New Opportunities in Heart Failure with Preserved Ejection Fraction: From Bench to Bedside… and Back
Abstract
1. Introduction
2. Definitions in HF
3. Clinical Presentation and Diagnosis
4. Non-Invasive Complementary Evaluation
5. Hemodynamic Abnormalities
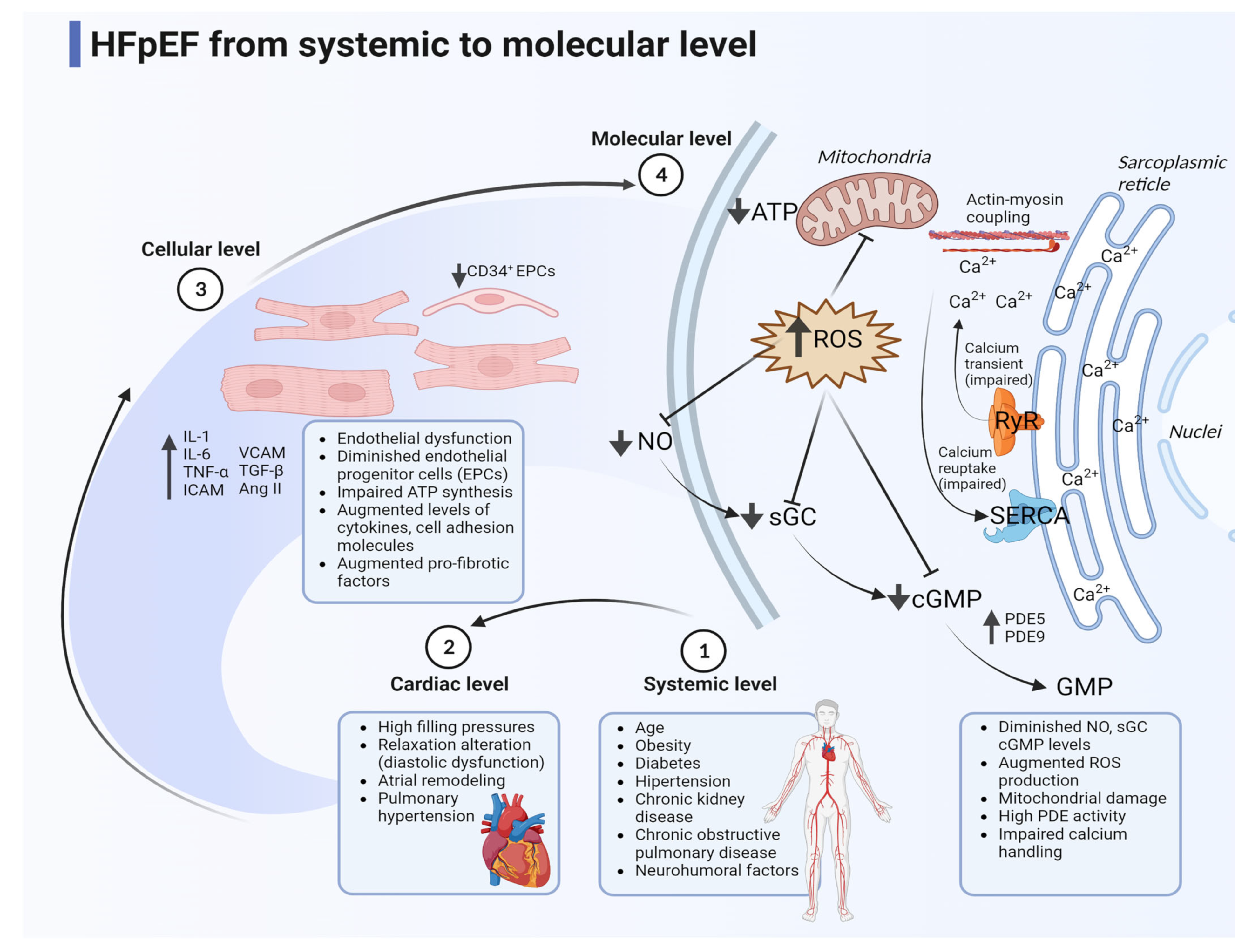
6. General Pathophysiology in HFpEF
7. The First Choice Drug: Diuretics
8. Calcium Handling
8.1. Physiology and Pathology Pathway
8.2. Calcium Dynamics Approach Therapies
8.2.1. Calcium Channels Blockers
8.2.2. Ranolazine
8.2.3. B-Blockers
8.2.4. Istaroxima
8.2.5. Cardiac Glycosides
9. Antifibrotic and Anti-Inflammatory Agents
9.1. Role of Inflammation
9.2. Antifibrotic Agents
9.2.1. Pirfenidone
9.2.2. Spironolactone
9.2.3. Sacubitril/Valsartan
9.2.4. Novel Therapies
9.3. Anti-Inflammatory Agents
9.3.1. Anti-TNF-α
9.3.2. Anti-IL-1
9.3.3. Anti-IL-6
9.3.4. Colchicine
9.3.5. iSGLT2
9.3.6. Metformin
10. Nitric Oxide Pathway and Endothelial Function
10.1. NO Pathophysiology and Dysfunction in HF
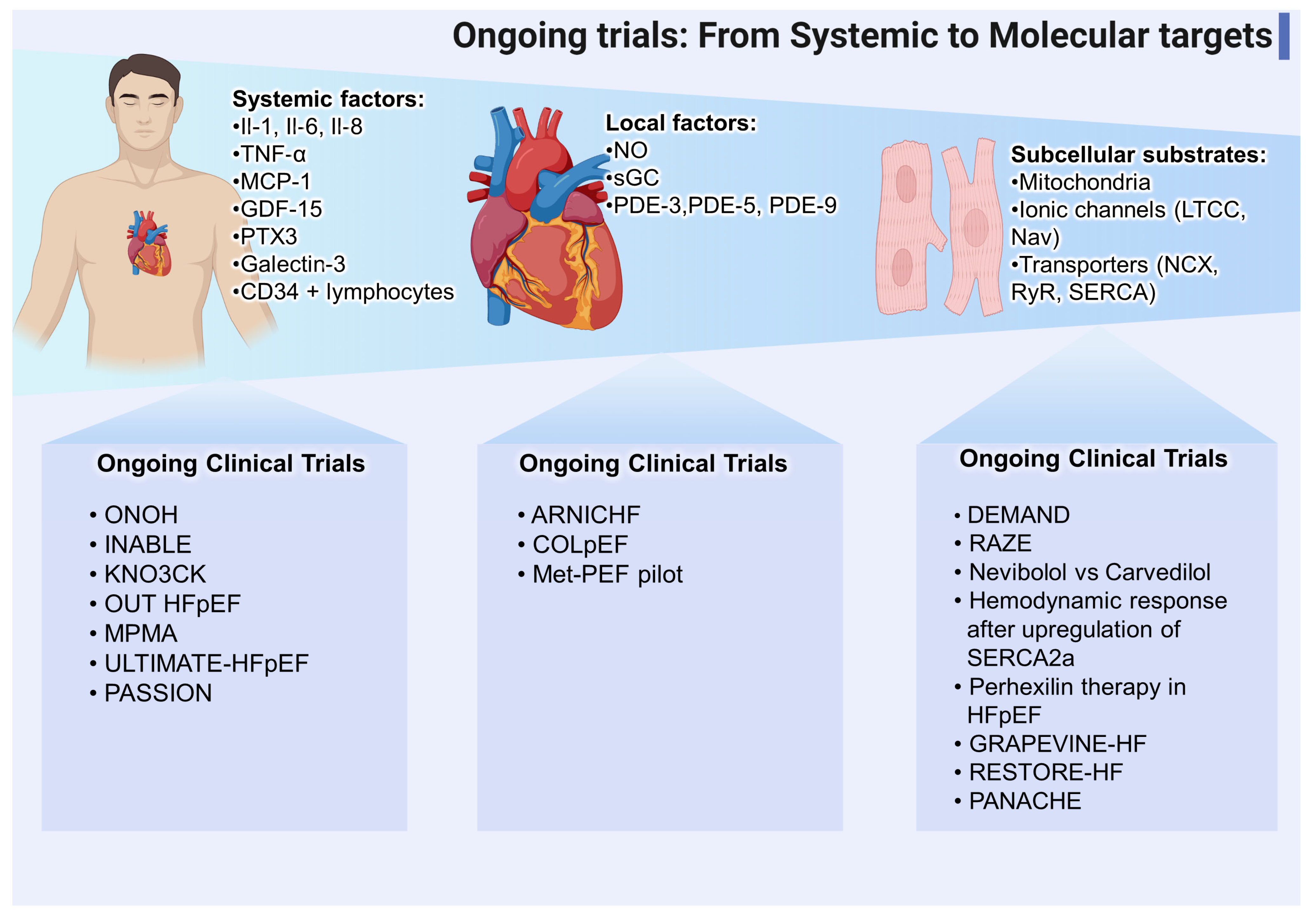
10.2. Novel Trials and Applications Related to the NO-sGC-cGMP Pathway
10.2.1. Nitric Oxide Donors
10.2.2. Modulators of sGC
10.2.3. PDE Inhibitors
11. Mitochondrial and Metabolic Defects
11.1. Some Physiology Aspects
11.2. Mitochondrial Dysfunction and Pharmacological Targets
11.2.1. Perhexiline
11.2.2. Resveratrol
11.2.3. Elamipretide
11.2.4. Neladenoson
12. Cell Therapy
12.1. How It Works?
12.2. Cell Therapy and the Future
13. Device-Based Therapies
13.1. Can a Device Ameliorate Impaired Mechanisms in HFpEF?
13.2. Interatrial Shunt Devices
13.3. V-Wave Shunt
13.4. Atrial Flow Regulator (AFR)
13.5. Trans-Catheter Atrial Shunt Systems
13.6. Left Ventricle Expanders (LVEs)
13.7. Mechanical Circulatory Support Devices (MCS)
13.7.1. Left Atrial Decompression Pumps
13.7.2. CoPulse
13.7.3. Left Atrial Assist Device (LAAD)
13.8. Autonomic Regulation
13.8.1. BAROSTIM NEO and BAROSTIM BAT
13.8.2. Cardiac Contractility Modulation (CCM)
13.8.3. Cardiac Resynchronization Therapy (CRT)
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Zakeri, R.; Cowie, M. Heart failure with preserved ejection fraction: Controversies, challenges and future directions. Heart 2018, 104, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.-C.; Ahn, Y.; Jung, H.O. Pathophysiology of Heart Failure with Preserved Ejection Fraction. Heart Fail. Clin. 2021, 17, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Shah, A.M.; Borlaug, B. Heart Failure with Preserved Ejection Fraction in Perspective. Circ. Res. 2019, 124, 1598–1617. [Google Scholar] [CrossRef] [PubMed]
- Margulies, K.B. Delivering Progress in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1138–1140. [Google Scholar] [CrossRef]
- Cortes, L.T.; Hong, L. Heart failure with preserved ejection fraction—Out with the old and out with the new? Front. Cardiovasc. Med. 2022, 9, 943572. [Google Scholar] [CrossRef]
- Abebe, T.B.; Gebreyohannes, E.A.; Tefera, Y.G.; Abegaz, T.M. Patients with HFpEF and HFrEF have different clinical characteristics but similar prognosis: A retrospective cohort study. BMC Cardiovasc. Disord. 2016, 16, 1–8. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Patel, R.; Michel, A.; Shah, S.; Senni, M.; Gheorghiade, M.; Butler, J. Mode of Death in Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2017, 69, 556–569. [Google Scholar] [CrossRef]
- McKee, P.A.; Castelli, W.P.; McNamara, P.M.; Kannel, W.B. The Natural History of Congestive Heart Failure: The Framingham Study. N. Engl. J. Med. 1971, 285, 1441–1446. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Olson, T.P.; Reddy, Y.N.V.; Melenovsky, V.; Kane, G.C.; Borlaug, B.A. Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. Eur. Heart J. 2018, 39, 2810–2821. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A Simple, Evidence-Based Approach to Help Guide Diagnosis of Heart Failure with Preserved Ejection Fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA–PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef]
- Aizpurua, A.B.; Wijk, S.S.; Rocca, H.B.; Henkens, M.; Heymans, S.; Mhs, L.B.; Shah, S.; Van Empel, V.P. Validation of the HFA-PEFF score for the diagnosis of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2019, 22, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Myhre, P.L.; Vaduganathan, M.; Claggett, B.L.; Lam, C.S.; Desai, A.S.; Anand, I.S.; Sweitzer, N.K.; Fang, J.C.; O’Meara, E.; Shah, S.J.; et al. Application of the H2 FPEF score to a global clinical trial of patients with heart failure with preserved ejection fraction: The TOPCAT trial. Eur. J. Heart Fail. 2019, 21, 1288–1291. [Google Scholar] [CrossRef] [PubMed]
- Nikorowitsch, J.; der Kellen, R.B.; Kirchhof, P.; Magnussen, C.; Jagodzinski, A.; Schnabel, R.B.; Blankenberg, S.; Wenzel, J. Applying the ESC 2016, H2 FPEF, and HFA-PEFF diagnostic algorithms for heart failure with preserved ejection fraction to the general population. ESC Heart Fail. 2021, 8, 3603–3612. [Google Scholar] [CrossRef]
- Shah, A.M.; Claggett, B.; Sweitzer, N.K.; Shah, S.J.; Anand, I.S.; O’Meara, E.; Desai, A.S.; Heitner, J.F.; Li, G.; Fang, J.; et al. Cardiac Structure and Function and Prognosis in Heart Failure with Preserved Ejection Fraction: Findings from the Echocardiographic Study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circ. Heart Fail. 2014, 7, 740–751. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39.e14. [Google Scholar] [CrossRef]
- Almeida, P.; Rodrigues, J.; Lourenço, P.; Maciel, M.J.; Bettencourt, P. Left atrial volume index is critical for the diagnosis of heart failure with preserved ejection fraction. J. Cardiovasc. Med. 2018, 19, 304–309. [Google Scholar] [CrossRef]
- Kasner, M.; Westermann, D.; Lopez, B.; Gaub, R.; Escher, F.; Kühl, U.; Schultheiss, H.-P.; Tschöpe, C. Diastolic Tissue Doppler Indexes Correlate with the Degree of Collagen Expression and Cross-Linking in Heart Failure and Normal Ejection Fraction. J. Am. Coll. Cardiol. 2011, 57, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J.-Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Reddy, Y.N.V.; Borlaug, B.A. The Role of Echocardiography in Heart Failure with Preserved Ejection Fraction: What Do We Want from Imaging? Heart Fail Clin. 2019, 15, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Kasner, M.; Gaub, R.; Sinning, D.; Westermann, D.; Steendijk, P.; Hoffmann, W.; Schultheiss, H.-P.; Tschöpe, C. Global strain rate imaging for the estimation of diastolic function in HFNEF compared with pressure-volume loop analysis. Eur. J. Echocardiogr. 2010, 11, 743–751. [Google Scholar] [CrossRef][Green Version]
- Zuber, M.; Cuculi, F.; Attenhofer Jost, C.H.; Kipfer, P.; Buser, P.; Seifert, B.; Erne, P. Value of brain natriuretic peptides in primary care patients with the clinical diagnosis of chronic heart failure. Scand. Cardiovasc. J. 2009, 43, 324–329. [Google Scholar] [CrossRef]
- Januzzi, J.L.; van Kimmenade, R.; Lainchbury, J.; Bayes-Genis, A.; Ordonez-Llanos, J.; Santalo-Bel, M.; Pinto, Y.M.; Richards, M. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: An international pooled analysis of 1256 patients: The International Collaborative of NT-proBNP Study. Eur. Heart J. 2005, 27, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Mallick, A.; Januzzi, J.L., Jr. Biomarkers in acute heart failure. Rev. Esp. Cardiol. 2015, 68, 514–525. [Google Scholar] [CrossRef]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.; Kozhuharov, N.; Coats, A.J.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef]
- Leong, D.P.; De Pasquale, C.G.; Selvanayagam, J.B. Heart Failure with Normal Ejection Fraction: The Complementary Roles of Echocardiography and CMR Imaging. JACC Cardiovasc. Imaging 2010, 3, 409–420. [Google Scholar] [CrossRef]
- Gori, M.; Iacovoni, A.; Senni, M. Haemodynamics of Heart Failure with Preserved Ejection Fraction: A Clinical Perspective. Card. Fail. Rev. 2016, 2, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Wilhelm, M.; Halle, M.; Van Craenenbroeck, E.; Kemps, H.; de Boer, R.A.; Coats, A.J.; Lund, L.; Mancini, D.; Borlaug, B.; et al. Exercise testing in heart failure with preserved ejection fraction: An appraisal through diagnosis, pathophysiology and therapy—A clinical consensus statement of the Heart Failure Association and European Association of Preventive Cardiology of the European Society of Cardiology. Eur. J. Heart Fail. 2022, 24, 1327–1345. [Google Scholar] [CrossRef] [PubMed]
- Baratto, C.; Caravita, S.; Soranna, D.; Faini, A.; Dewachter, C.; Zambon, A.; Perego, G.B.; Bondue, A.; Senni, M.; Badano, L.P.; et al. Current Limitations of Invasive Exercise Hemodynamics for the Diagnosis of Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2021, 14, e007555. [Google Scholar] [CrossRef] [PubMed]
- Obokata, M.; Kane, G.C.; Reddy, Y.N.V.; Olson, T.P.; Melenovsky, V.; Borlaug, B.A. Role of Diastolic Stress Testing in the Evaluation for Heart Failure with Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation 2017, 135, 825–838. [Google Scholar] [CrossRef] [PubMed]
- Shim, C.Y. Heart Failure with Preserved Ejection Fraction: The Major Unmet Need in Cardiology. Korean Circ. J. 2020, 50, 1051–1061. [Google Scholar] [CrossRef]
- Kessler, E.L.; Oerlemans, M.I.; Hoogen, P.V.D.; Yap, C.; Sluijter, J.P.; de Jager, S.C. Immunomodulation in Heart Failure with Preserved Ejection Fraction: Current State and Future Perspectives. J. Cardiovasc. Transl. Res. 2020, 14, 63–74. [Google Scholar] [CrossRef]
- Sweeney, M.; Corden, B.; Cook, S.A. Targeting cardiac fibrosis in heart failure with preserved ejection fraction: Mirage or miracle? EMBO Mol. Med. 2020, 12, e10865. [Google Scholar] [CrossRef]
- Wilck, N.; Markó, L.; Balogh, A.; Kräker, K.; Herse, F.; Bartolomaeus, H.; Szijártó, I.A.; Gollasch, M.; Reichhart, N.; Strauss, O.; et al. Nitric oxide–sensitive guanylyl cyclase stimulation improves experimental heart failure with preserved ejection fraction. J. Clin. Investig. 2018, 3, e96006. [Google Scholar] [CrossRef]
- Kumar, A.A.; Kelly, D.P.; Chirinos, J.A. Mitochondrial Dysfunction in Heart Failure with Preserved Ejection Fraction. Circulation 2019, 139, 1435–1450. [Google Scholar] [CrossRef]
- Frisk, M.; Le, C.; Shen, X.; Røe, T.; Hou, Y.; Manfra, O.; Silva, G.J.; van Hout, I.; Norden, E.S.; Aronsen, J.M.; et al. Etiology-Dependent Impairment of Diastolic Cardiomyocyte Calcium Homeostasis in Heart Failure with Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 405–419. [Google Scholar] [CrossRef]
- Golab-Janowska, M.; Paczkowska, E.; Machalinski, B.; Kotlega, D.; Meller, A.; Safranow, K.; Wankowicz, P.; Nowacki, P. Elevated Inflammatory Parameter Levels Negatively Impact Populations of Circulating Stem Cells (CD133+), Early Endothelial Progenitor Cells (CD133+/VEGFR2+), and Fibroblast Growth Factor in Stroke Patients. Curr. Neurovascular Res. 2019, 16, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Frljak, S.; Poglajen, G.; Vrtovec, B. Cell Therapy in Heart Failure with Preserved Ejection Fraction. Card. Fail. Rev. 2022, 8, e08. [Google Scholar] [CrossRef] [PubMed]
- Lim, G.B. Neurohormonal activation in HFpEF. Nat. Rev. Cardiol. 2019, 16, 700. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aimo, A.; Senni, M.; Barison, A.; Panichella, G.; Passino, C.; Bayes-Genis, A.; Emdin, M. Management of heart failure with preserved ejection fraction: From neurohormonal antagonists to empagliflozin. Heart Fail. Rev. 2022. [Google Scholar] [CrossRef] [PubMed]
- Faris, R.; Flather, M.; Purcell, H.; Henein, M.; Poole-Wilson, P.; Coats, A. Current evidence supporting the role of diuretics in heart failure: A meta analysis of randomised controlled trials. Int. J. Cardiol. 2002, 82, 149–158. [Google Scholar] [CrossRef]
- Schwartzenberg, S.; Redfield, M.M.; From, A.M.; Sorajja, P.; Nishimura, R.A.; Borlaug, B.A. Effects of Vasodilation in Heart Failure with Preserved or Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2012, 59, 442–451. [Google Scholar] [CrossRef]
- Miller, W.L.; Mullan, B.P. Volume Overload Profiles in Patients with Preserved and Reduced Ejection Fraction Chronic Heart Failure. JACC Heart Fail. 2016, 4, 453–459. [Google Scholar] [CrossRef]
- Felker, G.M.; Lee, K.L.; Bull, D.A.; Redfield, M.M.; Stevenson, L.W.; Goldsmith, S.R.; LeWinter, M.M.; Deswal, A.; Rouleau, J.L.; Ofili, E.O.; et al. Diuretic Strategies in Patients with Acute Decompensated Heart Failure. N. Engl. J. Med. 2011, 364, 797–805. [Google Scholar] [CrossRef]
- He, X.; Dong, B.; Xue, R.; Zhao, J.; Wu, Z.; Wu, Y.; Zhou, Y.; Wu, D.; Dong, Y.; He, J.; et al. Effect of aggressive diuresis in acute heart failure with reduced and preserved ejection fraction. ESC Heart Fail. 2021, 8, 3248–3256. [Google Scholar] [CrossRef]
- Longobardi, S.; Sher, A.; Niederer, S.A. In silico identification of potential calcium dynamics and sarcomere targets for recovering left ventricular function in rat heart failure with preserved ejection fraction. PLOS Comput. Biol. 2021, 17, e1009646. [Google Scholar] [CrossRef]
- Eisner, D.A.; Caldwell, J.L.; Trafford, A.W.; Hutchings, D. The Control of Diastolic Calcium in the Heart. Circ. Res. 2020, 126, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Ríos, E. Calcium-induced release of calcium in muscle: 50 years of work and the emerging consensus. J. Gen. Physiol. 2018, 150, 521–537. [Google Scholar] [CrossRef]
- Peana, D.; Domeier, T.L. Cardiomyocyte Ca2+ homeostasis as a therapeutic target in heart failure with reduced and preserved ejection fraction. Curr. Opin. Pharmacol. 2017, 33, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Kilfoil, P.J.; Lotteau, S.; Zhang, R.; Yue, X.; Aynaszyan, S.; Solymani, R.E.; Cingolani, E.; Marbán, E.; Goldhaber, J.I. Distinct features of calcium handling and β-adrenergic sensitivity in heart failure with preserved versus reduced ejection fraction. J. Physiol. 2020, 598, 5091–5108. [Google Scholar] [CrossRef] [PubMed]
- Benitah, J.-P.; Perrier, R.; Mercadier, J.-J.; Pereira, L.; Gómez, A.M. RyR2 and Calcium Release in Heart Failure. Front. Physiol. 2021, 12, 734210. [Google Scholar] [CrossRef] [PubMed]
- Adeniran, I.; MacIver, D.H.; Hancox, J.C.; Zhang, H. Abnormal calcium homeostasis in heart failure with pre-served ejection fraction is related to both reduced contractile function and incomplete relaxation: An electromecha-nically detailed biophysical modeling study. Front. Physiol. 2015, 6, 78. [Google Scholar] [CrossRef]
- Rouhana, S.; Farah, C.; Roy, J.; Finan, A.; de Araujo, G.R.; Bideaux, P.; Scheuermann, V.; Saliba, Y.; Reboul, C.; Cazorla, O.; et al. Early calcium handling imbalance in pressure overload-induced heart failure with nearly normal left ventricular ejection fraction. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 230–242. [Google Scholar] [CrossRef]
- Wijk, S.S.-V.; Tromp, J.; Beussink-Nelson, L.; Hage, C.; Svedlund, S.; Saraste, A.; Swat, S.A.; Sanchez, C.; Njoroge, J.; Tan, R.-S.; et al. Proteomic Evaluation of the Comorbidity-Inflammation Paradigm in Heart Failure with Preserved Ejection Fraction. Circulation 2020, 142, 2029–2044. [Google Scholar] [CrossRef]
- Lai, P.; Nikolaev, V.O.; De Jong, K.A. Understanding the Role of SERCA2a Microdomain Remodeling in Heart Failure Induced by Obesity and Type 2 Diabetes. J. Cardiovasc. Dev. Dis. 2022, 9, 163. [Google Scholar] [CrossRef]
- Maier, L.S.; Layug, B.; Karwatowska-Prokopczuk, E.; Belardinelli, L.; Lee, S.; Sander, J.; Lang, C.; Wachter, R.; Edelmann, F.; Hasenfuss, G.; et al. RAnoLazIne for the treatment of diastolic heart failure in patients with preserved ejection fraction: The RA-LI-DHF proof-of-concept study. JACC Heart Fail. 2013, 1, 115–122. [Google Scholar] [CrossRef]
- Flather, M.D.; Shibata, M.C.; Coats, A.J.; Van Veldhuisen, D.J.; Parkhomenko, A.; Borbola, J.; Cohen-Solal, A.; Dumitrascu, D.; Ferrari, R.; Lechat, P.; et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS). Eur. Heart J. 2005, 26, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Torre, E.; Arici, M.; Lodrini, A.M.; Ferrandi, M.; Barassi, P.; Hsu, S.-C.; Chang, G.-J.; Boz, E.; Sala, E.; Vagni, S.; et al. SERCA2a stimulation by istaroxime improves intracellular Ca2+ handling and diastolic dysfunction in a model of diabetic cardiomyopathy. Cardiovasc. Res. 2021, 118, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Rich, M.W.; Fleg, J.L.; Zile, M.; Young, J.B.; Kitzman, D.W.; Love, T.E.; Aronow, W.S.; Adams, K.F.; Gheorghiade, M. Effects of Digoxin on Morbidity and Mortality in Diastolic Heart Failure. Circulation 2006, 114, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Zile, M.R.; Baicu, C.F.; Ikonomidis, J.S.; Stroud, R.E.; Nietert, P.J.; Bradshaw, A.D.; Slater, R.; Palmer, B.M.; Van Buren, P.; Meyer, M.; et al. Myocardial Stiffness in Patients with Heart Failure and a Preserved Ejection Fraction. Circulation 2015, 131, 1247–1259. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kass, D.A. Heart Failure with Preserved Ejection Fraction. Circ. Res. 2014, 115, 79–96. [Google Scholar] [CrossRef]
- Levine, B.; Kalman, J.; Mayer, L.; Fillit, H.M.; Packer, M. Elevated Circulating Levels of Tumor Necrosis Factor in Severe Chronic Heart Failure. N. Engl. J. Med. 1990, 323, 236–241. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Weston, S.A.; Redfield, M.M.; Killian, J.M.; Roger, V.L. Tumor Necrosis Factor-α and Mortality in Heart Failure. Circulation 2008, 118, 625–631. [Google Scholar] [CrossRef]
- Glezeva, N.; Baugh, J.A. Role of inflammation in the pathogenesis of heart failure with preserved ejection fraction and its potential as a therapeutic target. Heart Fail. Rev. 2013, 19, 681–694. [Google Scholar] [CrossRef]
- Westermann, D.; Lindner, D.; Kasner, M.; Zietsch, C.; Savvatis, K.; Escher, F.; von Schlippenbach, J.; Skurk, C.; Steendijk, P.; Riad, A.; et al. Cardiac Inflammation Contributes to Changes in the Extracellular Matrix in Patients with Heart Failure and Normal Ejection Fraction. Circ. Heart Fail. 2011, 4, 44–52. [Google Scholar] [CrossRef]
- Izumiya, Y.; Hanatani, S.; Kimura, Y.; Takashio, S.; Yamamoto, E.; Kusaka, H.; Tokitsu, T.; Rokutanda, T.; Araki, S.; Tsujita, K.; et al. Growth Differentiation Factor-15 Is a Useful Prognostic Marker in Patients with Heart Failure with Preserved Ejection Fraction. Can. J. Cardiol. 2014, 30, 338–344. [Google Scholar] [CrossRef]
- Fernandez, A.B.M.; Ferrero-Gregori, A.; Garcia-Osuna, A.; Mirabet-Perez, S.; Pirla-Buxo, M.J.; Cinca-Cuscullola, J.; Ordonez-Llanos, J.; Minguell, E.R. Growth differentiation factor 15 as mortality predictor in heart failure patients with non-reduced ejection fraction. ESC Heart Fail. 2020, 7, 2223–2229. [Google Scholar] [CrossRef] [PubMed]
- Santhanakrishnan, R.; Chong, J.P.; Ng, T.P.; Ling, L.H.; Sim, D.; Leong, K.T.G.; Yeo, P.S.D.; Ong, H.Y.; Jaufeerally, F.; Wong, R.; et al. Growth differentiation factor 15, ST2, high-sensitivity troponin T, and N-terminal pro brain natriuretic peptide in heart failure with preserved vs. reduced ejection fraction. Eur. J. Heart Fail. 2012, 14, 1338–1347. [Google Scholar] [CrossRef]
- Sanada, S.; Hakuno, D.; Higgins, L.J.; Schreiter, E.; McKenzie, A.N.; Lee, R.T. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J. Clin. Investig. 2007, 117, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- AbouEzzeddine, O.F.; McKie, P.M.; Dunlay, S.M.; Stevens, S.R.; Felker, G.M.; Borlaug, B.A.; Chen, H.H.; Tracy, R.P.; Braunwald, E.; Redfield, M.M. Soluble ST2 in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e004382. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, A.; Raza, S.; Sun, J.L.; Anstrom, K.J.; Tracy, R.; Steiner, J.; VanBuren, P.; LeWinter, M.M. Pro-Inflammatory Biomarkers in Stable Versus Acutely Decompensated Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2018, 7, e007385. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Rodolico, D.; Hill, J.A. Metabolic inflammation in heart failure with preserved ejection fraction. Cardiovasc. Res. 2020, 117, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Ather, S.; Chan, W.; Bozkurt, B.; Aguilar, D.; Ramasubbu, K.; Zachariah, A.A.; Wehrens, X.H.; Deswal, A. Impact of Noncardiac Comorbidities on Morbidity and Mortality in a Predominantly Male Population with Heart Failure and Preserved Versus Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2012, 59, 998–1005. [Google Scholar] [CrossRef]
- Jin, X.; Yao, T.; Zhou, Z.; Zhu, J.; Zhang, S.; Hu, W.; Shen, C. Advanced Glycation End Products Enhance Macrophages Polarization into M1 Phenotype through Activating RAGE/NF-κB Pathway. BioMed Res. Int. 2015, 2015, 732450. [Google Scholar] [CrossRef]
- DeBerge, M.; Shah, S.J.; Wilsbacher, L.; Thorp, E.B. Macrophages in Heart Failure with Reduced versus Preserved Ejection Fraction. Trends Mol. Med. 2019, 25, 328–340. [Google Scholar] [CrossRef]
- Glezeva, N.; Voon, V.; Watson, C.; Horgan, S.; McDonald, K.; Ledwidge, M.; Baugh, J. Exaggerated Inflammation and Monocytosis Associate with Diastolic Dysfunction in Heart Failure with Preserved Ejection Fraction: Evidence of M2 Macrophage Activation in Disease Pathogenesis. J. Card. Fail. 2014, 21, 167–177. [Google Scholar] [CrossRef]
- Hulsmans, M.; Sager, H.B.; Roh, J.D.; Valero-Muñoz, M.; Houstis, N.E.; Iwamoto, Y.; Sun, Y.; Wilson, R.M.; Wojtkiewicz, G.; Tricot, B.; et al. Cardiac macrophages promote diastolic dysfunction. J. Exp. Med. 2018, 215, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.A.; Dodd, S.; Clayton, D.; Bedson, E.; Eccleson, H.; Schelbert, E.B.; Naish, J.H.; Jimenez, B.D.; Williams, S.G.; Cunnington, C.; et al. Pirfenidone in heart failure with preserved ejection fraction: A randomized phase 2 trial. Nat. Med. 2021, 27, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Roy, C.; Slimani, A.; de Meester, C.; Amzulescu, M.; Pasquet, A.; Vancraeynest, D.; Beauloye, C.; Vanoverschelde, J.; Gerber, B.L.; Pouleur, A. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic re-sonance in heart failure with preserved ejection fraction. J. Cardiovasc. Magn. Reson. 2018, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.A.; Rosala-Hallas, A.; Dodd, S.; Schelbert, E.B.; Williams, S.G.; Cunnington, C.; McDonagh, T.; Miller, C.A. Characteristics Associated with Growth Differentiation Factor 15 in Heart Failure with Preserved Ejection Fraction and the Impact of Pirfenidone. J. Am. Heart Assoc. 2022, 11, e024668. [Google Scholar] [CrossRef] [PubMed]
- Savill, P. Spironolactone in heart failure with preserved ejection fraction. Practitioner 2014, 258, 10. [Google Scholar] [CrossRef]
- de Denus, S.; O’Meara, E.; Desai, A.S.; Claggett, B.; Lewis, E.F.; Leclair, G.; Jutras, M.; Lavoie, J.; Solomon, S.D.; Pitt, B.; et al. Spironolactone Metabolites in TOPCAT—New Insights into Regional Variation. N. Engl. J. Med. 2017, 376, 1690–1692. [Google Scholar] [CrossRef]
- López, B.; Ravassa, S.; Moreno, M.U.; José, G.S.; Beaumont, J.; González, A.; Díez, J. Diffuse myocardial fibrosis: Mechanisms, diagnosis and therapeutic approaches. Nat. Rev. Cardiol. 2021, 18, 479–498. [Google Scholar] [CrossRef]
- Rol, N.; De Raaf, M.A.; Sun, X.Q.; Kuiper, V.P.; Bos, D.D.S.G.; Happé, C.; Kurakula, K.; Dickhoff, C.; Thuillet, R.; Tu, L.; et al. Nintedanib improves cardiac fibrosis but leaves pulmonary vascular remodelling unaltered in experimental pulmonary hypertension. Cardiovasc. Res. 2018, 115, 432–439. [Google Scholar] [CrossRef]
- Burke, R.M.; Dirkx, R.A., Jr.; Quijada, P.; Lighthouse, J.K.; Mohan, A.; O’Brien, M.; Wojciechowski, W.; Woeller, C.F.; Phipps, R.P.; Alexis, J.D.; et al. Prevention of Fibrosis and Pathological Cardiac Remodeling by Salinomycin. Circ. Res. 2021, 128, 1663–1678. [Google Scholar] [CrossRef]
- Yu, L.; Ruifrok, W.P.; Meissner, M.; Bos, E.M.; van Goor, H.; Sanjabi, B.; van der Harst, P.; Pitt, B.; Goldstein, I.J.; Koerts, J.A.; et al. Genetic and Pharmacological Inhibition of Galectin-3 Prevents Cardiac Remodeling by Interfering with Myocardial Fibrogenesis. Circ. Heart Fail. 2013, 6, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Gehlken, C.P.G.; van der Velde, A.R.; Meijers, W.C.; Silljé, H.H.; Muntendam, P.; Dokter, M.M.; van Gilst, W.H.; Schols, H.A.; de Boer, R.A. Pectins from various sources inhibit galectin-3-related cardiac fibrosis. Curr. Res. Transl. Med. 2021, 70, 103321. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.L.; Mcmurray, J.; Packer, M.; Swedberg, K.; Borer, J.S.; Colucci, W.; Djian, J.; Drexler, H.; Feldman, A.; Kober, L.; et al. Targeted Anticytokine Therapy in Patients with Chronic Heart Failure. Circulation 2004, 109, 1594–1602. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.S.; Packer, M.; Lo, K.H.; Fasanmade, A.A.; Willerson, J.T. Randomized, Double-Blind, Placebo-Controlled, Pilot Trial of Infliximab, a Chimeric Monoclonal Antibody to Tumor Necrosis Factor-α, in Patients with Moderate-to-Severe Heart Failure. Circulation 2003, 107, 3133–3140. [Google Scholar] [CrossRef]
- Van Tassell, B.W.; Trankle, C.R.; Canada, J.M.; Carbone, S.; Buckley, L.; Kadariya, D.; Del Buono, M.G.; Billingsley, H.; Wohlford, G.; Viscusi, M.; et al. IL-1 Blockade in Patients with Heart Failure with Preserved Ejection Fraction. Circ. Heart Fail. 2018, 11, e005036. [Google Scholar] [CrossRef]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef]
- Shen, S.; Duan, J.; Hu, J.; Qi, Y.; Kang, L.; Wang, K.; Chen, J.; Wu, X.; Xu, B.; Gu, R. Colchicine alleviates inflammation and improves diastolic dysfunction in heart failure rats with preserved ejection fraction. Eur. J. Pharmacol. 2022, 929, 175126. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Muscoli, S.; Barillà, F.; Tajmir, R.; Meloni, M.; Della Morte, D.; Bellia, A.; Di Daniele, N.; Lauro, D.; Andreadi, A. The New Role of SGLT2 Inhibitors in the Management of Heart Failure: Current Evidence and Future Perspective. Pharmaceutics 2022, 14, 1730. [Google Scholar] [CrossRef] [PubMed]
- Kolijn, D.; Pabel, S.; Tian, Y.; Lódi, M.; Herwig, M.; Carrizzo, A.; Zhazykbayeva, S.; Kovács, Á.; Fülöp, G.; Falcão-Pires, I.; et al. Empagliflozin improves endothelial and cardiomyocyte function in human heart failure with preserved ejection fraction via reduced pro-inflammatory-oxidative pathways and protein kinase Gα oxidation. Cardiovasc. Res. 2020, 117, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Cappetta, D.; Russo, R.; Rivellino, A.; Ciuffreda, L.P.; Roviezzo, F.; Piegari, E.; Berrino, L.; Rossi, F.; De Angelis, A.; et al. Sitagliptin reduces inflammation, fibrosis and preserves diastolic function in a rat model of heart failure with preserved ejection fraction. J. Cereb. Blood Flow Metab. 2017, 174, 4070–4086. [Google Scholar] [CrossRef]
- Cameron, A.R.; Morrison, V.; Levin, D.; Mohan, M.; Forteath, C.; Beall, C.; McNeilly, A.; Balfour, D.J.; Savinko, T.; Wong, A.K.; et al. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ. Res. 2016, 119, 652–665. [Google Scholar] [CrossRef]
- Clementi, E.; Nisoli, E. Nitric oxide and mitochondrial biogenesis: A key to long-term regulation of cellular metabolism. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 142, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Kojda, G.; Kottenberg, K.; Nix, P.; Schlüter, K.D.; Piper, H.M.; Noack, E. Low Increase in cGMP Induced by Organic Nitrates and Nitrovasodilators Improves Contractile Response of Rat Ventricular Myocytes. Circ. Res. 1996, 78, 91–101. [Google Scholar] [CrossRef]
- Paulus, W.J.; Bronzwaer, J.G.F. Nitric oxide’s role in the heart: Control of beating or breathing? Am. J. Physiol. Circ. Physiol. 2004, 287, H8–H13. [Google Scholar] [CrossRef]
- Mishra, S.; Kass, D.A. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2021, 18, 400–423, Correction in Nat. Rev. Cardiol. 2021, 18, 735. [Google Scholar] [CrossRef]
- Singh, P.; Vijayakumar, S.; Kalogeroupoulos, A.; Butler, J. Multiple Avenues of Modulating the Nitric Oxide Pathway in Heart Failure Clinical Trials. Curr. Heart Fail. Rep. 2018, 15, 44–52. [Google Scholar] [CrossRef]
- Greene, S.J.; Gheorghiade, M.; Borlaug, B.A.; Pieske, B.; Vaduganathan, M.; Burnett, J.C.; Roessig, L.; Stasch, J.; Solomon, S.D.; Paulus, W.J.; et al. The cGMP Signaling Pathway as a Therapeutic Target in Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2013, 2, e000536. [Google Scholar] [CrossRef]
- Gundewar, S.; Calvert, J.; Jha, S.; Toedt-Pingel, I.; Ji, S.Y.; Nunez, D.; Ramachandran, A.; Anaya-Cisneros, M.; Tian, R.; Lefer, D.J. Activation of AMP-Activated Protein Kinase by Metformin Improves Left Ventricular Function and Survival in Heart Failure. Circ. Res. 2009, 104, 403–411. [Google Scholar] [CrossRef]
- van Heerebeek, L.; Hamdani, N.; Falcão-Pires, I.; Leite-Moreira, A.F.; Begieneman, M.P.; Bronzwaer, J.G.; van der Velden, J.; Stienen, G.J.; Laarman, G.J.; Somsen, A.; et al. Low Myocardial Protein Kinase G Activity in Heart Failure with Preserved Ejection Fraction. Circulation 2012, 126, 830–839. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.C.; Sanker, S.; Wood, K.C.; Durgin, B.G.; Straub, A.C. Redox regulation of soluble guanylyl cyclase. Nitric Oxide 2018, 76, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Redfield, M.M.; Anstrom, K.J.; Levine, J.A.; Koepp, G.A.; Borlaug, B.A.; Chen, H.H.; LeWinter, M.M.; Joseph, S.M.; Shah, S.J.; Semigran, M.J.; et al. Isosorbide Mononitrate in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2015, 373, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Zamani, P.; Akers, S.; Soto-Calderon, H.; Beraun, M.; Koppula, M.R.; Varakantam, S.; Rawat, D.; Shiva-Kumar, P.; Haines, P.G.; Chittams, J.; et al. Isosorbide Dinitrate, with or without Hydralazine, Does Not Reduce Wave Reflections, Left Ventricular Hypertrophy, or Myocardial Fibrosis in Patients with Heart Failure with Preserved Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e004262. [Google Scholar] [CrossRef]
- Ullah, W.; Mukhtar, M.; Al-Mukhtar, A.; Saeed, R.; Boigon, M.; Haas, D.; Rame, E. Safety and efficacy of soluble guanylate cyclase stimulators in patients with heart failure: A systematic review and meta-analysis. World J. Cardiol. 2020, 12, 501–512. [Google Scholar] [CrossRef]
- Cordwin, D.J.; Berei, T.J.; Pogue, K.T. The Role of sGC Stimulators and Activators in Heart Failure with Reduced Ejection Fraction. J. Cardiovasc. Pharmacol. Ther. 2021, 26, 593–600. [Google Scholar] [CrossRef]
- Pieske, B.; Maggioni, A.P.; Lam, C.S.; Pieske-Kraigher, E.; Filippatos, G.; Butler, J.; Ponikowski, P.; Shah, S.; Solomon, S.D.; Scalise, A.-V.; et al. Vericiguat in patients with worsening chronic heart failure and preserved ejection fraction: Results of the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED EF (SOCRATES-PRESERVED) study. Eur. Heart J. 2017, 38, 1119–1127. [Google Scholar] [CrossRef]
- John, G.F. Cleland, Christian Mueller, What can we learn from SOCRATES: More questions than answers? Eur. Heart J. 2017, 38, 1128–1131. [Google Scholar] [CrossRef]
- Filippatos, G.; Maggioni, A.P.; Lam, C.S.; Pieske-Kraigher, E.; Butler, J.; Spertus, J.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; Scalise, A.-V.; et al. Patient-reported outcomes in the SOluble guanylate Cyclase stimulatoR in heArT failurE patientS with PRESERVED ejection fraction (SOCRATES-PRESERVED) study. Eur. J. Heart Fail. 2017, 19, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.W.; Lam, C.S.P.; Anstrom, K.J.; Ezekowitz, J.; Hernandez, A.F.; O’Connor, C.M.; Pieske, B.; Ponikowski, P.; Shah, S.J.; Solomon, S.D.; et al. Effect of Vericiguat vs Placebo on Quality of Life in Patients with Heart Failure and Preserved Ejection Frac-tion: The VITALITY-HFpEF Randomized Clinical Trial. JAMA 2020, 324, 1512–1521. [Google Scholar] [CrossRef] [PubMed]
- Udelson, J.E.; Lewis, G.D.; Shah, S.J.; Zile, M.R.; Redfield, M.M.; Burnett, J.; Parker, J.; Seferovic, J.P.; Wilson, P.; Mittleman, R.S.; et al. Effect of Praliciguat on Peak Rate of Oxygen Consumption in Patients With Heart Failure With Preserved Ejection Fraction: The CAPACITY HFpEF Randomized Clinical Trial. JAMA 2020, 324, 1522–1531. [Google Scholar] [CrossRef]
- Ghofrani, H.-A.; Galiè, N.; Grimminger, F.; Grünig, E.; Humbert, M.; Jing, Z.-C.; Keogh, A.M.; Langleben, D.; Kilama, M.O.; Fritsch, A.; et al. Riociguat for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2013, 369, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Dachs, T.M.; Duca, F.; Rettl, R.; Binder-Rodriguez, C.; Dalos, D.; Ligios, L.C.; Kammerlander, A.; Grünig, E.; Pretsch, I.; Steringer-Mascherbauer, R.; et al. Riociguat in pulmonary hypertension and heart failure with preserved ejection fraction: The haemoDYNAMIC trial. Eur. Heart J. 2022, 43, 3402–3413. [Google Scholar] [CrossRef] [PubMed]
- Emdin, M.; Aimo, A.; Castiglione, V.; Vergaro, G.; Georgiopoulos, G.; Saccaro, L.F.; Lombardi, C.M.; Passino, C.; Cerbai, E.; Metra, M.; et al. Targeting Cyclic Guanosine Monophosphate to Treat Heart Failure. J. Am. Coll. Cardiol. 2020, 76, 1795–1807. [Google Scholar] [CrossRef]
- Infante, T.; Costa, D.; Napoli, C. Novel Insights Regarding Nitric Oxide and Cardiovascular Diseases. Angiology 2021, 72, 411–425. [Google Scholar] [CrossRef]
- Guazzi, M.; Vicenzi, M.; Arena, R.; Guazzi, M.D. PDE5 inhibition with sildenafil improves left ventricular diastolic function, cardiac geometry, and clinical status in patients with stable systolic heart failure: Results of a 1-year, prospective, randomized, place-bo-controlled study. Circ. Heart Fail. 2011, 4, 8–17. [Google Scholar] [CrossRef]
- Andersen, M.J.; Ersbøll, M.; Axelsson, A.; Gustafsson, F.; Hassager, C.; Køber, L.; Borlaug, B.A.; Boesgaard, S.; Skovgaard, L.T.; Møller, J.E. Sildenafil and Diastolic Dysfunction after Acute Myocardial Infarction in Patients with Preserved Ejection Fraction. Circulation 2013, 127, 1200–1208. [Google Scholar] [CrossRef]
- Redfield, M.M.; Chen, H.H.; Borlaug, B.A.; Semigran, M.J.; Lee, K.L.; Lewis, G.D.; LeWinter, M.M.; Rouleau, J.L.; Bull, D.A.; Mann, D.; et al. Effect of Phosphodiesterase-5 Inhibition on Exercise Capacity and Clinical Status in Heart Failure with Preserved Ejection Fraction. JAMA 2013, 309, 1268–1277. [Google Scholar] [CrossRef]
- Liu, L.C.; Hummel, Y.M.; van der Meer, P.; Berger, R.M.; Damman, K.; van Veldhuisen, D.J.; Voors, A.A.; Hoendermis, E.S. Effects of sildenafil on cardiac structure and function, cardiopulmonary exercise testing and health-related quality of life measures in heart failure patients with preserved ejection fraction and pulmonary hypertension. Eur. J. Heart Fail. 2016, 19, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, H.N.; Gupta, R.C.; Singh-Gupta, V.; Zhang, K.; Lanfear, D.E. Abnormalities of Mitochondrial Dynamics in the Failing Heart: Normalization Following Long-Term Therapy with Elamipretide. Cardiovasc. Drugs Ther. 2018, 32, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Luptak, I.; Sverdlov, A.L.; Panagia, M.; Qin, F.; Pimentel, D.R.; Croteau, D.; Siwik, D.A.; Ingwall, J.S.; Bachschmid, M.M.; Balschi, J.A.; et al. Decreased ATP production and myocardial contractile reserve in metabolic heart disease. J. Mol. Cell. Cardiol. 2018, 116, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Krueger, K.J.; Rahman, F.K.; Shen, Q.; Vacek, J.; Hiebert, J.B.; Pierce, J.D. Mitochondrial bioenergetics and D-ribose in HFpEF: A brief narrative review. Ann. Transl. Med. 2021, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.T.; Abozguia, K.; Shivu, G.N.; Mahadevan, G.; Ahmed, I.; Williams, L.; Dwivedi, G.; Patel, K.; Steendijk, P.; Ashrafian, H.; et al. Heart failure with preserved ejection fraction is characterized by dynamic impairment of active relaxation and contraction of the left ventricle on exercise and associated with myocardial energy deficiency. J. Am. Coll. Cardiol. 2009, 54, 402–409. [Google Scholar] [CrossRef]
- Haykowsky, M.J.; Brubaker, P.H.; Morgan, T.M.; Kritchevsky, S.; Eggebeen, J.; Kitzman, D.W. Impaired aerobic capacity and physical functional performance in older heart failure patients with pre-served ejection fraction: Role of lean body mass. J. Gerontol. Ser. A 2013, 68, 968–975. [Google Scholar] [CrossRef]
- Singh, S.; Beadle, R.; Cameron, D.; Rudd, A.; Bruce, M.; Jagpal, B.; Schwarz, K.; Brindley, G.; Mckiddie, F.; Lang, C.; et al. Randomized double-blind placebo-controlled trial of perhexiline in heart failure with preserved ejection fraction syndrome. Futur. Cardiol. 2014, 10, 693–698. [Google Scholar] [CrossRef]
- Sabbah, H.N. Targeting the Mitochondria in Heart Failure. JACC Basic Transl. Sci. 2020, 5, 88–106. [Google Scholar] [CrossRef]
- Sparagna, G.C.; Lesnefsky, E.J. Cardiolipin Remodeling in the Heart. J. Cardiovasc. Pharmacol. 2009, 53, 290–301. [Google Scholar] [CrossRef]
- Shah, S.J.; Voors, A.A.; McMurray, J.J.V.; Kitzman, D.W.; Viethen, T.; Wirtz, A.B.; Huang, E.; Pap, A.F.; Solomon, S.D. Effect of Neladenoson Bialanate on Exercise Capacity among Patients with Heart Failure with Preserved Ejection Fraction. JAMA 2019, 321, 2101–2112. [Google Scholar] [CrossRef]
- Buzhor, E.; Leshansky, L.; Blumenthal, J.; Barash, H.; Warshawsky, D.; Mazor, Y.; Shtrichman, R. Cell-based therapy approaches: The hope for incurable diseases. Regen. Med. 2014, 9, 649–672. [Google Scholar] [CrossRef] [PubMed]
- Leor, J.; Patterson, M.; Quiñones, M.; Kedes, L.; Kloner, R. Transplantation of fetal myocardial tissue into the in-farcted myocardium of rat. A potential method for repair of infarcted myocardium? Circulation 1996, 94, II332–II336. [Google Scholar]
- Taylor, D.A.; Atkins, B.Z.; Hungspreugs, P.; Jones, T.R.; Reedy, M.C.; Hutcheson, K.A.; Glower, D.D.; Kraus, W.E. Regenerating functional myocardium: Improved performance after skeletal myoblast transplantation. Nat. Med. 1998, 4, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Psaltis, P.J.; Schwarz, N.; Toledo-Flores, D.; Nicholls, S.J. Cellular therapy for heart failure. Curr. Cardiol. Rev. 2016, 12, 195–215. [Google Scholar] [CrossRef] [PubMed]
- Bartunek, J.; Terzic, A.; Davison, B.A.; Filippatos, G.S.; Radovanovic, S.; Beleslin, B.; Merkely, B.; Musialek, P.; Wojakowski, W.; Andreka, P.; et al. Cardiopoietic cell therapy for advanced ischemic heart failure: Results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. Eur. Heart J. 2016, 38, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Bartunek, J.; Behfar, A.; Dolatabadi, D.; Vanderheyden, M.; Ostojic, M.; Dens, J.; El Nakadi, B.; Banovic, M.; Beleslin, B.; Vrolix, M.; et al. Cardiopoietic stem cell therapy in heart failure: The C-CURE (cardiopoietic stem cell therapy in heart fail-URE) multicenter randomized trial with lineage-specified biologics. J. Am. Coll. Cardiol. 2013, 61, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.M.; Fishman, J.E.; Gerstenblith, G.; Velazquez, D.L.D.; Zambrano, J.P.; Suncion, V.Y.; Tracy, M.; Ghersin, E.; Johnston, P.V.; Brinker, J.A.; et al. Comparison of Allogeneic vs. Autologous Bone Marrow–Derived Mesenchymal Stem Cells Delivered by Transendocardial Injection in Patients with Ischemic Cardiomyopathy. JAMA 2012, 308, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ruiz, R.; Fernández-Avilés, F. Intracoronary ALLogeneic heart STem cells to Achieve myocardial Regeneration (ALLSTAR): A randomized, placebo-controlled, double-blinded trial. Eur. Heart J. 2020, 41, 3459–3461. [Google Scholar] [CrossRef]
- Toma, C.; Pittenger, M.F.; Cahill, K.S.; Byrne, B.J.; Kessler, P.D. Human Mesenchymal Stem Cells Differentiate to a Cardiomyocyte Phenotype in the Adult Murine Heart. Circulation 2002, 105, 93–98. [Google Scholar] [CrossRef]
- Yoshioka, T.; Ageyama, N.; Shibata, H.; Yasu, T.; Misawa, Y.; Takeuchi, K.; Matsui, K.; Yamamoto, K.; Terao, K.; Shimada, K.; et al. Repair of Infarcted Myocardium Mediated by Transplanted Bone Marrow–Derived CD34+ Stem Cells in a Nonhuman Primate Model. Stem Cells 2005, 23, 355–364. [Google Scholar] [CrossRef]
- Kinnaird, T.; Stabile, E.; Burnett, M.S.; Shou, M.; Lee, C.W.; Barr, S.; Fuchs, S.; Epstein, S.E. Local Delivery of Marrow-Derived Stromal Cells Augments Collateral Perfusion through Paracrine Mechanisms. Circulation 2004, 109, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Gnecchi, M.; He, H.; Liang, O.D.; Melo, L.G.; Morello, F.; Mu, H.; Noiseux, N.; Zhang, L.; Pratt, R.E.; Ingwall, J.S.; et al. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat. Med. 2005, 11, 367–368. [Google Scholar] [CrossRef] [PubMed]
- Menasche, P.; Alfieri, O.; Janssens, S.; McKenna, W.; Reichenspurner, H.; Trinquart, L.; Vilquin, J.-T.; Marolleau, J.-P.; Seymour, B.; Larghero, J.; et al. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) Trial. Circulation 2008, 117, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Gongora, E. Stem cell therapy in heart failure: Where do we stand today? Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1866, 165489. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.-L.; Li, Q.; Rokosh, G.; Sanganalmath, S.K.; Chen, N.; Ou, Q.; Stowers, H.; Hunt, G.; Bolli, R. Long-Term Outcome of Administration of c-kitPOS Cardiac Progenitor Cells after Acute Myocardial Infarction: Transplanted Cells Do Not Become Cardiomyocytes, Structural and Functional Improvement and Prolifer-ation of Endogenous Cells Persist for at Least One Year. Circ. Res. 2016, 118, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Johnston, P.V.; Sasano, T.; Mills, K.; Evers, R.; Lee, S.-T.; Smith, R.R.; Lardo, A.C.; Lai, S.; Steenbergen, C.; Gerstenblith, G.; et al. Engraftment, Differentiation, and Functional Benefits of Autologous Cardiosphere-Derived Cells in Porcine Ischemic Cardiomyopathy. Circulation 2009, 120, 1075–1083. [Google Scholar] [CrossRef]
- Malliaras, K.; Makkar, R.R.; Smith, R.R.; Cheng, K.; Wu, E.; Bonow, R.O.; Marbán, L.; Mendizabal, A.; Cingolani, E.; Johnston, P.V.; et al. Intracoronary Cardiosphere-Derived Cells after Myocardial Infarction. J. Am. Coll. Cardiol. 2014, 63, 110–122. [Google Scholar] [CrossRef]
- Gallet, R.; de Couto, G.; Simsolo, E.; Valle, J.; Sun, B.; Liu, W.; Tseliou, E.; Zile, M.R.; Marbán, E. Cardiosphere-Derived Cells Reverse Heart Failure with Preserved Ejection Fraction in Rats by Decreasing Fibrosis and Inflammation. JACC Basic Transl. Sci. 2016, 1, 14–28. [Google Scholar] [CrossRef]
- Cho, J.H.; Kilfoil, P.J.; Zhang, R.; Solymani, R.E.; Bresee, C.; Kang, E.M.; Luther, K.; Rogers, R.G.; De Couto, G.; Goldhaber, J.I.; et al. Reverse electrical remodeling in rats with heart failure and preserved ejection fraction. J. Clin. Investig. 2018, 3, e121123. [Google Scholar] [CrossRef]
- Valgimigli, M.; Rigolin, G.M.; Fucili, A.; Della Porta, M.; Soukhomovskaia, O.; Malagutti, P.; Bugli, A.M.; Bragotti, L.Z.; Francolini, G.; Mauro, E.; et al. CD34+ and Endothelial Progenitor Cells in Patients with Various Degrees of Congestive Heart Failure. Circulation 2004, 110, 1209–1212. [Google Scholar] [CrossRef]
- Poglajen, G.; Frljak, S.; Zemljič, G.; Cerar, A.; Okrajšek, R.; Šebeštjen, M.; Vrtovec, B. Stem Cell Therapy for Chronic and Advanced Heart Failure. Curr. Heart Fail. Rep. 2020, 17, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Vrtovec, B.; Frljak, S.; Poglajen, G.; Zemljic, G.; Cerar, A.; Sever, M.; Haddad, F.; Wu, J.C. A pilot clinical trial of cell therapy in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2022, 24, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Hasenfuß, G.; Hayward, C.; Burkhoff, D.; Silvestry, F.E.; McKenzie, S.; Gustafsson, F.; Malek, F.; Van der Heyden, J.; Lang, I.; Petrie, M.C.; et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): A multicentre, open-label, single-arm, phase 1 trial. Lancet 2016, 387, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Rosalia, L.; Ozturk, C.; Shoar, S.; Fan, Y.; Malone, G.; Cheema, F.H.; Conway, C.; Byrne, R.A.; Duffy, G.P.; Malone, A.; et al. Device-Based Solutions to Improve Cardiac Physiology and Hemodynamics in Heart Failure with Preserved Ejection Fraction. JACC Basic Transl. Sci. 2021, 6, 772–795. [Google Scholar] [CrossRef]
- Burkhoff, D.; Maurer, M.S.; Joseph, S.M.; Rogers, J.G.; Birati, E.Y.; Rame, J.E.; Shah, S.J. Left Atrial Decompression Pump for Severe Heart Failure with Preserved Ejection Fraction. JACC Heart Fail. 2015, 3, 275–282. [Google Scholar] [CrossRef]
- Moscato, F.; Wirrmann, C.; Granegger, M.; Eskandary, F.; Zimpfer, D.; Schima, H. Use of continuous flow ventricular assist devices in patients with heart failure and a normal ejection fraction: A computer-simulation study. J. Thorac. Cardiovasc. Surg. 2012, 145, 1352–1358. [Google Scholar] [CrossRef]
- Søndergaard, L.; Reddy, V.; Kaye, D.; Malek, F.; Walton, A.; Mates, M.; Franzen, O.; Neuzil, P.; Ihlemann, N.; Gustafsson, F. Transcatheter treatment of heart failure with preserved or mildly reduced ejection fraction using a novel interatrial implant to lower left atrial pressure. Eur. J. Heart Fail. 2014, 16, 796–801. [Google Scholar] [CrossRef]
- Feldman, T.; Mauri, L.; Kahwash, R.; Litwin, S.; Ricciardi, M.J.; Van Der Harst, P.; Penicka, M.; Fail, P.S.; Kaye, D.M.; Petrie, M.C.; et al. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure with Preserved Ejection Fraction (REDUCE LAP-HF i [Reduce Elevated Left Atrial Pressure in Patients with Heart Failure]): A Phase 2, Ran-domized, Sham-Controlled Trial. Circulation 2018, 137, 364–375. [Google Scholar] [CrossRef]
- Shah, S.J.; Feldman, T.; Ricciardi, M.J.; Kahwash, R.; Lilly, S.; Litwin, S.; Nielsen, C.D.; Van Der Harst, P.; Hoendermis, E.; Penicka, M.; et al. One-Year Safety and Clinical Outcomes of a Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure with Preserved Ejection Fraction in the Reduce Elevated Left Atrial Pressure in Patients with Heart Failure (REDUCE LAP-HF I) Trial. JAMA Cardiol. 2018, 3, 968–977. [Google Scholar] [CrossRef]
- Berry, N.; Mauri, L.; Feldman, T.; Komtebedde, J.; van Veldhuisen, D.J.; Solomon, S.D.; Massaro, J.M.; Shah, S.J. Transcatheter InterAtrial Shunt Device for the treatment of heart failure: Rationale and design of the pivotal randomized trial to REDUCE Elevated Left Atrial Pressure in Patients with Heart Failure II (REDUCE LAP-HF II): Rationale and design of REDUCE LAP-HF II. Am. Heart J. 2019, 226, 222–231. [Google Scholar] [CrossRef] [PubMed]
- del Rio, C.L.; McConnell, P.I.; Nitzan, Y.; Ueyama, Y.; Kloepfer, P.; Youngblood, B.L.; George, R.; Jacoby, M.; Hamlin, R.L.; Oz, O.; et al. Chronic pressure-dependent cardiac unloading with a novel intra-atrial shunt (v-wave device) in an ovine model of ischemic heart failure: Evidence for shunt-mediated improvements in function and survivability. Circulation 2013, 128, A18354. [Google Scholar]
- Del Trigo, M.; Bergeron, S.; Bernier, M.; Amat-Santos, I.J.; Puri, R.; Campelo-Parada, F.; Altisent, O.A.-J.; Regueiro, A.; Eigler, N.; Rozenfeld, E.; et al. Unidirectional left-to-right interatrial shunting for treatment of patients with heart failure with reduced ejection fraction: A safety and proof-of-principle cohort study. Lancet 2016, 387, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Rodés-Cabau, J.; Bernier, M.; Amat-Santos, I.J.; Ben Gal, T.; Nombela-Franco, L.; del Blanco, B.G.; Kerner, A.; Bergeron, S.; del Trigo, M.; Pibarot, P.; et al. Interatrial Shunting for Heart Failure. JACC Cardiovasc. Interv. 2018, 11, 2300–2310. [Google Scholar] [CrossRef]
- Patel, M.B.; Samuel, B.P.; Girgis, R.E.; Parlmer, M.A.; Vettukattil, J.J. Implantable atrial flow regulator for severe, irreversible pulmonary arterial hypertension. EuroIntervention 2015, 11, 706–709. [Google Scholar] [CrossRef]
- Paitazoglou, C.; Bergmann, M.W.; Özdemir, R.; Pfister, R.; Bartunek, J.; Kilic, T.; Lauten, A.; Schmeisser, A.; Zoghi, M.; Anker, S.D.; et al. One-year results of the first-in-man study investigating the Atrial Flow Regulator for left atrial shunting in symptomatic heart failure patients: The PRELIEVE study. Eur. J. Heart Fail. 2021, 23, 800–810. [Google Scholar] [CrossRef]
- Simard, T.; Labinaz, M.; Zahr, F.; Nazer, B.; Gray, W.; Hermiller, J.; Chaudhry, S.-P.; Guimaraes, L.; Philippon, F.; Eckman, P.; et al. Percutaneous Atriotomy for Levoatrial–to–Coronary Sinus Shunting in Symptomatic Heart Failure. JACC Cardiovasc. Interv. 2020, 13, 1236–1247. [Google Scholar] [CrossRef]
- Nussinovitch, U. (Ed.) Front matter. In Emerging Technologies for Heart Diseases; Academic Press: Cambridge, MA, USA, 2020; pp. 95–127. [Google Scholar] [CrossRef]
- Feld, Y.; Reisner, Y.; Meyer-Brodnitz, G.; Hoefler, R. The CORolla device for energy transfer from systole to diastole: A novel treatment for heart failure with preserved ejection fraction. Heart Fail. Rev. 2021; ahead of print. [Google Scholar] [CrossRef]
- Escher, A.; Choi, Y.; Callaghan, F.; Thamsen, B.; Kertzscher, U.; Schweiger, M.; Hübler, M.; Granegger, M. A Valveless Pulsatile Pump for Heart Failure with Preserved Ejection Fraction: Hemo- and Fluid Dynamic Feasibility. Ann. Biomed. Eng. 2020, 48, 1821–1836. [Google Scholar] [CrossRef]
- Miyagi, C.; Kuban, B.D.; Flick, C.R.; Polakowski, A.R.; Miyamoto, T.; Karimov, J.H.; Starling, R.C.; Fukamachi, K. Left atrial assist device for heart failure with preserved ejection fraction: Initial results with torque control mode in diastolic heart failure model. Heart Fail. Rev. 2021; ahead of print. [Google Scholar] [CrossRef]
- Badrov, M.B.; Mak, S.; Floras, J.S. Cardiovascular Autonomic Disturbances in Heart Failure with Preserved Ejection Fraction. Can. J. Cardiol. 2020, 37, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.M.; Nanayakkara, S.; Wang, B.; Shihata, W.; Marques, F.Z.; Esler, M.; Lambert, G.; Mariani, J. Characterization of Cardiac Sympathetic Nervous System and Inflammatory Activation in HFpEF Patients. JACC Basic Transl. Sci. 2022, 7, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Halbach, M.; Abraham, W.T.; Butter, C.; Ducharme, A.; Klug, D.; Little, W.C.; Reuter, H.; Schafer, J.E.; Senni, M.; Swarup, V.; et al. Baroreflex activation therapy for the treatment of heart failure with reduced ejection fraction in patients with and without coronary artery disease. Int. J. Cardiol. 2018, 266, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Bisognano, J.D.; De Leeuw, P.; Bach, D.S.; Lovett, E.G.; Kaufman, C.L. Improved Functional Capacity and Cardiovascular Structure after Baroreflex Activation Therapy™ in Resistant Hypertension Patients with Symptomatic Heart Failure: Results from European and United States Trials of the Rheos® System. J. Card. Fail. 2009, 15, S63. [Google Scholar] [CrossRef]
- Zile, M.R.; Lindenfeld, J.; Weaver, F.A.; Zannad, F.; Galle, E.; Rogers, T.; Abraham, W.T. Baroreflex Activation Therapy in Patients with Heart Failure with Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2020, 76, 1–13. [Google Scholar] [CrossRef]
- Borggrefe, M.; Mann, D.L. Cardiac Contractility Modulation in 2018. Circulation 2018, 138, 2738–2740. [Google Scholar] [CrossRef]
- Chung, E.S.; Katra, R.P.; Ghio, S.; Bax, J.; Gerritse, B.; Hilpisch, K.; Peterson, B.J.; Feldman, D.S.; Abraham, W.T. Cardiac resynchronization therapy may benefit patients with left ventricular ejection fraction >35%: A PROSPECT trial substudy. Eur. J. Heart Fail. 2010, 12, 581–587. [Google Scholar] [CrossRef]

| Non-Invasive Complementary Evaluation | Parameters | Cut-Off Values | Comments |
|---|---|---|---|
| Echocardiographic (morphological) [18,19,20] | Left ventricle mass index (g/m2) Wall relative thickness Left atrial volume index (mL/m2) | Female ≥ 95 Male ≥ 115 >0.42 >34 (sinus rhythm) >40 (atrial fibrillation) | Concentric remodeling and LV hypertrophy support HFpEF diagnosis, but absence of hypertrophy does not exclude it. Left atrial enlargement reflects chronic high filling pressures of LV. |
| Echocardiographic (functional) [21,22,23,24,25] | E/e’ ratio at rest | >9 | The sensitivity and specificity of an E/e’ ratio > 9 was 78% and 59%, compared with 46% and 86% for E/e’ > 13. The mitral E/e’ index correlates with LV stiffness and fibrosis and is less age-dependent than e’. |
| E/e’ ratio at peak stress | >15 | Exercise echocardiography should be considered abnormal if average E/e’ ratio at peak stress increases to >15, with or without a peak TR velocity > 3.4 m/s. | |
| TR velocity at rest (m/s) | >2.8 | A TR peak velocity > 2.8 m/s indicates increased PASP and is an indirect marker of LV diastolic dysfunction. It has sensitivity 54% and specificity 85% for the presence of HFpEF. | |
| TR velocity at peak stress (m/s) | >3.4 | An increase only in TR velocity in stress should not be used to diagnose HFpEF because it might be caused by a normal hyperdynamic response to exercise (with increased pulmonary blood flow) in the absence of LV diastolic dysfunction. | |
| PA systolic pressure (mmHg) | >35 | PAP > 35 mmHg (derived from tricuspid regurgitation (TR) velocity) was 46% sensitive and 86% specific for HFpEF. | |
| LV global longitudinal strain | <16% | Reduced LV longitudinal systolic strain and LV early diastolic strain rate have both been identified in HFpEF. The utility of GLS < 16% was moderate (sensitivity 62% and specificity 56%) |
| Parameters | Cut-Off Values | Comments | |||
|---|---|---|---|---|---|
| BNP (pg/mL) [26] | Acute dyspnea (acute setting) | These are hemodynamic cardiac stress biomarkers. ESC guidelines recommend its use for diagnostic and prognosis in HF. | |||
| <100 | HF unlikely | ||||
| 100–400 | Grey zone | ||||
| >400 | HF likely | ||||
| Mild symptoms (chronic setting) | ACC/AHA suggest it for diagnosis, risk stratification (at diagnosis and prior to discharge), and HF prevention. | ||||
| <35 | HF unlikely | ||||
| 35–150 | Grey zone | ||||
| >150 | HF likely | ||||
| NT-proBNP (pg/mL) [27] | Acute dyspnea (acute setting) | Atrial fibrillation, age, acute and chronic kidney disease can reduce its diagnostic accuracy. Lower levels are present in obese patients. | |||
| <50 years | 50–75 years | >75 years | |||
| <300 | <300 | <300 | HF unlikely | ||
| 300–450 | 300–900 | 300–1800 | Grey zone | ||
| >450 | >900 | >1800 | HF likely | ||
| Mild symptoms (chronic setting) | In acute heart failure there is age adjusted cut-off values for NT-proBNP. | ||||
| <125 | HF unlikely | ||||
| 125–600 | Grey zone | ||||
| >600 | HF likely | ||||
| MR-proANP (ng/L) [28] | <120 | The BACH and PRIDE trials showed MR-proANP use in the diagnosis of acute HF was similar to BNP and slightly inferior to NT-proBNP, respectively. ESC suggests its use in acute heart failure | |||
| Etiology | Main Radiological Characteristics |
|---|---|
| Ischemic | Subendocardial enhancement in specific coronary artery territory with myocardial fibrosis/scar. |
| Inflammation (myocarditis, sarcoidosis) | Patchy pattern, late gadolinium enhancement and myocardial oedema. Enhanced T1 and T2 sequences. |
| Hypertrophic cardiomyopathy | Basal asymmetrical septal hypertrophy (basal anterior septal thickness ≥ 15 mm at end-diastole; ratio of septal to inferolateral wall thickness ≥ 1.3), focal fibrosis on late gadolinium enhancement. |
| Amyloidosis | Abnormal gadolinium kinetics in the myocardium nulling before the blood pool, enhanced in T1 and ECV. |
| Hypereosinophilic syndromes | Nonischemic subendocardial scar pattern. |
| Parameter | Rest | Exercise # | ||
|---|---|---|---|---|
| Healthy | HFpEF | Healthy | HFpEF | |
| PCWP (mmHg) | <12 | 15 | <23 | ≥25 |
| LVEDP (mmHg) | <16 | >16 | <25 | >25 |
| RAP (mmHg) | 0–6 | >10 | >PCWP * | |
| Mean PAP (mmHg) | <20 | ≥25 | <30 | >30 |
| PAP/CO slope (mmHg/L/min) + | <3 | >3 | ||
| PCPW/CO slope (mmHg/L/min) + | <2 | >2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Lucares, A.; Romero-Hernández, E.; Villa, E.; Weitz-Muñoz, S.; Vizcarra, G.; Reyes, M.; Vergara, D.; Bustamante, S.; Llancaqueo, M.; Toro, L. New Opportunities in Heart Failure with Preserved Ejection Fraction: From Bench to Bedside… and Back. Biomedicines 2023, 11, 70. https://doi.org/10.3390/biomedicines11010070
Parra-Lucares A, Romero-Hernández E, Villa E, Weitz-Muñoz S, Vizcarra G, Reyes M, Vergara D, Bustamante S, Llancaqueo M, Toro L. New Opportunities in Heart Failure with Preserved Ejection Fraction: From Bench to Bedside… and Back. Biomedicines. 2023; 11(1):70. https://doi.org/10.3390/biomedicines11010070
Chicago/Turabian StyleParra-Lucares, Alfredo, Esteban Romero-Hernández, Eduardo Villa, Sebastián Weitz-Muñoz, Geovana Vizcarra, Martín Reyes, Diego Vergara, Sergio Bustamante, Marcelo Llancaqueo, and Luis Toro. 2023. "New Opportunities in Heart Failure with Preserved Ejection Fraction: From Bench to Bedside… and Back" Biomedicines 11, no. 1: 70. https://doi.org/10.3390/biomedicines11010070
APA StyleParra-Lucares, A., Romero-Hernández, E., Villa, E., Weitz-Muñoz, S., Vizcarra, G., Reyes, M., Vergara, D., Bustamante, S., Llancaqueo, M., & Toro, L. (2023). New Opportunities in Heart Failure with Preserved Ejection Fraction: From Bench to Bedside… and Back. Biomedicines, 11(1), 70. https://doi.org/10.3390/biomedicines11010070





