Factors of Persistent Limited Exercise Tolerance in Patients after COVID-19 with Normal Left Ventricular Ejection Fraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Basic Characteristics
2.2. Laboratory Tests
2.3. Echocardiography
2.4. Spiroergometry
2.5. Body Mass Analysis
2.6. Statistical Analysis
3. Results
3.1. Evaluation of Basic Characteristics
3.2. Evaluation of Laboratory Tests
3.3. Evaluation of Echocardiography
3.4. Evaluation of Spiroergometry
3.5. Evaluation of Body Mass Analysis
3.6. Multivariate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carotti, M.; Salaffi, F.; Sarzi-Puttini, P.; Agostini, A.; Borgheresi, A.; Minorati, D.; Galli, M.; Marotto, D.; Giovagnoni, A. Chest CT features of coronavirus disease 2019 (COVID-19) pneumonia: Key points for radiologists. Radiol. Med. 2020, 125, 636–646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zamorano Cuervo, N.; Grandvaux, N. ACE2: Evidence of role as entry receptor for SARS-CoV-2 and implications in comorbidities. eLife 2020, 9, e61390. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Danilczyk, U.; Penninger, J.M. Angiotensin-converting enzyme II in the heart and the kidney. Circ. Res. 2006, 98, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, I.; Bertram, S.; Herzog, P.; Pfefferle, S.; Steffen, I.; Muench, M.O.; Simmons, G.; Hofmann, H.; Kuri, T.; Weber, F.; et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J. Virol. 2010, 84, 1198–1205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- South, A.M.; Diz, D.I.; Chappell, M.C. COVID-19, ACE2, and the cardiovascular consequences. Am. J. Physiol. Heart Circ. Physiol. 2020, 318, H1084–H1090. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, K.I.; Feng, G.; Liu, W.Y.; Targher, G.; Byrne, C.D.; Zheng, M.H. Extrapulmonary complications of COVID-19: A multisystem disease? J. Med. Virol. 2021, 93, 323–335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bielecka-Dabrowa, A.; Cichocka-Radwan, A.; Lewek, J.; Pawliczak, F.; Maciejewski, M.; Banach, M. Cardiac manifestations of COVID-19. Rev. Cardiovasc. Med. 2021, 22, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Lewek, J.; Jatczak-Pawlik, I.; Maciejewski, M.; Jankowski, P.; Banach, M. COVID-19 and cardiovascular complications-preliminary results of the LATE-COVID study. Arch. Med. Sci. 2021, 17, 818–822. [Google Scholar] [CrossRef]
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, Á.; Martínez-Cava, A.; Courel-Ibáñez, J. Chronotropic Incompetence in Non-Hospitalized Patients with Post-COVID-19 Syndrome. J. Clin. Med. 2021, 10, 5434. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, Á.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz Martínez, B.J.; Bernal-Morel, E.; Courel-Ibáñez, J. Post-COVID-19 Syndrome and the Potential Benefits of Exercise. Int. J. Environ. Res. Public Health 2021, 18, 5329. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Telles, A.; López-Romero, S.; Figueroa-Hurtado, E.; Pou-Aguilar, Y.N.; Wong, A.W.; Milne, K.M.; Ryerson, C.J.; Guenette, J.A. Pulmonary function and functional capacity in COVID-19 survivors with persistent dyspnoea. Respir. Physiol. Neurobiol. 2020, 288, 103644. [Google Scholar] [CrossRef] [PubMed]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s guide to cardiopulmonary exercise testing in adults. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2019, 32, 1–64. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, A.; Riegler, L.; Rucco, M.A.; Cocchia, R.; Scarafile, R.; Salerno, G.; Martone, F.; Vriz, O.; Caso, P.; Calabrò, R.; et al. Left atrial volume index in healthy subjects: Clinical and echocardiographic correlates. Echocardiography 2013, 30, 1001–1007. [Google Scholar] [CrossRef]
- Daskalov, I.R.; Petrovsky, P.D.; Demirevska, L.D. Mitral annular systolic velocity as a marker of preclinical systolic dysfunction among patients with arterial hypertension. Cardiovasc. Ultrasound. 2012, 10, 46. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tousignant, C.; Kim, H.; Papa, F.; Mazer, C.D. Evaluation of TAPSE as a measure of right ventricular output. Can. J. Anaesth. 2012, 59, 376–383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Datta, D.; Normandin, E.; ZuWallack, R. Cardiopulmonary exercise testing in the assessment of exertional dyspnea. Ann. Thorac. Med. 2015, 10, 77–86. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Jaafar, Z.A.; Kreidieh, D.; Itani, L.; Tannir, H.; El Masri, D.; El Ghoch, M. Cross-validation of prediction equations for estimating the body fat percentage in adults with obesity. Clin. Nutr. ESPEN 2021, 41, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Yoh, K.; Enomoto, H.; Ishii, N.; Iwata, Y.; Nakano, C.; Takata, R.; Nishimura, T.; Aizawa, N.; Sakai, Y.; et al. Extracellular water to total body water ratio in viral liver diseases: A study using bioimpedance analysis. Nutrients 2018, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Blume, G.G.; Mcleod, C.J.; Barnes, M.E.; Seward, J.B.; Pellikka, P.A.; Bastiansen, P.M.; Tsang, T.S.M. Left atrial function: Physiology, assessment, and clinical implications. Eur. J. Echocardiogr. 2011, 12, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Hurst, J.R.; Suissa, S. Cardiovascular disease and COPD: Dangerous liaisons? Eur. Respir. Rev. 2018, 27, 180057. [Google Scholar] [CrossRef] [PubMed]
- Singh, I.; Joseph, P.; Heerdt, P.M.; Cullinan, M.; Lutchmansingh, D.D.; Gulati, M.; Possick, J.D.; Systrom, D.M.; Waxman, A.B. Persistent Exertional Intolerance After COVID-19: Insights From Invasive Cardiopulmonary Exercise Testing. Chest 2021, 161, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Motiejunaite, J.; Balagny, P.; Arnoult, F.; Mangin, L.; Bancal, C.; Vidal-Petiot, E.; Flamant, M.; Jondeau, G.; Cohen-Solal, A.; d’Ortho, M.P.; et al. Hyperventilation as one of the mechanisms of persistent dyspnoea in SARS-CoV-2 survivors. Eur. Respir. J. 2021, 58, 2101578. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baratto, C.; Caravita, S.; Faini, A. Impact of COVID-19 on exercise pathophysiology a combined cardiopulmonary and echocardiographic exercise study. J. Appl. Physiol. 2021, 130, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Martínez-Cava, A.; Buendía-Romero, A.; Franco-López, F.; Sánchez-Agar, J.A.; Sánchez-Alcaraz, B.J.; Tufano, J.J.; Pallarés, J.G.; Courel-Ibáñez, J. Relationship between the severity of persistent symptoms, physical fitness, and cardiopulmonary function in post-COVID-19 condition. A population-based analysis. Intern. Emerg. Med. 2022, 17, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Franco-López, F.; Buendía-Romero, Á.; Martínez-Cava, A.; Sánchez-Agar, J.A.; Sánchez-Alcaraz Martínez, B.J.; Courel-Ibáñez, J.; Pallarés, J.G. Rehabilitation for post-COVID-19 condition through a supervised exercise intervention: A randomized controlled trial. Scand. J. Med. Sci. Sports 2022, 32, 1791–1801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tangen, J.; Aukrust, P.; Barratt-Due, A.; Skulstad, H.; Edvardsen, T. Reduced cardiac function by echocardiography in a minority of COVID-19 patients 3 months after hospitalization. J. Am. Soc. Echocardiogr. 2022, 35, 243–244. [Google Scholar] [CrossRef]
- Baruch, G.; Rothschild, E.; Sadon, S.; Szekely, Y.; Lichter, Y.; Kaplan, A.; Taieb, P.; Banai, A.; Hochstadt, A.; Merdler, I.; et al. Evolution of right and left ventricle routine and speckle-tracking echocardiography in patients recovering from coronavirus diseasea longitudinal study. Eur. Heart J. Cardiovasc. Imaging. 2019, 23, 1055–1065. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, H.; Liu, X.; Zhao, Y.; Zhang, P. Low tricuspid annular plane systolic excursion is associated with a poor outcome in patients with COVID-19: A systematic review and meta-analysis. Medicine 2022, 101, e28971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pelà, G.; Goldoni, M.; Solinas, E.; Cavalli, C.; Tagliaferri, S.; Ranzieri, S.; Frizzelli, A.; Marchi, L.; Mori, P.A.; Majori, M.; et al. Sex-Related Differences in Long-COVID-19 Syndrome. J. Women′s Health 2022, 31, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, M.; Lewek, J.; Kapusta, J.; Banach, M.; Jankowski, P.; Bielecka-Dabrowa, A. Predictors of Long COVID in Patients without Comorbidities: Data from the Polish Long-COVID Cardiovascular (PoLoCOV-CVD) Study. J. Clin. Med. 2022, 11, 4980. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cornejo-Pareja, I.; Vegas-Aguilar, I.M.; Lukaski, H.; Talluri, A.; Bellido-Guerrero, D.; Tinahones, F.J.; García-Almeida, J.M. Overhydration Assessed Using Bioelectrical Impedance Vector Analysis Adversely Affects 90-Day Clinical Outcome among SARS-CoV2 Patients: A New Approach. Nutrients 2022, 14, 2726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Parameter | Peak VO2 < 80% VO2 Predicted n = 47 | Peak VO2 ≥ 80% VO2 Predicted n = 73 | p |
|---|---|---|---|
| Age | (30–65), 49 * | (47–64), 55 * | 0.08 |
| Height (cm) | (164–176), 170 * | (164–173), 169 * | 0.39 |
| Body mass (kg) | (65–89), 75 * | (69–89), 77 * | 0.51 |
| BMI (kg/m2) | (22.70–29.98), 25.97 * | (24.61–30.49), 27.28 * | 0.07 |
| BSA (m2) | (1.69–2.04), 1.86 * | (1.75–2.01), 1.85 * | 0.94 |
| hs-cTnT (pg/mL) | (3.70–9.85), 4.90 * | (3.0–6.9), 4.6 * | 0.14 |
| NT-proBNP (pg/mL) | (31–125), 73 * | (39–106), 73 * | 0.48 |
| Hemoglobin (g/dL) | (12.9–14.6), 13.9 * | (12.4–14.3), 13.3 * | 0.11 |
| Creatinine (mg/dL) | (0.65–0.90), 0.78 * | (0.66–0.88), 0.73 * | 0.27 |
| GFR (mL/min/1.73 m2) | (79.9–107.7), 98.1 * | (79.0–98.7), 89.9 * | 0.07 |
| Glucose (mg/dL) | (86–99), 91 * | (86–93), 91 * | 0.44 |
| HDL cholesterol (mg/dL) | (35–58), 49 * | (42–59), 50 * | 0.38 |
| LDL cholesterol (mg/dL) | 92.29 (±34.64) | 100.00 (±29.72) | 0.20 |
| Triglycerides (mg/dL) | (76–164), 101 * | (90–158), 114 * | 0.28 |
| Total cholesterol (mg/dL) | 163.83 (±39.12) | 179.00 (±36.22) | 0.03 |
| ALT (U/L) | (15–25), 22 * | (17–35), 22 * | 0.25 |
| AST (U/L) | (24–29), 27 * | (24–32), 26 * | 0.47 |
| CRP (mg/L) | (0.5–0.5), 0.5 * | (0.5–0.5), 0.5 * | 0.66 |
| EF (%) | (55–66), 62 * | (59–66), 62 * | 0.44 |
| LA volume (mL) | (39.0–78.5), 52.0 * | (49–78), 61 * | 0.20 |
| LAVi (mL/m2) | (21.9–39.5), 30.0 * | (27.0–39.3), 31.9 * | 0.32 |
| E (cm/s) | (61–87), 72 * | (60–83), 72 * | 0.59 |
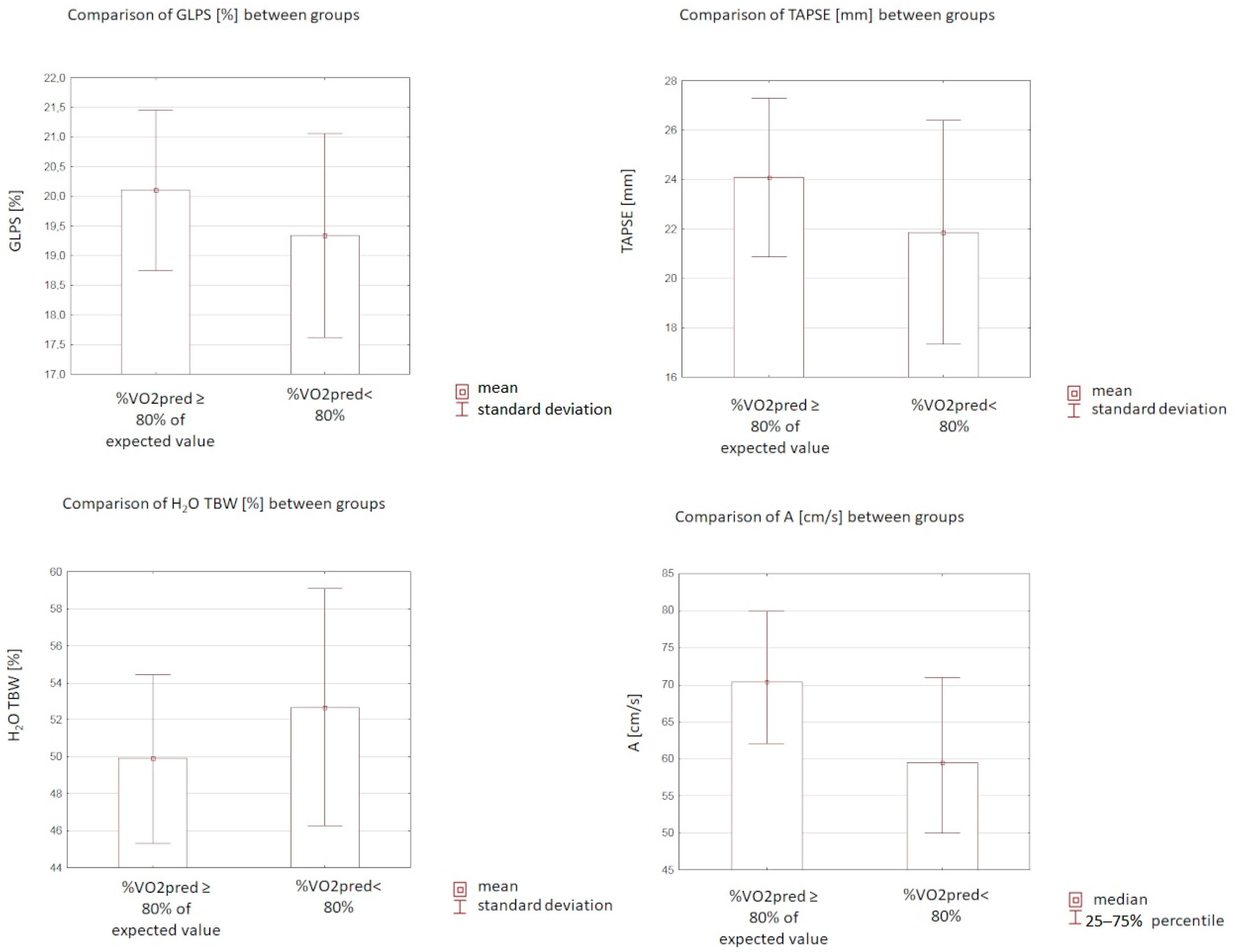
| A (cm/s) | (50.0–71.0), 59.5 * | (62.0–80.0), 70.5 * | 0.004 |
| E/A | (0.98–1.70), 1.23 * | (0.80–1.22), 1.01 * | 0.006 |
| GLPS (%) | 19.34 (±1.72) | 20.10 (±1.35) | 0.03 |
| TAPSE (mm) | 21.86 (±4.53) | 24.08 (±3.2) | 0.002 |
| TDE S’ (cm/s) | (11–15), 14 * | (12–16), 14 * | 0.17 |
| Parameter | Peak VO2 < 80% VO2 Predicted n = 47 | Peak VO2 ≥ 80% VO2 Predicted n = 73 | p |
|---|---|---|---|
| Exercise time (s) | (378–642), 507 * | (439–697), 580 * | 0.06 |
| HR max | 132.87 (±33.61) | 146.63 (±20.46) | 0.006 |
| Peripheral SBP max (mmHg) | (130–170), 150 * | (150–200), 180 * | <0.0001 |
| Peripheral DBP max (mmHg) | (70–90), 80 * | (80–90), 80 * | 0.29 |
| FEV1 (L) | (2.55–3.64), 2.99 * | (2.59–3.54), 3.03 * | 0.79 |
| FVC (L) | 3.90 (±1.05) | 3.80 (±0.94) | 0.62 |
| FVC% | 99.61 (±14.74) | 111.71 (±16.95) | <0.0001 |
| FEV1/FVC | (76.0–87.0), 82.5 * | (77–86), 83 * | 0.78 |
| FEV1/FVC% | (96–110), 104 * | (97–110), 105 * | 0.57 |
| FEF 25–75 (L/s) | 3.25 (±1.23) | 2.66 (±1.13) | 0.01 |
| RER | (1.01–1.10), 1.08 * | (1.03–1.12), 1.09 * | 0.14 |
| VO2max (mL/min/kg) | (14–25), 17 * | (20–26), 23 * | <0.0001 |
| VO2AT (mL/min/kg) | (10–15), 12 * | (13–16), 15 * | 0.001 |
| Peak VO2max (L) | (0.98–1.71), 1.29 * | (1.42–2.08), 1.73 * | <0.0001 |
| VE/VCO2 slope | (26.2–34.6), 29.6 * | (25.5–32.6), 29.5 * | 0.54 |
| Fat (%) | 28.46 (±7.75) | 30.92 (±5.47) | 0.1 |
| Fat (kg) | (15.7–28.4), 20.5 * | (18.6–29.8), 25.3 * | 0.1 |
| FFM (kg) | (47–59.1), 56.5 * | (47.3–63.5), 52.7 * | 0.82 |
| TBW (kg) | (34.2–44.0), 41.4 * | (33.7–45.3), 38.5 * | 0.84 |
| TBW (%) | 52.67 (±6.41) | 49.89 (±4.59) | 0.02 |
| ECW (kg) | (15.8–19.3), 18.0 * | (14.7–19.8), 17.2 * | (0.96) |
| ICW (kg) | (19.4–25.5), 24.1 * | (19.1–26.2), 21.7 * | 0.8 |
| ECW/TBW × 100% | 43.17 (±3.23) | 43.77 (±2.37) | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryglewska-Wawrzak, K.; Sakowicz, A.; Banach, M.; Maciejewski, M.; Bielecka-Dabrowa, A. Factors of Persistent Limited Exercise Tolerance in Patients after COVID-19 with Normal Left Ventricular Ejection Fraction. Biomedicines 2022, 10, 3257. https://doi.org/10.3390/biomedicines10123257
Gryglewska-Wawrzak K, Sakowicz A, Banach M, Maciejewski M, Bielecka-Dabrowa A. Factors of Persistent Limited Exercise Tolerance in Patients after COVID-19 with Normal Left Ventricular Ejection Fraction. Biomedicines. 2022; 10(12):3257. https://doi.org/10.3390/biomedicines10123257
Chicago/Turabian StyleGryglewska-Wawrzak, Katarzyna, Agata Sakowicz, Maciej Banach, Marek Maciejewski, and Agata Bielecka-Dabrowa. 2022. "Factors of Persistent Limited Exercise Tolerance in Patients after COVID-19 with Normal Left Ventricular Ejection Fraction" Biomedicines 10, no. 12: 3257. https://doi.org/10.3390/biomedicines10123257
APA StyleGryglewska-Wawrzak, K., Sakowicz, A., Banach, M., Maciejewski, M., & Bielecka-Dabrowa, A. (2022). Factors of Persistent Limited Exercise Tolerance in Patients after COVID-19 with Normal Left Ventricular Ejection Fraction. Biomedicines, 10(12), 3257. https://doi.org/10.3390/biomedicines10123257







