Acute COVID-19 Management in Heart Failure Patients: A Specific Setting Requiring Detailed Inpatient and Outpatient Hospital Care
Abstract
:1. Introduction
2. Impact of COVID on the Stable HF Population
3. Difference in Cardiovascular Comorbidities, Cardiovascular Complications, and Survival across the Pandemic Waves
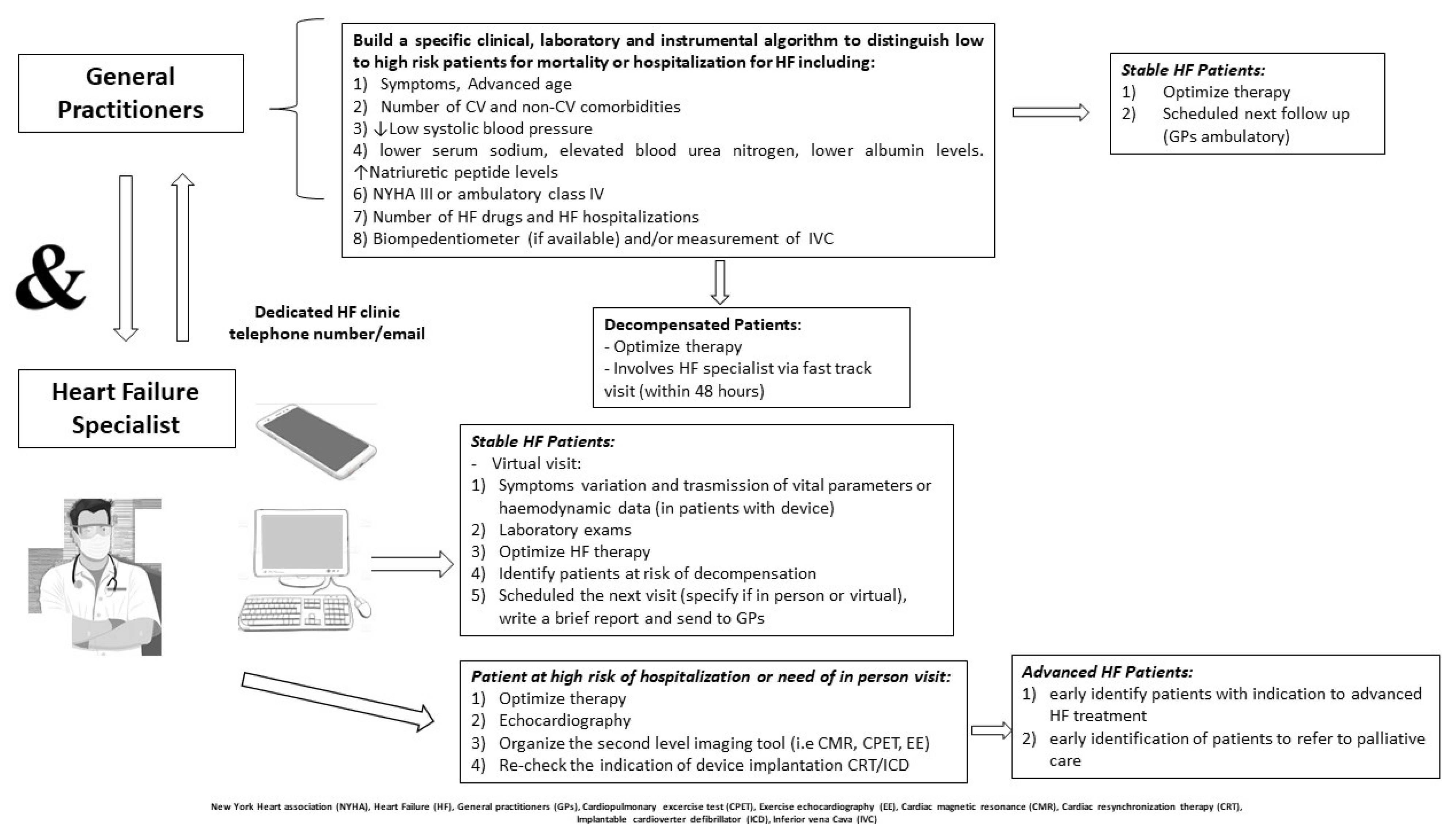
4. Care of Heart Failure Patients with COVID-19
4.1. HF Gaps in COVID-19
4.2. Treatment Effects of Current Antiviral Treatments
4.3. The Role of Anti-Arrhythmic Agents
4.4. Corticosteroids, Diuretic Agents and SGLT-2 Inibhitors
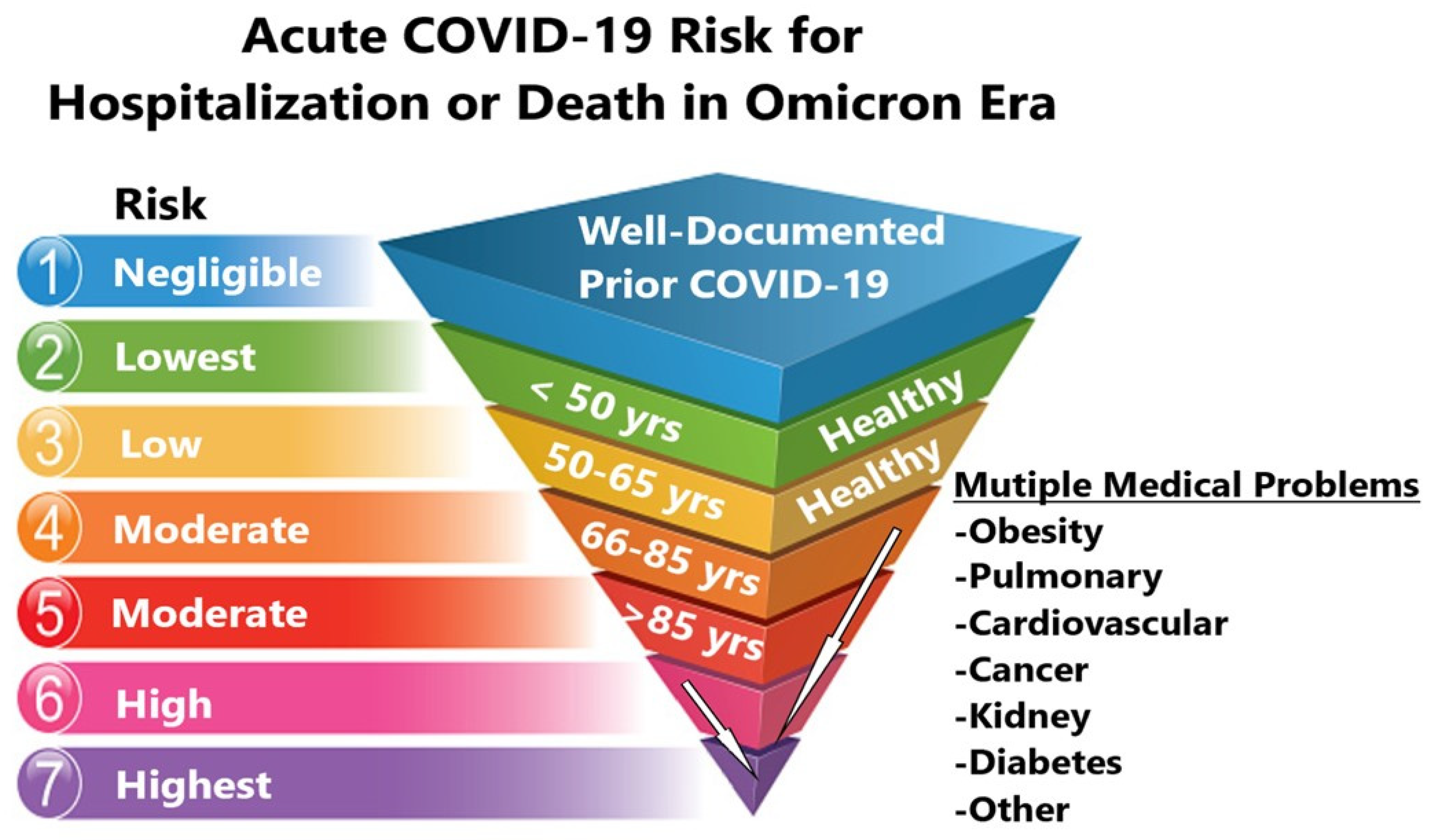
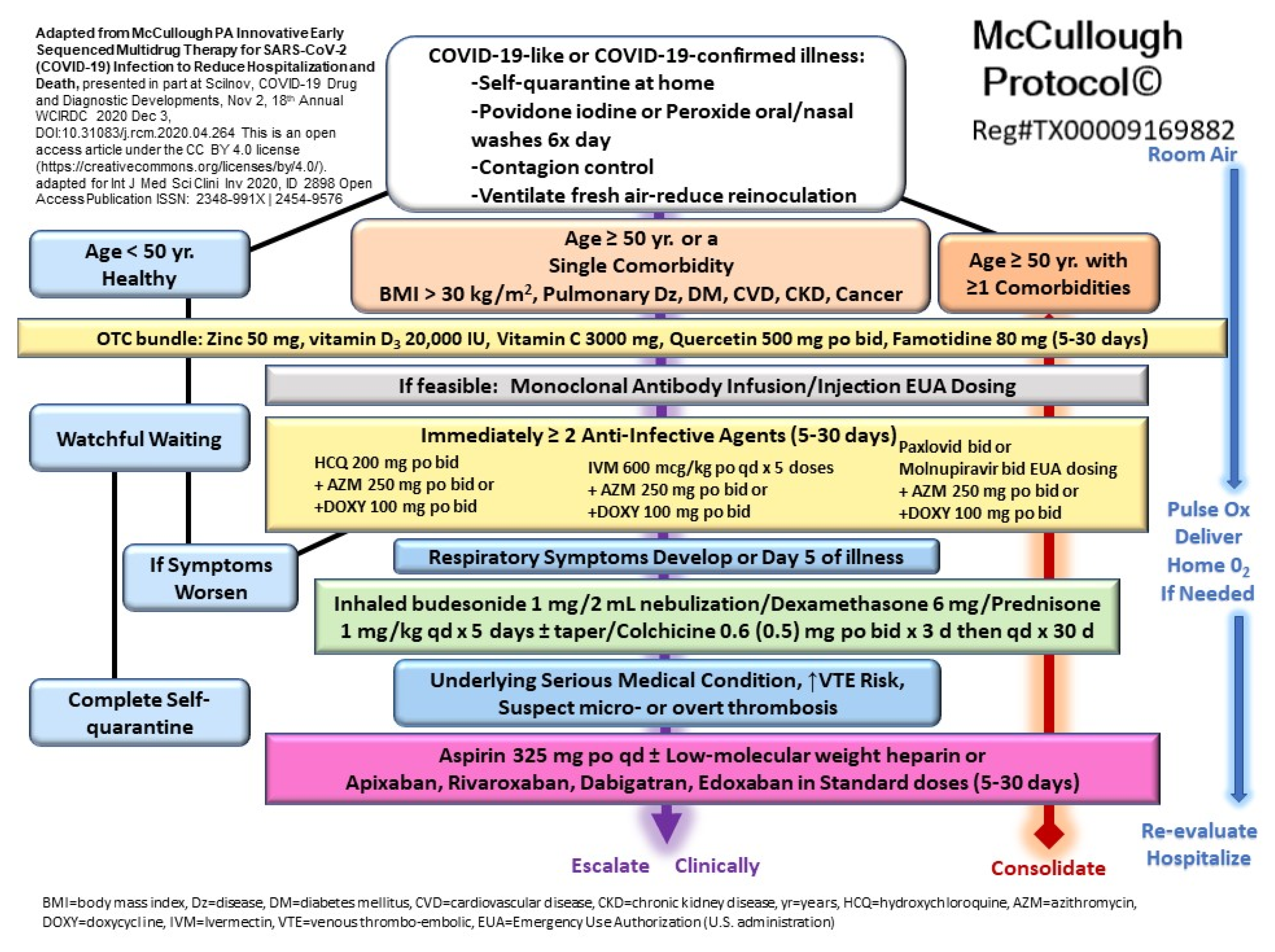
5. Community Standard of Care for Outpatient COVID-19 in High-Risk Patients
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Inciardi, R.M.; Adamo, M.; Lupi, L.; Cani, D.S.; Di Pasquale, M.; Tomasoni, D.; Italia, L.; Zaccone, G.; Tedino, C.; Fabbricatore, D.; et al. Characteristics and outcomes of patients hospi-talized for COVID-19 and cardiac disease in Northern Italy. Eur. Heart J. 2020, 41, 1821–1829. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, D.; Inciardi, R.M.; Lombardi, C.M.; Tedino, C.; Agostoni, P.; Ameri, P.; Barbieri, L.; Bellasi, A.; Camporotondo, R.; Canale, C.; et al. Impact of heart failure on the clinical course and outcomes of patients hospitalized for COVID-19. Results of the Cardio-COVID-Italy multicentre study. Eur. J. Heart Fail. 2020, 22, 2238–2247. [Google Scholar] [CrossRef]
- Bhatt, A.S.; Jering, K.S.; Vaduganathan, M.; Claggett, B.L.; Cunningham, J.W.; Rosenthal, N.; Signorovitch, J.; Thune, J.J.; Vardeny, O.; Solomon, S.D. Clinical Outcomes in Patients with Heart Failure Hospitalized With COVID-19. JACC Heart Fail. 2020, 9, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Clark, R.A.; Pellicori, P.; Inglis, S.C.; Rn, S.C.I. Caring for people with heart failure and many other medical problems through and beyond the COVID-19 pandemic: The advantages of universal access to home telemonitoring. Eur. J. Heart Fail. 2020, 22, 995–998. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Marchandot, B.; Carmona, A.; Curtiaud, A.; El Idrissi, A.; Trimaille, A.; Kibler, M.; Cardi, T.; Heger, J.; Hess, S.; et al. Increased susceptibility to SARS-CoV-2 infection in patients with reduced left ventricular ejection fraction. ESC Heart Fail. 2021, 8, 380–389. [Google Scholar] [CrossRef]
- Bromage, D.I.; Cannatà, A.; Rind, I.A.; Gregorio, C.; Piper, S.; Shah, A.M.; McDonagh, T.A. The impact of COVID-19 on heart failure hospitalization and management: Report from a Heart Failure Unit in London during the peak of the pandemic. Eur. J. Heart Fail. 2020, 22, 978–984. [Google Scholar] [CrossRef]
- Rumery, K.; Seo, A.; Jiang, L.; Choudhary, G.; Shah, N.R.; Rudolph, J.L.; Wu, W.; Erqou, S. Outcomes of coronavirus disease-2019 among veterans with pre-existing diagnosis of heart failure. ESC Heart Fail. 2021, 8, 2338–2344. [Google Scholar] [CrossRef]
- Lassen, M.C.H.; Skaarup, K.G.; Lind, J.N.; Alhakak, A.S.; Sengeløv, M.; Nielsen, A.B.; Espersen, C.; Ravnkilde, K.; Hauser, R.; Schöps, L.B.; et al. Echocardiographic abnormalities and predictors of mortality in hospitalized COVID-19 patients: The ECHOVID-19 study. ESC Heart Fail. 2020, 7, 4189–4197. [Google Scholar] [CrossRef]
- Zhang, Y.; Coats, A.J.; Zheng, Z.; Adamo, M.; Ambrosio, G.; Anker, S.D.; Butler, J.; Xu, D.; Mao, J.; Khan, M.S.; et al. Management of heart failure patients withCOVID-19: A joint position paper of the Chinese Heart Failure Association & National Heart Failure Committee and the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 941–956. [Google Scholar] [CrossRef]
- Baigent, C.; Windecker, S.; Andreini, D.; Arbelo, E.; Barbato, E.; Bartorelli, A.L.; Baumbach, A.; Behr, E.R.; Berti, S.; Bueno, H.; et al. The Task Force for the management of COVID-19 of the European Society of Cardiology. ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic: Part 2—Care pathways, treatment, and follow-up. Eur. Heart J. 2021, 43, 1059–1103. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Metra, M.; Collins, S.P.; Adamo, M.; Ambrosy, A.P.; Antohi, L.E.; Ben Gal, T.; Farmakis, D.; Gustafsson, F.; Hill, L.; et al. Heart failure during the COVID-19 pandemic: Clinical, diagnostic, management, and organizational dilemmas. ESC Heart Fail. 2022, 9, 3713–3736. [Google Scholar] [CrossRef] [PubMed]
- Christensen, D.M.; Butt, J.H.; Fosbøl, E.; Køber, L.; Torp-Pedersen, C.; Gislason, G.; Phelps, M. Nationwide cardiovascular disease admission rates during a second COVID-19 lockdown. Am. Heart J. 2021, 241, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Chagué, F.; Boulin, M.; Eicher, J.; Bichat, F.; Jalmes, M.S.; Cransac-Miet, A.; Soudry-Faure, A.; Danchin, N.; Cottin, Y.; Zeller, M. Impact of lockdown on patients with congestive heart failure during the coronavirus disease 2019 pandemic. ESC Heart Fail. 2020, 7, 4420–4423. [Google Scholar] [CrossRef] [PubMed]
- Sankaranarayanan, R.; Hartshorne-Evans, N.; Redmond-Lyon, S.; Wilson, J.; Essa, H.; Gray, A.; Clayton, L.; Barton, C.; Ahmed, F.Z.; Cunnington, C.; et al. The impact of COVID-19 on the management of heart failure: A United Kingdom patient questionnaire study. ESC Heart Fail. 2021, 8, 1324–1332. [Google Scholar] [CrossRef]
- Beltrami, M.; Bartolini, S.; Milli, M.; Palazzuoli, A. The relevance of specific heart failure outpatient programs in the COVID era: An appropriate model for every disease. Rev. Cardiovasc. Med. 2021, 22, 677–690. [Google Scholar] [CrossRef]
- Mitter, S.S.; Alvarez-Garcia, J.; Miller, M.A.; Moss, N.; Lala, A. Insights From HeartLogic Multisensor Monitoring During the COVID-19 Pandemic in New York City. JACC Heart Fail. 2020, 8, 1053–1055. [Google Scholar] [CrossRef]
- Schulman, S.; Sholzberg, M.; Spyropoulos, A.C.; Zarychanski, R.; Resnick, H.E.; Bradbury, C.A.; Connors, J.M.; Falanga, A.; Iba, T.; Kaatz, S.; et al. ISTH guidelines for antithrombotic treatment in COVID-19. J. Thromb. Haemost. 2022, 20, 2214–2225. [Google Scholar] [CrossRef]
- Parenica, J.; Benesova, K.; Radvan, M.; Sanca, O.; Hlasensky, J.; Lokaj, P.; Ondrus, T.; Helanova, K.; Kala, P.; Dusek, L.; et al. COVID-19 vaccine booster significantly decreases the risk of intensive care unit hospitalization in heart failure patients during the Omicron variant wave: A population-based study. Front. Cardiovasc. Med. 2022, 9, 3075. [Google Scholar] [CrossRef]
- Case, B.C.; Shea, C.; Rappaport, H.; Cellamare, M.; Zhang, C.; Zhu, M.; Medranda, G.A.; Satler, L.F.; Ben-Dor, I.; Hashim, H.; et al. The Evolving Impact of Myocardial Injury in Patients With COVID-19 Amid the Omicron Wave of the Pandemic. Am. J. Cardiol. 2022, 190, 54–60. [Google Scholar] [CrossRef]
- Kaptein, F.H.J.; Stals, M.A.M.; Grootenboers, M.; Braken, S.J.E.; Burggraaf, J.L.I.; van Bussel, B.C.T.; Cannegieter, S.C.; Ten Cate, H.; Endeman, H.; Gommers, D.A.M.P.J.; et al. Incidence of thrombotic complications and overall survival in hospitalized patients with COVID-19 in the second and first wave. Thromb. Res. 2021, 199, 143–148. [Google Scholar] [CrossRef]
- Rind, I.A.; Cannata, A.; McDonaugh, B.; Cassimon, B.; Bannister, C.; Scott, P.A.; Piper, S.; Bromage, D.I.; McDonagh, T.A. Patients hospitalised with heart failure across different waves of the COVID-19 pandemic show consistent clinical characteristics and outcomes. Int. J. Cardiol. 2021, 350, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Chubachi, S.; Namkoong, H.; Asakura, T.; Tanaka, H.; Otake, S.; Nakagawara, K.; Morita, A.; Fukushima, T.; Watase, M.; et al. Characteristics of hospitalized patients with COVID-19 during the first to fifth waves of infection: A report from the Japan COVID-19 Task Force. BMC Infect. Dis. 2022, 22, 935. [Google Scholar] [CrossRef] [PubMed]
- Grippo, F.; Grande, E.; Maraschini, A.; Navarra, S.; Pappagallo, M.; Marchetti, S.; Crialesi, R.; Frova, L.; Orsi, C.; Simeoni, S.; et al. Evolution of Pathology Patterns in Persons Who Died From COVID-19 in Italy: A National Study Based on Death Certificates. Front. Med. 2021, 8, 645543. [Google Scholar] [CrossRef]
- Giacomelli, A.; Ridolfo, A.L.; Pezzati, L.; Oreni, L.; Carrozzo, G.; Beltrami, M.; Poloni, A.; Caloni, B.; Lazzarin, S.; Colombo, M.; et al. Mortality rates among COVID-19 patients hospitalised during the first three waves of the epidemic in Milan, Italy: A prospective observational study. PLoS ONE 2022, 17, e0263548. [Google Scholar] [CrossRef] [PubMed]
- Dell’Antonio, L.S.; Leite, F.M.C.; Dell’Antonio, C.S.d.S.; de Souza, C.B.; Garbin, J.R.T.; dos Santos, A.P.B.; Junior, N.F.d.M.; Lopes-Júnior, L.C. COVID-19 Mortality in Public Hospitals in a Brazilian State: An Analysis of the Three Waves of the Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 14077. [Google Scholar] [CrossRef]
- Lee, T.; Cheng, M.P.; Vinh, D.C.; Lee, T.C.; Tran, K.C.; Winston, B.W.; Sweet, D.; Boyd, J.H.; Walley, K.R.; Haljan, G.; et al. Organ dysfunction and death in patients admitted to hospital with COVID-19 in pandemic waves 1 to 3 in British Columbia, Ontario and Quebec, Canada: A cohort study. CMAJ Open 2022, 10, E379–E389. [Google Scholar] [CrossRef]
- Martinot, M.; Eyriey, M.; Gravier, S.; Kayser, D.; Ion, C.; Mohseni-Zadeh, M.; Ongagna, J.; Schieber, A.; Kempf, C. Evolution of baseline characteristics and severe outcomes in COVID-19 inpatients during the first and second waves in Northeastern France. Infect. Dis. Now 2021, 52, 35–39. [Google Scholar] [CrossRef]
- Stidsen, J.V.; Green, A.; Rosengaard, L.; Højlund, K. Risk of severe COVID-19 infection in persons with diabetes during the first and second waves in Denmark: A nationwide cohort study. Front. Endocrinol. 2022, 13, 1025699. [Google Scholar] [CrossRef]
- Fericean, R.M.; Citu, C.; Manolescu, D.; Rosca, O.; Bratosin, F.; Tudorache, E.; Oancea, C. Characterization and Outcomes of SARS-CoV-2 Infection in Overweight and Obese Patients: A Dynamic Comparison of COVID-19 Pandemic Waves. J. Clin. Med. 2022, 11, 2916. [Google Scholar] [CrossRef]
- Macedo, A.V.S.; Silva, P.G.M.D.B.E.; de Paula, T.C.; Moll-Bernardes, R.J.; dos Santos, T.M.; Mazza, L.; Feldman, A.; Arruda, G.D.S.; de Albuquerque, D.C.; de Sousa, A.S.; et al. Discontinuing vs continuing ACEIs and ARBs in hospitalized patients with COVID-19 according to disease severity: Insights from the BRACE CORONA trial. Am. Heart J. 2022, 249, 86–97. [Google Scholar] [CrossRef]
- Palazzuoli, A.; Tecson, K.M.; Vicenzi, M.; D’Ascenzo, F.; De Ferrari, G.M.; Monticone, S.; Secco, G.G.; Tavazzi, G.; Forleo, G.; Severino, P.; et al. Usefulness of Combined Renin-Angiotensin System Inhibitors and Diuretic Treatment In Patients Hospitalized with COVID-19. Am. J. Cardiol. 2022, 167, 133–138. [Google Scholar] [CrossRef]
- DeFilippis, E.M.; Reza, N.; Donald, E.; Givertz, M.M.; Lindenfeld, J.; Jessup, M. Considerations for Heart Failure Care During the COVID-19 Pandemic. JACC Heart Fail. 2020, 8, 681–691. [Google Scholar] [CrossRef]
- Geleris, J.; Sun, Y.; Platt, J.; Zucker, J.; Baldwin, M.; Hripcsak, G.; Labella, A.; Manson, D.K.; Kubin, C.; Barr, R.G.; et al. Observational Study of Hydroxychloroquine in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2020, 382, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.V.; Roman, Y.M.; Pasupuleti, V.; Barboza, M.J.J.; White, C.M. Hydroxychloroquine or Chloroquine for Treatment or Prophylaxis of COVID-19: A Living Systematic Review. Ann. Intern. Med. 2020, 173, 287–296. [Google Scholar] [CrossRef]
- Barnabas, R.V.; Brown, E.; Bershteyn, A.; Miller, R.S.; Wener, M.; Celum, C.; Wald, A.; Chu, H.; Wesche, D.; Baeten, J.M. Efficacy of hydroxychloroquine for post-exposure prophylaxis to prevent severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among adults exposed to coronavirus disease (COVID-19): A structured summary of a study protocol for a randomised controlled trial. Trials 2020, 21, 475. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.; Gabriels, J.; Chang, D.; Kim, B.S.; Mansoor, A.; Mahmood, E.; Makker, P.; Ismail, H.; Goldner, B.; Willner, J.; et al. Effect of Chloroquine, Hydroxychloroquine, and Azithromycin on the Corrected QT Interval in Patients With SARS-CoV-2 Infection. Circ. Arrhythmia Electrophysiol. 2020, 13, e008662. [Google Scholar] [CrossRef] [PubMed]
- Ramireddy, A.; Chugh, H.; Reinier, K.; Ebinger, J.; Park, E.; Thompson, M.; Cingolani, E.; Cheng, S.; Marban, E.; Albert, C.; et al. Experience With Hydroxychloroquine and Azithromycin in the Coronavirus Disease 2019 Pandemic: Implications for QT Interval Monitoring. J. Am. Heart Assoc. 2020, 9, e017144. [Google Scholar] [CrossRef] [PubMed]
- Chang, D.; Saleh, M.; Gabriels, J.; Ismail, H.; Goldner, B.; Willner, J.; Beldner, S.; Mitra, R.; John, R.; Epstein, L.M. Inpatient Use of Ambulatory Telemetry Monitors for COVID-19 Patients Treated with Hydroxychloroquine and/or Azithromycin. J. Am. Coll. Cardiol. 2020, 75, 2992–2993. [Google Scholar] [CrossRef]
- Moreno-González, G.; Mussetti, A.; Albasanz-Puig, A.; Salvador, I.; Sureda, A.; Gudiol, C.; Salazar, R.; Marin, M.; Garcia, M.; Navarro, V.; et al. A Phase I/II Clinical Trial to evaluate the efficacy of baricitinib to prevent respiratory insufficiency progression in onco-hematological patients affected with COVID19: A structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 116. [Google Scholar] [CrossRef]
- Baldini, C.; Moriconi, F.R.; Galimberti, S.; Libby, P.; De Caterina, R. The JAK–STAT pathway: An emerging target for cardiovascular disease in rheumatoid arthritis and myeloproliferative neoplasms. Eur. Heart J. 2021, 42, 4389–4400. [Google Scholar] [CrossRef]
- Caricchio, R.; Abbate, A.; Gordeev, I.; Meng, J.; Hsue, P.Y.; Neogi, T.; Arduino, R.; Fomina, D.; Bogdanov, R.; Stepanenko, T.; et al. Effect of Canakinumab vs Placebo on Survival Without Invasive Mechanical Ventilation in Patients Hospitalized with Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 326, 230–239. [Google Scholar] [CrossRef]
- Ammirati, E.; Bizzi, E.; Veronese, G.; Groh, M.; Van de Heyning, C.M.; Lehtonen, J.; de Chambrun, M.P.; Cereda, A.; Picchi, C.; Trotta, L.; et al. Immunomodulating Therapies in Acute Myocarditis and Recurrent/Acute Pericarditis. Front. Med. 2022, 9, 357. [Google Scholar] [CrossRef]
- Guzik, T.J.; Mohiddin, S.A.; DiMarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the cardiovascular system: Implications for risk assessment, diagnosis, and treatment options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef] [PubMed]
- Rattanawong, P.; Shen, W.; El Masry, H.; Sorajja, D.; Srivathsan, K.; Valverde, A.; Scott, L.R. Guidance on Short-Term Management of Atrial Fibrillation in Coronavirus Disease 2019. J. Am. Heart Assoc. 2020, 9, e017529. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; Elmahi, E.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Siemieniuk, R.A.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Izcovich, A.; Kum, E.; Pardo-Hernandez, H.; Qasim, A.; Martinez, J.P.D.; Rochwerg, B.; et al. Drug treatments for COVID-19: Living systematic review and network meta-analysis. BMJ 2020, 370, m2980. [Google Scholar] [CrossRef]
- Isidori, A.M.; Pofi, R.; Hasenmajer, V.; Lenzi, A.; Pivonello, R. Use of glucocorticoids in patients with adrenal insufficiency and COVID-19 infection. Lancet Diabetes Endocrinol. 2020, 8, 472–473. [Google Scholar] [CrossRef]
- Tsolaki, V.; Zakynthinos, G.E.; Mantzarlis, K.; Makris, D. Increased mortality among hypertensive COVID-19 patients: Pay a closer look on diuretics in mechanically ventilated patients. Heart Lung 2020, 49, 894–895. [Google Scholar] [CrossRef]
- Alshnbari, A.; Idris, I. Can sodium-glucose co-transporter-2 (SGLT-2) inhibitor reduce the risk of adverse complications due to COVID-19?—Targeting hyperinflammation. Curr. Med. Res. Opin. 2022, 38, 357–364. [Google Scholar] [CrossRef]
- Permana, H.; Yanto, T.A.; Hariyanto, T.I. Pre-admission use of sodium glucose transporter-2 inhibitor (SGLT-2i) may significantly improves COVID-19 outcomes in patients with diabetes: A systematic review, meta-analysis, and meta-regression. Diabetes Res. Clin. Pract. 2022, 195, 110205. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Furtado, R.H.M.; Berwanger, O.; Koch, G.G.; Martinez, F.; Mukhtar, O.; Verma, S.; Gasparyan, S.B.; Tang, F.; Windsor, S.L.; et al. Dapagliflozin and Kidney Outcomes in Hospitalized Patients with COVID-19 Infection: An Analysis of the DARE-19 Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2022, 17, 643–654. [Google Scholar] [CrossRef]
- Procter, B.C.; Ross, C.; Pickard, V.; Smith, E.; Hanson, C.; McCullough, P.A. Clinical outcomes after early ambulatory multidrug therapy for high-risk SARS-CoV-2 (COVID-19) infection. Rev. Cardiovasc. Med. 2020, 21, 611–614. [Google Scholar] [CrossRef] [PubMed]
- McCullough, P.A.; Alexander, P.E.; Armstrong, R.; Arvinte, C.; Bain, A.F.; Bartlett, R.P.; Berkowitz, R.L.; Berry, A.C.; Borody, T.J.; Brewer, J.H.; et al. Multifaceted highly targeted sequential multidrug treatment of early ambulatory high-risk SARS-CoV-2 infection (COVID-19). Rev. Cardiovasc. Med. 2020, 21, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Alexander, P.E.; Armstrong, R.; Fareed, G.; Lotus, J.; Oskoui, R.; Prodromos, C.; Risch, H.A.; Tenenbaum, H.C.; Wax, C.M.; Dara, P.; et al. Early multidrug treatment of SARS-CoV-2 infection (COVID-19) and reduced mortality among nursing home (or outpatient/ambulatory) residents. Med. Hypotheses 2021, 153, 110622. [Google Scholar] [CrossRef] [PubMed]
- Fazio, S.; Bellavite, P.; Zanolin, E.; McCullough, P.A.; Pandolfi, S.; Affuso, F. Retrospective Study of Outcomes and Hospitalization Rates of Patients in Italy with a Confirmed Diagnosis of Early COVID-19 and Treated at Home Within 3 Days or After 3 Days of Symptom Onset with Prescribed and Non-Prescribed Treatments Between November 2020 and August 2021. Experiment 2021, 28, e935379-1. [Google Scholar] [CrossRef]
- Hazan, S.; Dave, S.; Gunaratne, A.W.; Dolai, S.; Clancy, R.L.; A McCullough, P.; Borody, T.J. Effectiveness of ivermectin-based multidrug therapy in severely hypoxic, ambulatory COVID-19 patients. Future Microbiol. 2022, 17, 339–350. [Google Scholar] [CrossRef] [PubMed]
- A McCullough, P.; Vijay, K. SARS-CoV-2 infection and the COVID-19 pandemic: A call to action for therapy and interventions to resolve the crisis of hospitalization, death, and handle the aftermath. Rev. Cardiovasc. Med. 2021, 22, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Gkioulekas, E.; McCullough, P.A.; Zelenko, V. Statistical Analysis Methods Applied to Early Outpatient COVID-19 Treatment Case Series Data. COVID 2022, 2, 84. [Google Scholar] [CrossRef]
| Drugs | Mechanism of Action | Cardiovascular Side Effects |
|---|---|---|
| Aminoquinoline | ||
| Hydroxychloroquine/chloroquine | -Interfere with lysosomal activity and autophagy, altering the PH of the lysosomes reduces low affinity self-antigen presentation. -Inhibits terminal glycosylation of ACE2 in SARS-COV-2. | -Conduction abnormalities (bundle branch block, atrioventricular block) -QTc prolongation -Hypoglycemia (mostly in diabetic patients) |
| Ivermectin | -Interfere with Spike protein attachment to RBCs -Block nuclear entry of SARS-CoV-2 -Modulate intracellular messengers of inflammation | None |
| Antiviral drug nucleoside analogue | ||
| Remdesevir | Remdesevir is a phosphoramidate prodrug that contains an active nucleoside triphosphate and inhibits the RNA-dependent RNA polymerase of coronaviruses. | -Sinus bradycardia -QTc prolongation and torsade de point -AF trigger -T-wave abnormalities -Cardiac arrest -Significantly increased risk of acute kidney injury |
| Janus kinase inhibitor | ||
| Baricitinib | -Reduces the inflammatory response through the inhibition of the Janus-Kinase signaling transducer and activator of transcription pathway. -It acts on AP2-associated protein kinase 1 inhibition, reducing viral endocytosis. | - Thrombosis, including deep venous thrombosis and pulmonary embolism -Higher rate of major adverse cardiovascular events (cardiovascular death, myocardial infarction, and stroke) compared to the placebo |
| Corticosteroids | ||
| Dexamethasone | -Inhibits the pro-inflammatory pathway that encodes for chemokines, cytokines, cell adhesion molecules, and the acute inflammatory activation in response to SARS-CoV-2 infection. | -Elevations of total plasma cholesterol and triglycerides -Abnormal glycemic control -Increased systolic blood pressure and weight -Fluid and sodium retention, HF occurrence |
| Interleukin antagonists | ||
| Anakinra | -Is a recombinant, non-glycosylated form of the human IL-1 receptor antagonist that binds the IL-1 receptor, reducing the inflammatory response. | Not described |
| Interleukin-6 inhibitors | ||
| Tolicizumab | Tocilizumab and sarilumab are both IL-6 receptor antagonists that prevent the downstream activation of IL-6. | -Hypertension -Increased levels of cholesterol and/or triglycerides |
| Sarilumab | -Cardiac failure -Embolic and thrombotic event | |
| Interleukin-1 inhibitors | ||
| Canakinumab | It is a human IgG1k monoclonal antibody that neutralizes soluble IL-1β. | Not described |
| Monoclonal Antibodies | ||
| Etesevimab | Monoclonal antibodies specifically bind the virus’ surface spike protein receptor binding domain. This high affinity is related to the strong binding of the ACE2 host cell surface receptor. | -Hypertension |
| Bamlanivimab | -Hypertension -Ischemic heart disease | |
| Casirivimab | -Hypertension -Ischemic heart disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palazzuoli, A.; Beltrami, M.; McCullough, P.A. Acute COVID-19 Management in Heart Failure Patients: A Specific Setting Requiring Detailed Inpatient and Outpatient Hospital Care. Biomedicines 2023, 11, 790. https://doi.org/10.3390/biomedicines11030790
Palazzuoli A, Beltrami M, McCullough PA. Acute COVID-19 Management in Heart Failure Patients: A Specific Setting Requiring Detailed Inpatient and Outpatient Hospital Care. Biomedicines. 2023; 11(3):790. https://doi.org/10.3390/biomedicines11030790
Chicago/Turabian StylePalazzuoli, Alberto, Matteo Beltrami, and Peter A. McCullough. 2023. "Acute COVID-19 Management in Heart Failure Patients: A Specific Setting Requiring Detailed Inpatient and Outpatient Hospital Care" Biomedicines 11, no. 3: 790. https://doi.org/10.3390/biomedicines11030790
APA StylePalazzuoli, A., Beltrami, M., & McCullough, P. A. (2023). Acute COVID-19 Management in Heart Failure Patients: A Specific Setting Requiring Detailed Inpatient and Outpatient Hospital Care. Biomedicines, 11(3), 790. https://doi.org/10.3390/biomedicines11030790






