R.; Berti, S.; Bueno, H.; et al. European Society of Cardiology guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 1—Epidemiology, pathophysiology, and diagnosis. Eur. Heart J. 2022, 43, 1033–1058. [Google Scholar] [CrossRef]
- Pareek, M.; Singh, A.; Vadlamani, L.; Eder, M.; Pacor, J.; Park, J.; Ghazizadeh, Z.; Heard, A.; Cruz-Solbes, A.S.; Nikooie, R.; et al. Relation of Cardiovascular Risk Factors to Mortality and Cardiovascular Events in Hospitalized Patients with Coronavirus Disease 2019 (from the Yale COVID-19 Cardiovascular Registry). Am. J. Cardiol. 2021, 146, 99–106. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Rola, P.; Doroszko, A.; Trocha, M.; Giniewicz, K.; Kujawa, K.; Gawryś, J.; Matys, T.; Gajecki, D.; Madziarski, M.; Zieliński, S.; et al. Usefulness of C2HEST Score in Predicting Clinical Outcomes of COVID-19 in Heart Failure and Non-Heart-Failure Cohorts. J. Clin. Med. 2022, 11, 3495. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Lala, A.; Johnson, K.W.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized with COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, S.; Aziz, R.; Al Mahri, S.; Malik, S.S.; Haji, E.; Khan, A.H.; Khatlani, T.S.; Bouchama, A. Obesity and COVID-19: What makes obese host so vulnerable? Immun. Ageing 2021, 18, 1. [Google Scholar] [CrossRef]
- Chang, W.-T.; Toh, H.S.; Liao, C.-T.; Yu, W.-L. Cardiac Involvement of COVID-19: A Comprehensive Review. Am. J. Med. Sci. 2021, 361, 14–22. [Google Scholar] [CrossRef]
- Guo, J.; Huang, Z.; Lin, L.; Lv, J. Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease: A Viewpoint on the Potential Influence of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers on Onset and Severity of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Am. Heart Assoc. 2020, 9, e016219. [Google Scholar] [CrossRef]
- Sokolski, M.; Reszka, K.; Suchocki, T.; Adamik, B.; Doroszko, A.; Drobnik, J.; Gorka-Dynysiewicz, J.; Jedrzejczyk, M.; Kaliszewski, K.; Kilis-Pstrusinska, K.; et al. History of Heart Failure in Patients Hospitalized Due to COVID-19: Relevant Factor of In-Hospital Complications and All-Cause Mortality up to Six Months. J. Clin. Med. 2022, 11, 241. [Google Scholar] [CrossRef]
- Bojkova, D.; Wagner, J.U.; Shumliakivska, M.; Aslan, G.S.; Saleem, U.; Hansen, A.; Luxán, G.; Günther, S.; Pham, M.D.; Krishnan, J.; et al. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc. Res. 2020, 116, 2207–2215. [Google Scholar] [CrossRef]
- Lindner, D.; Fitzek, A.; Bräuninger, H.; Aleshcheva, G.; Edler, C.; Meissner, K.; Scherschel, K.; Kirchhof, P.; Escher, F.; Schultheiss, H.-P.; et al. Association of Cardiac Infection with SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020, 5, 1281–1285. [Google Scholar] [CrossRef]
- Tavazzi, G.; Pellegrini, C.; Maurelli, M.; Belliato, M.; Sciutti, F.; Bottazzi, A.; Sepe, P.A.; Resasco, T.; Camporotondo, R.; Bruno, R.; et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020, 22, 911–915. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.A.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Fried, J.A.; Ramasubbu, K.; Bhatt, R.; Topkara, V.K.; Clerkin, K.J.; Horn, E.; Rabbani, L.R.; Brodie, D.; Jain, S.S.; Kirtane, A.J.; et al. The Variety of Cardiovascular Presentations of COVID-19. Circulation 2020, 141, 1930–1936. [Google Scholar] [CrossRef]
- Puntmann, V.O.; Carerj, M.L.; Wieters, I.; Fahim, M.; Arendt, C.; Hoffmann, J.; Shchendrygina, A.; Escher, F.; Vasa-Nicotera, M.; Zeiher, A.M.; et al. Outcomes of Cardiovascular Magnetic Resonance Imaging in Patients Recently Recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 1265–1273. [Google Scholar] [CrossRef]
- Joy, G.; Artico, J.; Kurdi, H.; Lau, C.; Adam, R.D.; Menacho, K.M.; Pierce, I.; Captur, G.; Davies, R.; Schelbert, E.B.; et al. Prospective Case-Control Study of Cardiovascular Abnormalities 6 Months Following Mild COVID-19 in Healthcare Workers. JACC Cardiovasc. Imaging 2021, 14, 2155–2166. [Google Scholar] [CrossRef]
- Kotecha, T.; Knight, D.S.; Razvi, Y.; Kumar, K.; Vimalesvaran, K.; Thornton, G.; Patel, R.; Chacko, L.; Brown, J.T.; Coyle, C.; et al. Patterns of myocardial injury in recovered troponin-positive COVID-19 patients assessed by cardiovascular magnetic resonance. Eur. Heart J. 2021, 42, 1866–1878. [Google Scholar] [CrossRef]
- Vakili, K.; Fathi, M.; Pezeshgi, A.; Mohamadkhani, A.; Hajiesmaeili, M.; Rezaei-Tavirani, M.; Sayehmiri, F. Critical complications of COVID-19: A descriptive meta-analysis study. Rev. Cardiovasc. Med. 2020, 21, 433–442. [Google Scholar] [CrossRef]
- Shoar, S.; Hosseini, F.; Naderan, M.; Mehta, J.L. Meta-analysis of Cardiovascular Events and Related Biomarkers Comparing Survivors Versus Non-survivors in Patients with COVID-19. Am. J. Cardiol. 2020, 15, 50–61. [Google Scholar] [CrossRef]
- Modin, D.; Claggett, B.; Sindet-Pedersen, C.; Lassen, M.C.; Skaarup, K.G.; Jensen, J.U.; Fralick, M.; Schou, M.; Lamberts, M.; Gerds, T.; et al. Acute COVID-19 and the Incidence of Ischemic Stroke and Acute Myocardial Infarction. Circulation 2020, 142, 2080–2082. [Google Scholar] [CrossRef]
- Bangalore, S.; Sharma, A.; Slotwiner, A.; Yatskar, L.; Harari, R.; Shah, B.; Ibrahim, H.; Friedman, G.H.; Thompson, C.; Alviar, C.L.; et al. ST-Segment Elevation in Patients with Covid-19—A Case Series. N. Engl. J. Med. 2020, 382, 2478–2480. [Google Scholar] [CrossRef]
- Bridwell, R.; Long, B.; Gottlieb, M. Neurologic complications of COVID-19. Am. J. Emerg. Med. 2020, 38, 1549.e3–1549.e7. [Google Scholar] [CrossRef]
- Avila, J.; Long, B.; Holladay, D.; Gottlieb, M. Thrombotic complications of COVID-19. Am. J. Emerg. Med. 2021, 39, 213–218. [Google Scholar] [CrossRef]
- Giustino, G.; Pinney, S.P.; Lala, A.; Reddy, V.Y.; Johnston-Cox, H.A.; Mechanick, J.I.; Halperin, J.L.; Fuster, V. Coronavirus and Cardiovascular Disease, Myocardial Injury, and Arrhythmia: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 76, 2011–2023. [Google Scholar] [CrossRef]
- Wichmann, D. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef]
- Protasiewicz, M.; Reszka, K.; Kosowski, W.; Adamik, B.; Bombala, W.; Doroszko, A.; Gajecki, D.; Gawryś, J.; Guziński, M.; Jedrzejczyk, M.; et al. Anticoagulation Prior to COVID-19 Infection Has No Impact on 6 Months Mortality: A Propensity Score-Matched Cohort Study. J. Clin. Med. 2022, 11, 352. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Kochav, S.M.; Coromilas, E.; Nalbandian, A.; Ranard, L.S.; Gupta, A.; Chung, M.K.; Gopinathannair, R.; Biviano, A.B.; Garan, H.; Wan, E.Y. Cardiac Arrhythmias in COVID-19 Infection. Circ. Arrhythm. Electrophysiol. 2020, 13, e008719. [Google Scholar] [CrossRef]
- Bhatla, A.; Mayer, M.M.; Adusumalli, S.; Hyman, M.C.; Oh, E.; Tierney, A.; Moss, J.; Chahal, A.A.; Anesi, G.; Denduluri, S.; et al. COVID-19 and cardiac arrhythmias. Heart Rhythm 2020, 17, 1439–1444. [Google Scholar] [CrossRef]
- Angeli, F.; Spanevello, A.; De Ponti, R.; Visca, D.; Marazzato, J.; Palmiotto, G.; Feci, D.; Reboldi, G.; Fabbri, L.M.; Verdecchia, P. Electrocardiographic features of patients with COVID-19 pneumonia. Eur. J. Intern. Med. 2020, 78, 101–106. [Google Scholar] [CrossRef]
- Davis, H.E.; Assaf, G.S.; McCorkell, L.; Wei, H.; Low, R.J.; Re’em, Y.; Redfield, S.; Austin, J.P.; Akrami, A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. eClinicalMedicine 2021, 38, 1010–1019. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F. Persistent Symptoms in Patients After Acute COVID-19. JAMA 2020, 324, 603–605. [Google Scholar] [CrossRef]
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 43, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.L.; Goßling, A.; Adam, G.; Aepfelbacher, M.; Behrendt, C.-A.; Cavus, E.; Cheng, B.; Fischer, N.; Gallinat, J.; Kühn, S.; et al. Multi-organ assessment in mainly non-hospitalized individuals after SARS-CoV-2 infection: The Hamburg City Health Study COVID programme. Eur. Heart J. 2022, 43, 1124–1137. [Google Scholar] [CrossRef] [PubMed]
- Alsaied, T.; Tremoulet, A.H.; Burns, J.C.; Saidi, A.; Dionne, A.; Lang, S.M.; Newburger, J.W.; de Ferranti, S.; Friedman, K.G. Review of Cardiac Involvement in Multisystem Inflammatory Syndrome in Children. Circulation 2021, 143, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Hoste, L.; Van Paemel, R.; Haerynck, F. Multisystem inflammatory syndrome in children related to COVID-19: A systematic review. Eur. J. Pediatr. 2021, 180, 2019–2034. [Google Scholar] [CrossRef]
- Kabeerdoss, J.; Pilania, R.K.; Karkhele, R.; Kumar, T.S.; Danda, D.; Singh, S. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: Immunological mechanisms, clinical manifestations and management. Rheumatol. Int. 2021, 41, 19–32. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, K.; Levin, M.; Irfan, O.; Morris, S.K.; Wilson, K.; Klein, J.D.; Bhutta, Z.A. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020, 20, e276–e288. [Google Scholar] [CrossRef]
- Pouletty, M.; Borocco, C.; Ouldali, N.; Caseris, M.; Basmaci, R.; Lachaume, N.; Bensaid, P.; Pichard, S.; Kouider, H.; Morelle, G.; et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): A multicentre cohort. Ann. Rheum. Dis. 2020, 79, 999–1006. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franczuk, P.; Tkaczyszyn, M.; Kulak, M.; Domenico, E.; Ponikowski, P.; Jankowska, E.A. Cardiovascular Complications of Viral Respiratory Infections and COVID-19. Biomedicines 2023, 11, 71. https://doi.org/10.3390/biomedicines11010071
Franczuk P, Tkaczyszyn M, Kulak M, Domenico E, Ponikowski P, Jankowska EA. Cardiovascular Complications of Viral Respiratory Infections and COVID-19. Biomedicines. 2023; 11(1):71. https://doi.org/10.3390/biomedicines11010071
Chicago/Turabian StyleFranczuk, Paweł, Michał Tkaczyszyn, Maria Kulak, Esabel Domenico, Piotr Ponikowski, and Ewa Anita Jankowska. 2023. "Cardiovascular Complications of Viral Respiratory Infections and COVID-19" Biomedicines 11, no. 1: 71. https://doi.org/10.3390/biomedicines11010071
APA StyleFranczuk, P., Tkaczyszyn, M., Kulak, M., Domenico, E., Ponikowski, P., & Jankowska, E. A. (2023). Cardiovascular Complications of Viral Respiratory Infections and COVID-19. Biomedicines, 11(1), 71. https://doi.org/10.3390/biomedicines11010071





