Inflammatory Cytokine-Induced HIF-1 Activation Promotes Epithelial–Mesenchymal Transition in Endometrial Epithelial Cells
Abstract
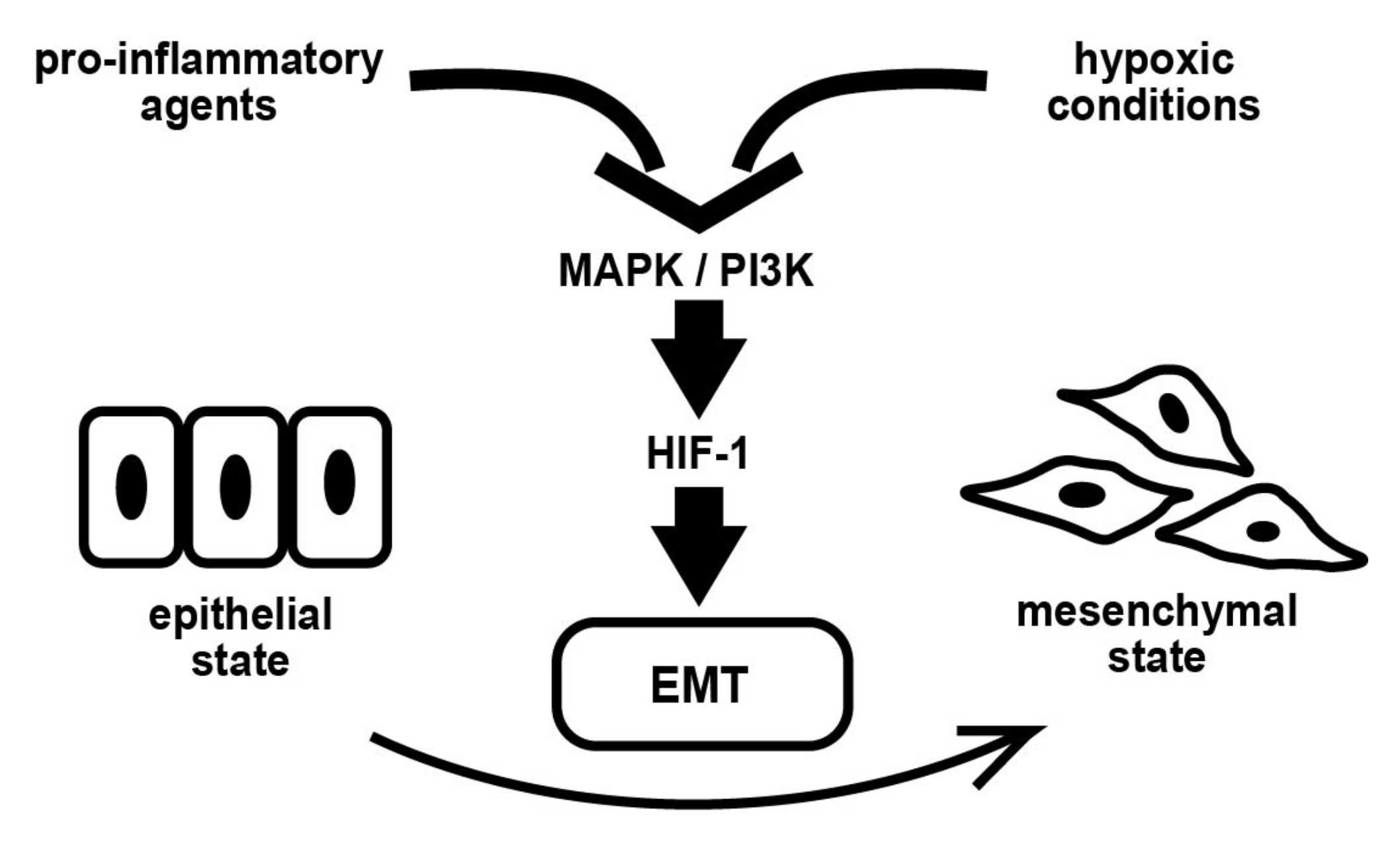
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Western Blot Analysis
2.4. Semi-Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
2.5. RNA-Seq
2.6. Transcriptomics Analysis
2.7. Wound Healing Assay
2.8. Statistical Analysis
3. Results
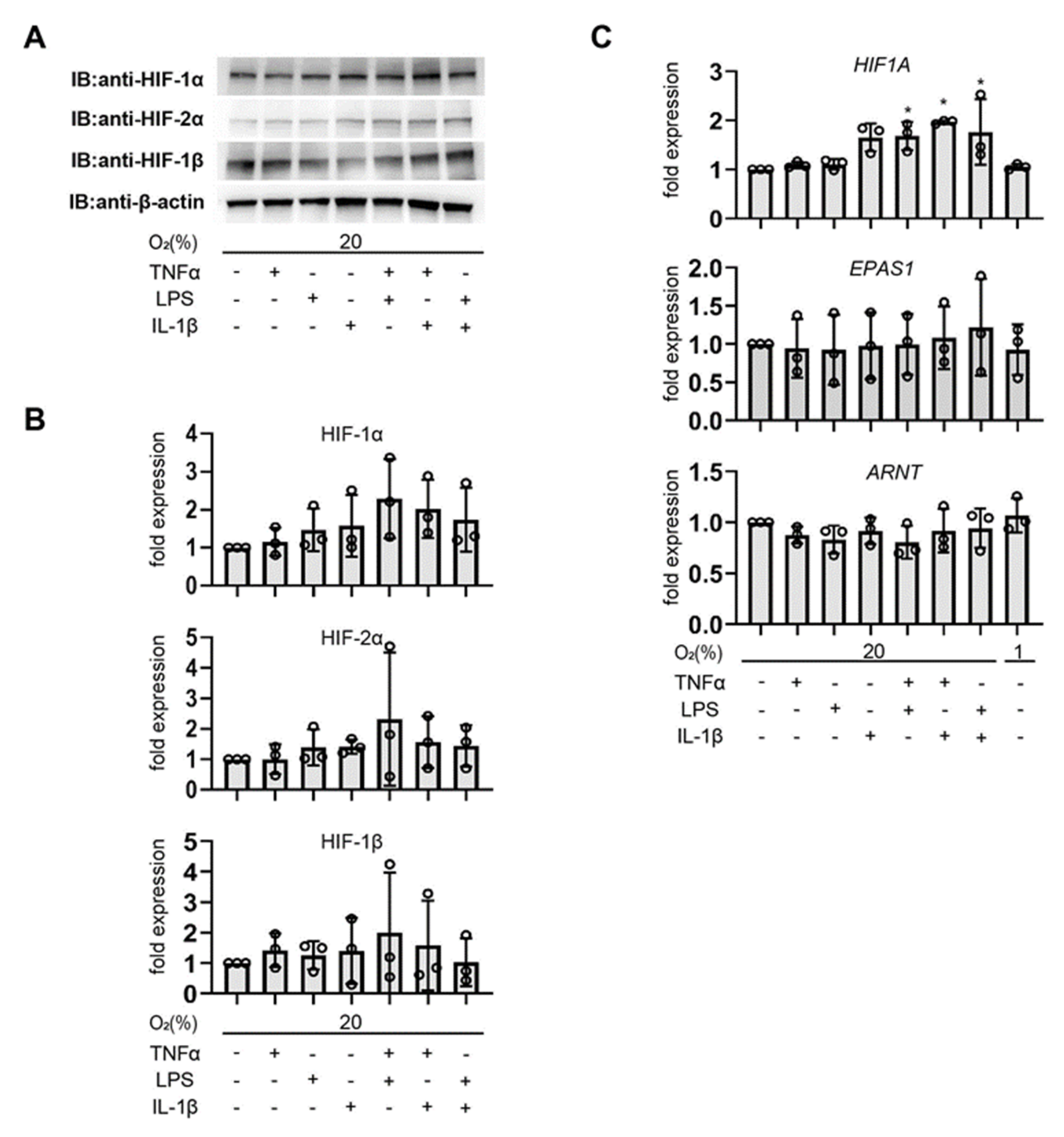
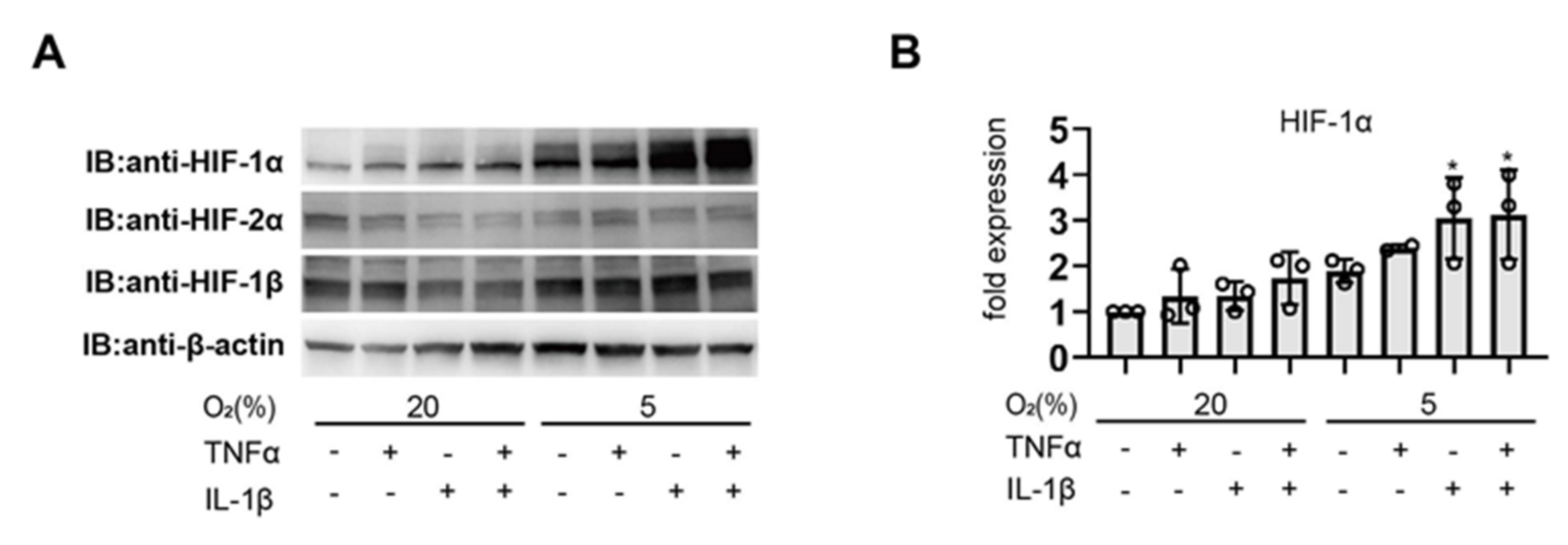
3.1. Pro-Inflammatory Agents Induce HIF-1α Protein Accumulation under Non-Hypoxic Conditions in Endometrial Epithelial Cells
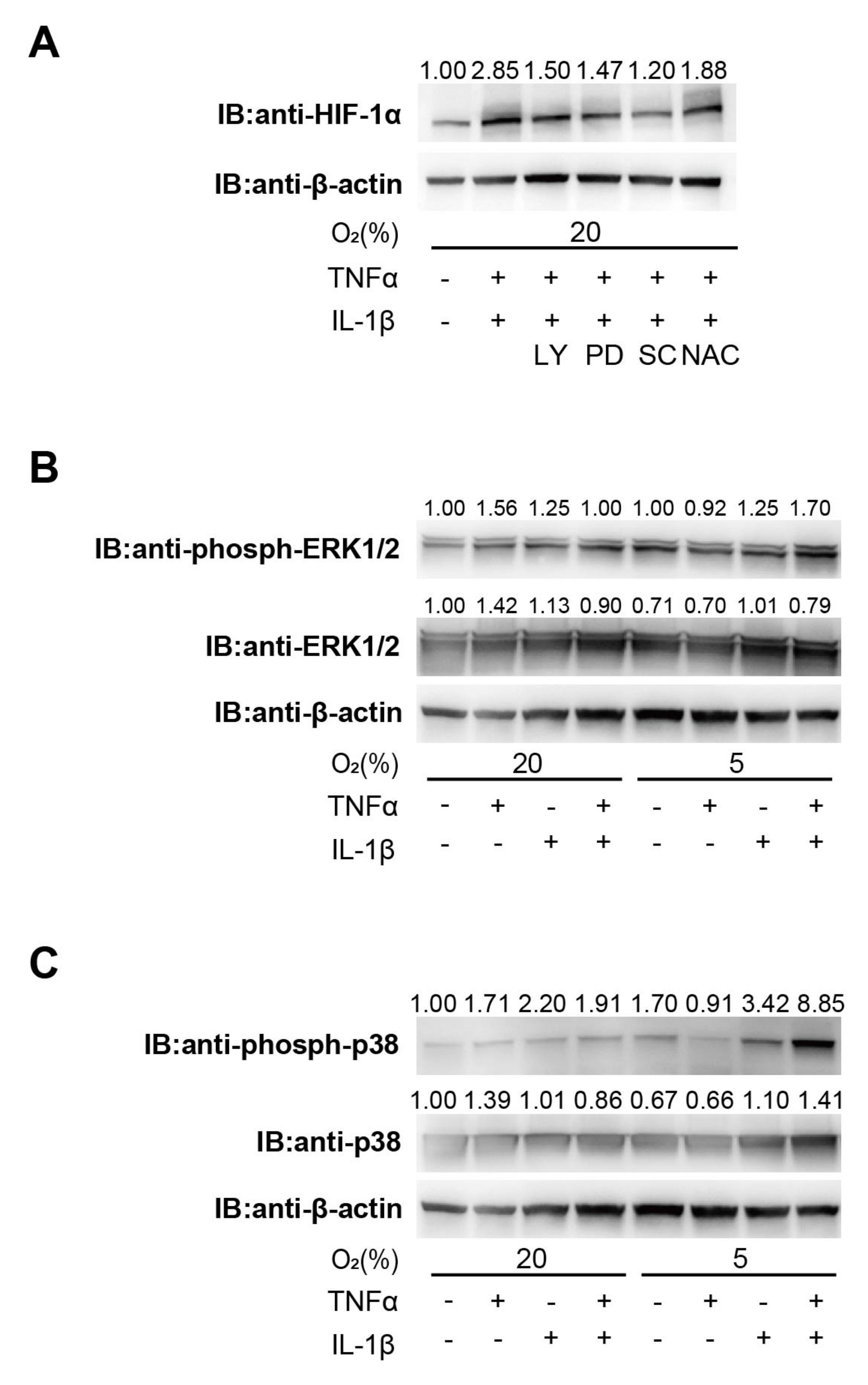
3.2. Involvement of the PI3K and MAPK Signaling Pathway in Cytokine-Induced HIF-1 Activation
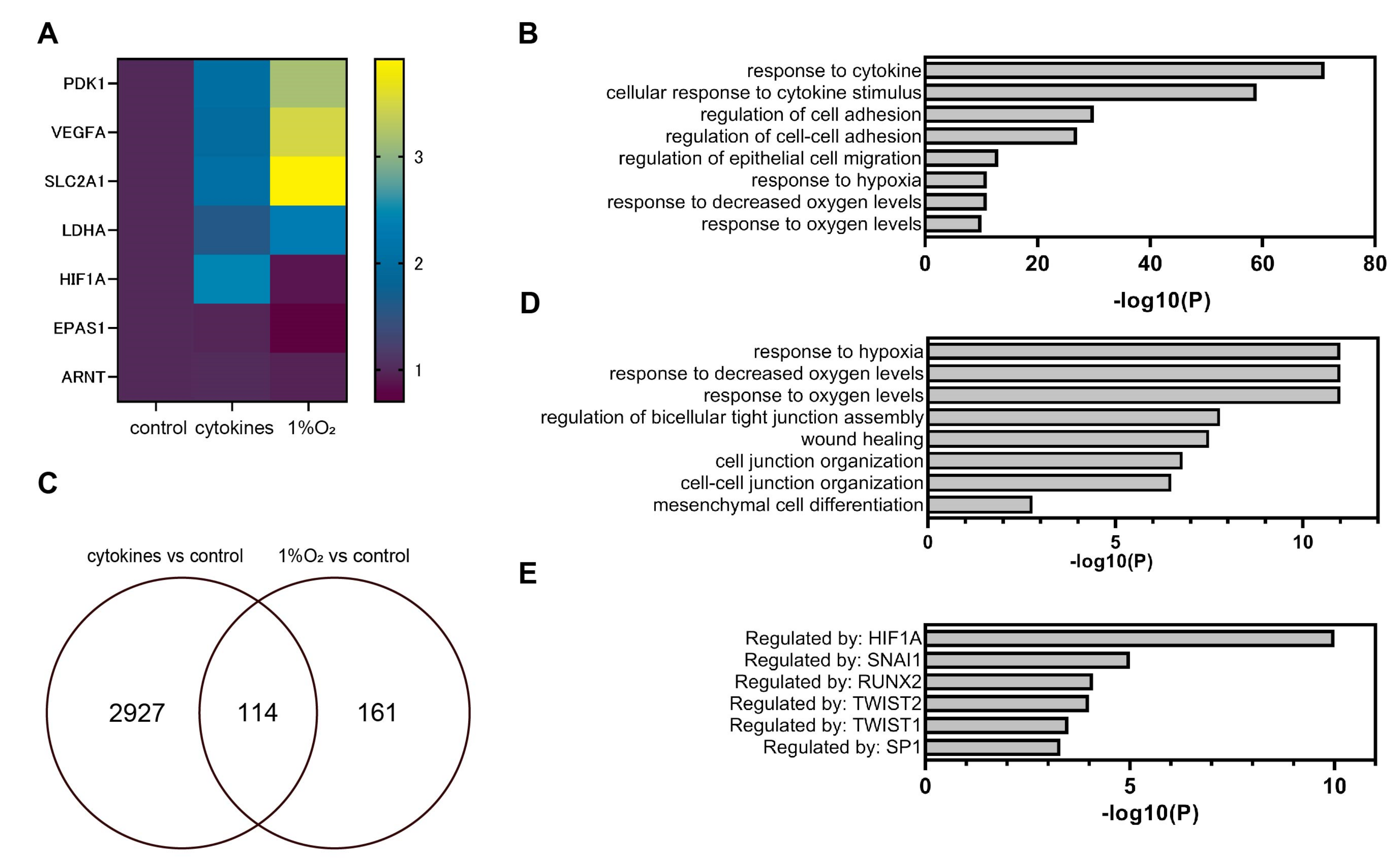
3.3. Global Gene Expression Analysis Using RNA-Seq
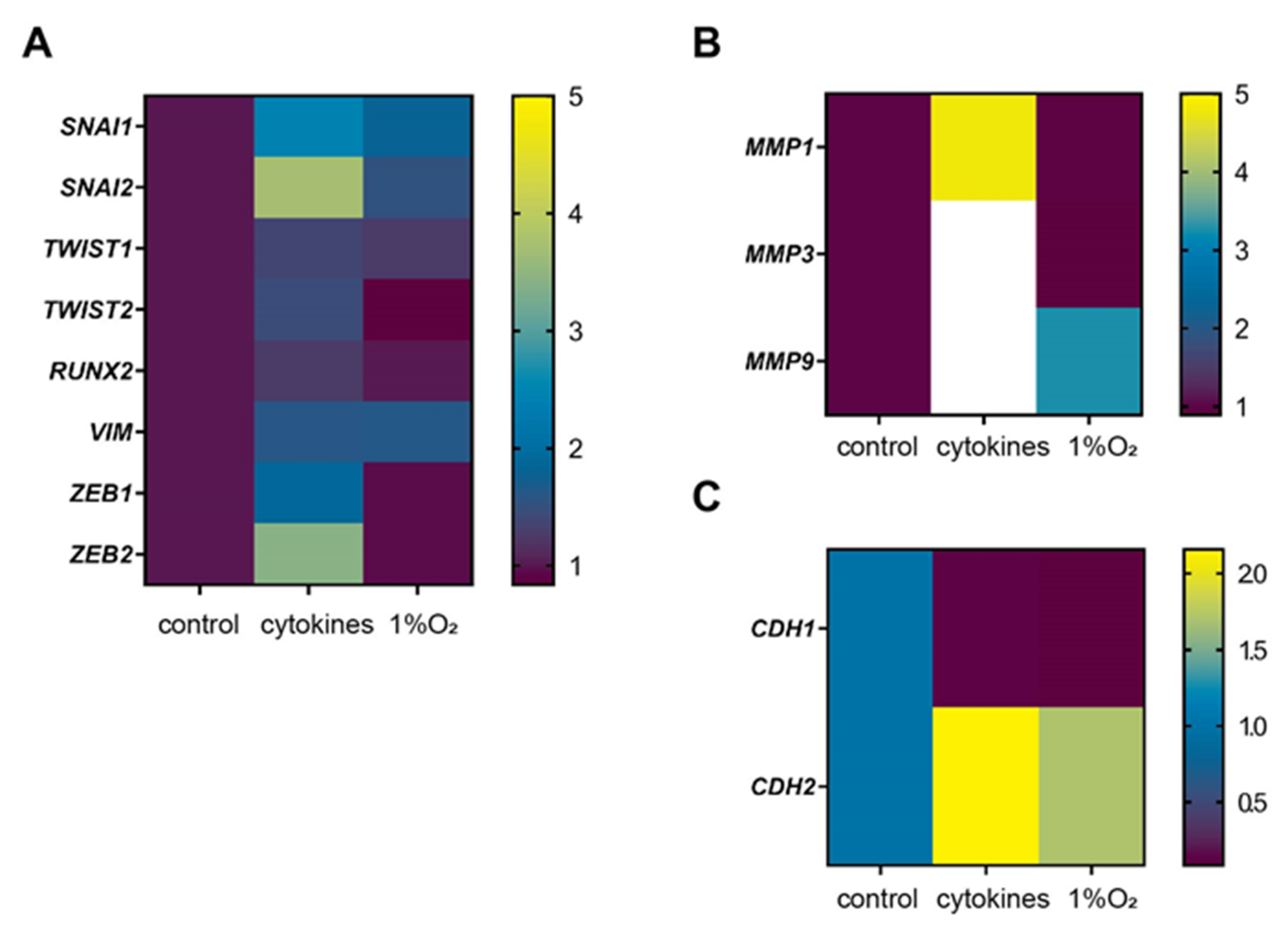
3.4. Representative Genes of EMT were Induced by Cytokines
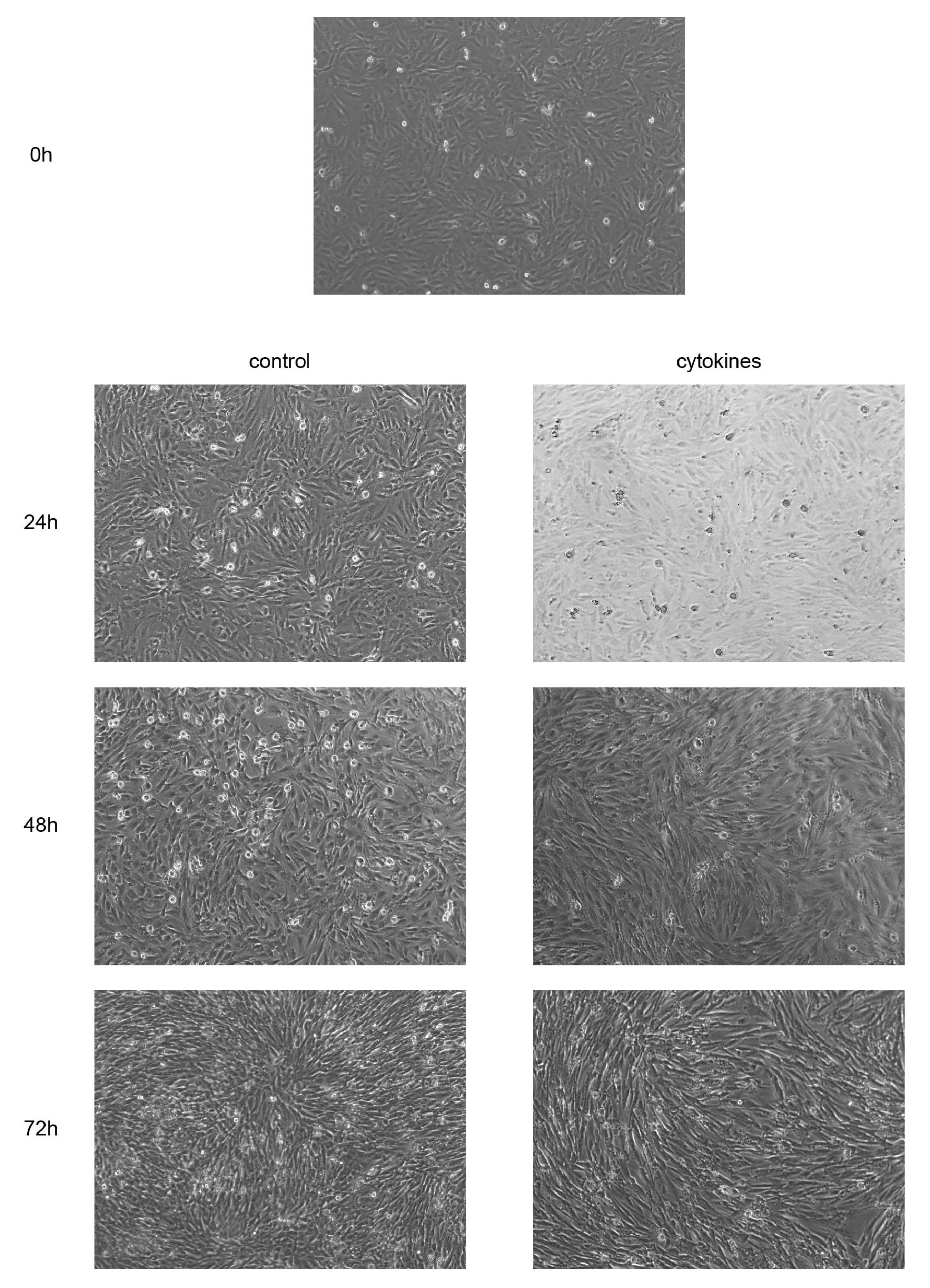
3.5. Cytokines Facilitate Morphological Changes in EM-E6/E7/TERT Cells
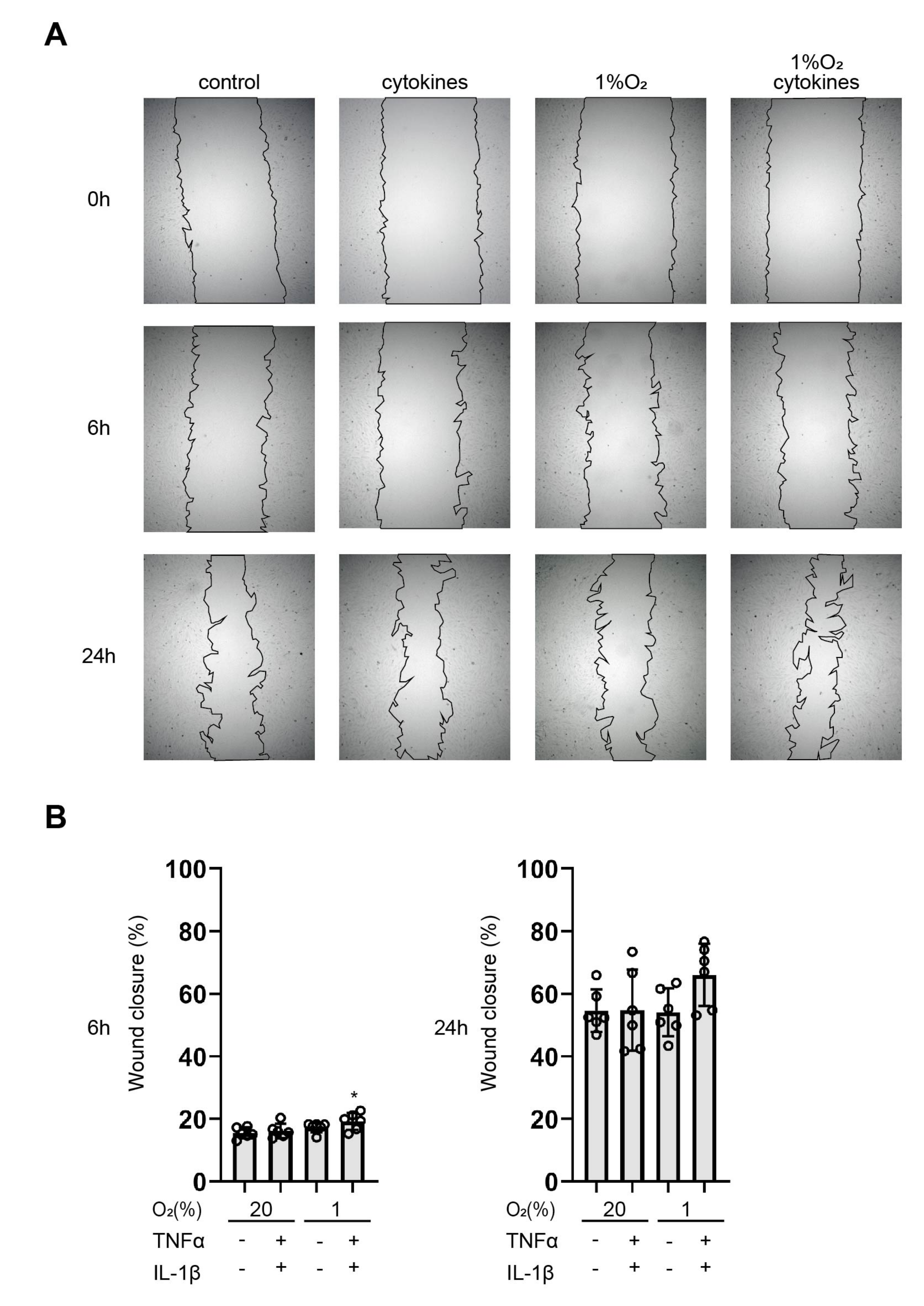
3.6. Cytokines Facilitate Wound Healing
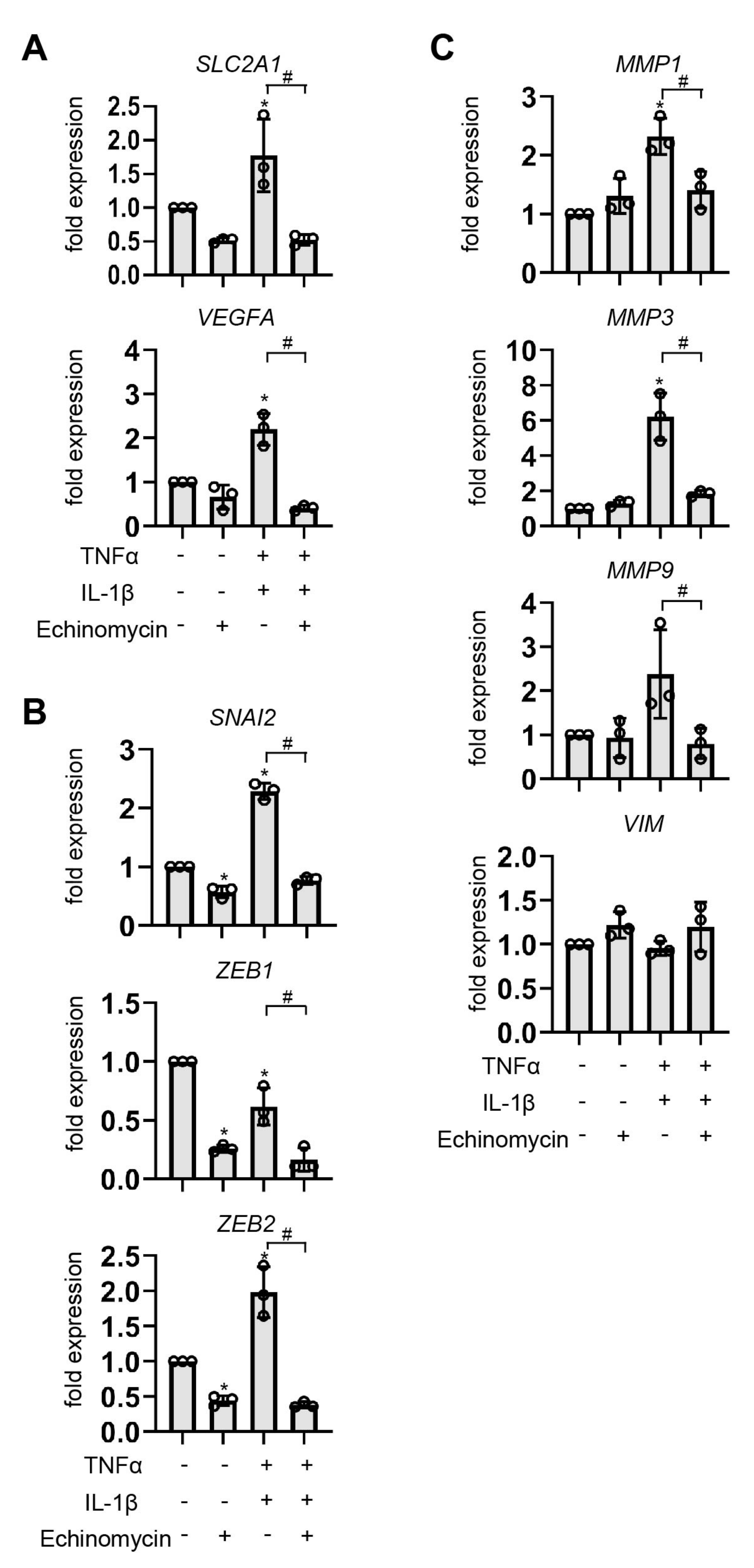
3.7. Effect of HIF-1 Suppression by Echinomycin on EMT-Related Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Reagents | Source | Identifier |
|---|---|---|
| 12% Mini-PROTEAN® TGXTM Precast Protein Gels | BIO-RAD | 4561043 |
| 30w/v% Albumin Solution, from Bovine Serum (BSA), Fatty Acid Free | FUJIFILM | 017-22231 |
| 7.5% Mini-PROTEAN® TGXTM Precast Protein Gels | BIO-RAD | 4561023 |
| Anti-rabbit IgG, HRP-linked Antibody | Cell Signaling TECHNOLOGY | 7074 |
| Anti-β-Actin antibody | Sigma-Aldrich | A5316 |
| Blocking One | Nacalai Tesque | 03953-95 |
| DCTM Protein Assay Reagents Package | BIO-RAD | 5000116JA |
| DMEM, low glucose, pyruvate | Gibco | 11885-084 |
| N-(2-methoxy-2-oxoacetyl) glycine methyl ester (DMOG) | Sigma-Aldrich | D3695 |
| Dimethyl Sulfoxide | FUJIFILM | 043-07216 |
| Echinomycin | Enzo Life Sciences | ALX-380-201 |
| ECLTM anti-mouse IgG HRP Linked Whole Ab | cytiva | NA931 |
| ECLTM Prime Western Blotting Detection Reagents | cytiva | RPN2232 |
| Fetal Bovine Serum Characterized | Hyclone | SH30396 |
| HIF-1β/ARNT (D28F3) XP® rabbit mAb | Cell Signaling TECHNOLOGY | 5537 |
| HIF-2 alpha/EPAS1 Antibody | Novus Biologicals | NB100-122 |
| LY294002 | FUJIFILM | 1290-4861 |
| Maxwell® RSC simply RNA Cells Kit | Promega | AS1390 |
| N-Acetyl-L-cysteine, ROS inhibitor | abcam | 143032 |
| p38 MAPK(D13E1) XP® Rabbit mAb | Cell Signaling TECHNOLOGY | 8690 |
| p44/42 MAPK(Erk1/2) (137F5) Rabbit mAb | Cell Signaling TECHNOLOGY | 4695 |
| PBS, pH 7.4 | Gibco | 10010-023 |
| PD98059 | FUJIFILM | 169-19211 |
| Penicillin-Streptomycin Mixed Solution (Stabilized) | Nacalai Tesque | 09367-34 |
| Phospho-p38MAPK(Thr180/Tyr182) (D3F9) XP® Rabbit mAb | Cell Signaling TECHNOLOGY | 4511 |
| Phospho-p44/42MAPK(Erk1/2) (Thr202/Tyr204) (D13.14.4E) XP® Rabbit mAb | Cell Signaling TECHNOLOGY | 4370 |
| Protease inhibitor cocktail | Calbiochem | 539134 |
| Purified Mouse Anti-Human HIF-1α | BD Transduction LaboratoriesTM | 610959 |
| Recombinant Human IL-1β | PeproTech | 200-01B |
| Recombinant Human TNF-α | PeproTech | 300-01A |
| ReverTra Ace® qPCR RT Master Mix with gDNA Remover | TOYOBO | FSQ-301 |
| RIPA Buffer | FUJIFILM | 182-02451 |
| Running Buffer Solution for SDS-PAGE | Nacalai Tesque | 30329-61 |
| Sample Buffer Solution with Reducing Reagent (6×) for SDS-PAGE | Nacalai Tesque | 09449-14 |
| SC-514 | Sigma-Aldrich | SML0557 |
| THUNDERBIRD® Next SYBR qPCR Mix | TOYOBO | QPX-201 |
| Trans-Blot® Turbo™ Mini PVDF Transfer Packs | BIO-RAD | 1704156 |
| Tris Bufferd Saline with Tween® 20 (TBS-T) Tablets, pH7.6 | TaKaRa | T9142 |
| Trypsin-EDTA (0.25%) | Gibco | 25200-056 |
| Ultrapure LPS, E. coli 0111: B4 | InvivoGen | tlrl-3pelps |
References
- Aberdeen, G.W.; Wiegand, S.J.; Bonagura, T.W.; Pepe, G.J.; Albrecht, E.D. Vascular endothelial growth factor mediates the estrogen-induced breakdown of tight junctions between and increase in proliferation of microvessel endothelial cells in the baboon endometrium. Endocrinology 2008, 149, 6076–6083. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Krieg, S.; Kuo, C.J.; Wiegand, S.J.; Rabinovitch, M.; Druzin, M.L.; Brenner, R.M.; Giudice, L.C.; Nayak, N.R. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 2008, 22, 3571–3580. [Google Scholar] [CrossRef] [PubMed]
- Salamonsen, L.A. Tissue injury and repair in the female human reproductive tract. Reproduction 2003, 125, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, Y.S.; Dou, G.R.; Hou, H.Y.; Shi, Y.Y.; Zhang, R.; Ma, K.; Wu, L.; Yao, L.B.; Cai, Y.; et al. Influence of Dll4 via HIF-1α-VEGF signaling on the angiogenesis of choroidal neovascularization under hypoxic conditions. PLoS ONE 2011, 6, e18481. [Google Scholar] [CrossRef]
- Tsuzuki, T.; Okada, H.; Cho, H.; Tsuji, S.; Nishigaki, A.; Yasuda, K.; Kanzaki, H. Hypoxic stress simultaneously stimulates vascular endothelial growth factor via hypoxia-inducible factor-1α and inhibits stromal cell-derived factor-1 in human endometrial stromal cells. Hum. Reprod. 2012, 27, 523–530. [Google Scholar] [CrossRef]
- Semenza, G.L. HIF-1 and human disease: One highly involved factor. Genes Dev. 2000, 14, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Ravi, R.; Mookerjee, B.; Bhujwalla, Z.M.; Sutter, C.H.; Artemov, D.; Zeng, Q.; Dillehay, L.E.; Madan, A.; Semenza, G.L.; Bedi, A. Regulation of tumor angiogenesis by p53-induced degradation of hypoxia-inducible factor 1alpha. Genes Dev. 2000, 14, 34–44. [Google Scholar] [CrossRef]
- Treins, C.; Giorgetti-Peraldi, S.; Murdaca, J.; Semenza, G.L.; Van Obberghen, E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J. Biol. Chem. 2002, 277, 27975–27981. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Carmeliet, P. Hypoxia and inflammation. N. Engl. J. Med. 2011, 364, 656–665. [Google Scholar] [CrossRef]
- Haddad, J.J.; Land, S.C. A non-hypoxic, ROS-sensitive pathway mediates TNF-alpha-dependent regulation of HIF-1alpha. FEBS Lett. 2001, 505, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Blouin, C.C.; Pagé, E.L.; Soucy, G.M.; Richard, D.E. Hypoxic gene activation by lipopolysaccharide in macrophages: Implication of hypoxia-inducible factor 1alpha. Blood 2004, 103, 1124–1130. [Google Scholar] [CrossRef]
- Oda, T.; Hirota, K.; Nishi, K.; Takabuchi, S.; Oda, S.; Yamada, H.; Arai, T.; Fukuda, K.; Kita, T.; Adachi, T.; et al. Activation of hypoxia-inducible factor 1 during macrophage differentiation. Am. J. Physiol. Cell Physiol. 2006, 291, C104–C113. [Google Scholar] [CrossRef] [PubMed]
- Hellwig-Bürgel, T.; Rutkowski, K.; Metzen, E.; Fandrey, J.; Jelkmann, W. Interleukin-1beta and tumor necrosis factor-alpha stimulate DNA binding of hypoxia-inducible factor-1. Blood 1999, 94, 1561–1567. [Google Scholar] [CrossRef]
- Zhou, J.; Schmid, T.; Brüne, B. Tumor necrosis factor-alpha causes accumulation of a ubiquitinated form of hypoxia inducible factor-1alpha through a nuclear factor-kappaB-dependent pathway. Mol. Biol. Cell 2003, 14, 2216–2225. [Google Scholar] [CrossRef]
- Tortorella, C.; Piazzolla, G.; Matteo, M.; Pinto, V.; Tinelli, R.; Sabbà, C.; Fanelli, M.; Cicinelli, E. Interleukin-6, interleukin-1β, and tumor necrosis factor α in menstrual effluents as biomarkers of chronic endometritis. Fertil. Steril. 2014, 101, 242–247. [Google Scholar] [CrossRef]
- Kimura, F.; Takebayashi, A.; Ishida, M.; Nakamura, A.; Kitazawa, J.; Morimune, A.; Hirata, K.; Takahashi, A.; Tsuji, S.; Takashima, A.; et al. Review: Chronic endometritis and its effect on reproduction. J. Obstet. Gynaecol. Res. 2019, 45, 951–960. [Google Scholar] [CrossRef]
- Johnston-MacAnanny, E.B.; Hartnett, J.; Engmann, L.L.; Nulsen, J.C.; Sanders, M.M.; Benadiva, C.A. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil. Steril. 2010, 93, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Resta, L.; Nicoletti, R.; Tartagni, M.; Marinaccio, M.; Bulletti, C.; Colafiglio, G. Detection of chronic endometritis at fluid hysteroscopy. J. Minim. Invasive Gynecol. 2005, 12, 514–518. [Google Scholar] [CrossRef]
- Cicinelli, E.; Matteo, M.; Trojano, G.; Mitola, P.C.; Tinelli, R.; Vitagliano, A.; Crupano, F.M.; Lepera, A.; Miragliotta, G.; Resta, L. Chronic endometritis in patients with unexplained infertility: Prevalence and effects of antibiotic treatment on spontaneous conception. Am. J. Reprod. Immunol. 2018, 79, e12782. [Google Scholar] [CrossRef]
- Kasius, J.C.; Fatemi, H.M.; Bourgain, C.; Sie-Go, D.M.; Eijkemans, R.J.; Fauser, B.C.; Devroey, P.; Broekmans, F.J. The impact of chronic endometritis on reproductive outcome. Fertil. Steril. 2011, 96, 1451–1456. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Huang, J.; Wang, C.C.; Yu, M.Y.; Laird, S.; Li, T.C. Comparison of the prevalence of chronic endometritis as determined by means of different diagnostic methods in women with and without reproductive failure. Fertil. Steril. 2018, 109, 832–839. [Google Scholar] [CrossRef]
- Bartley, J.; Jülicher, A.; Hotz, B.; Mechsner, S.; Hotz, H. Epithelial to mesenchymal transition (EMT) seems to be regulated differently in endometriosis and the endometrium. Arch. Gynecol. Obstet. 2014, 289, 871–881. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Darcha, C. Epithelial to mesenchymal transition-like and mesenchymal to epithelial transition-like processes might be involved in the pathogenesis of pelvic endometriosis. Hum. Reprod. 2012, 27, 712–721. [Google Scholar] [CrossRef]
- Furuya, M.; Masuda, H.; Hara, K.; Uchida, H.; Sato, K.; Sato, S.; Asada, H.; Maruyama, T.; Yoshimura, Y.; Katabuchi, H.; et al. ZEB1 expression is a potential indicator of invasive endometriosis. Acta Obstet. Gynecol. Scand. 2017, 96, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, K.; Delatorre, R.; Pedemonte, B.; McLeod, C.; Anderson, L.; Chambers, J. E-cadherin expression in endometrioid, papillary serous, and clear cell carcinoma of the endometrium. Obstet. Gynecol. 2002, 100, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Tanos, P.; Dimitriou, S.; Gullo, G.; Tanos, V. Biomolecular and Genetic Prognostic Factors That Can Facilitate Fertility-Sparing Treatment (FST) Decision Making in Early Stage Endometrial Cancer (ES-EC): A Systematic Review. Int. J. Mol. Sci. 2022, 23, 2653. [Google Scholar] [CrossRef]
- Giampaolino, P.; Cafasso, V.; Boccia, D.; Ascione, M.; Mercorio, A.; Viciglione, F.; Palumbo, M.; Serafino, P.; Buonfantino, C.; De Angelis, M.C.; et al. Fertility-Sparing Approach in Patients with Endometrioid Endometrial Cancer Grade 2 Stage IA (FIGO): A Qualitative Systematic Review. Biomed. Res. Int. 2022, 2022, 4070368. [Google Scholar] [CrossRef]
- Mutlu, L.; Manavella, D.D.; Gullo, G.; McNamara, B.; Santin, A.D.; Patrizio, P. Endometrial Cancer in Reproductive Age: Fertility-Sparing Approach and Reproductive Outcomes. Cancers 2022, 14, 5187. [Google Scholar] [CrossRef] [PubMed]
- Munksgaard, P.S.; Blaakaer, J. The association between endometriosis and ovarian cancer: A review of histological, genetic and molecular alterations. Gynecol. Oncol. 2012, 124, 164–169. [Google Scholar] [CrossRef]
- Terzic, M.; Aimagambetova, G.; Kunz, J.; Bapayeva, G.; Aitbayeva, B.; Terzic, S.; Laganà, A.S. Molecular Basis of Endometriosis and Endometrial Cancer: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2021, 22, 9274. [Google Scholar] [CrossRef]
- Zundel, W.; Schindler, C.; Haas-Kogan, D.; Koong, A.; Kaper, F.; Chen, E.; Gottschalk, A.R.; Ryan, H.E.; Johnson, R.S.; Jefferson, A.B.; et al. Loss of PTEN facilitates HIF-1-mediated gene expression. Genes Dev. 2000, 14, 391–396. [Google Scholar] [CrossRef]
- Berg, A.; Fasmer, K.E.; Mauland, K.K.; Ytre-Hauge, S.; Hoivik, E.A.; Husby, J.A.; Tangen, I.L.; Trovik, J.; Halle, M.K.; Woie, K.; et al. Tissue and imaging biomarkers for hypoxia predict poor outcome in endometrial cancer. Oncotarget 2016, 7, 69844–69856. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Liu, Y.; Xiong, W.; Zhang, L.; Liu, H.; Du, Y.; Li, N. Hypoxia-inducible factor 1α-induced epithelial-mesenchymal transition of endometrial epithelial cells may contribute to the development of endometriosis. Hum. Reprod. 2016, 31, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Wang, W.; Zhang, Y.; Song, E.; Fan, Y.; Wei, B. Hypoxia-inducible factor-1alpha: A promising therapeutic target in endometriosis. Biochimie 2016, 123, 130–137. [Google Scholar] [CrossRef]
- Kyo, S.; Nakamura, M.; Kiyono, T.; Maida, Y.; Kanaya, T.; Tanaka, M.; Yatabe, N.; Inoue, M. Successful immortalization of endometrial glandular cells with normal structural and functional characteristics. Am. J. Pathol. 2003, 163, 2259–2269. [Google Scholar] [CrossRef]
- Yoshida, T.; Okumura, T.; Matsuo, Y.; Okuyama, T.; Michiura, T.; Kaibori, M.; Umezaki, N.; Bono, H.; Hirota, K.; Sekimoto, M. Activation of transcription factor HIF inhibits IL-1β-induced NO production in primary cultured rat hepatocytes. Nitric Oxide 2022, 124, 1–14. [Google Scholar] [CrossRef]
- Kida, N.; Matsuo, Y.; Hashimoto, Y.; Nishi, K.; Tsuzuki-Nakao, T.; Bono, H.; Maruyama, T.; Hirota, K.; Okada, H. Cigarette Smoke Extract Activates Hypoxia-Inducible Factors in a Reactive Oxygen Species-Dependent Manner in Stroma Cells from Human Endometrium. Antioxidants 2021, 10, 48. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Yamada, K.; Kawasaki, Y.; Hirose, H.; Matsumoto, K.; Ishikawa, K.; Yasumizu, Y. ikra: RNAseq pipeline centered on Salmon. Zendo 2019. [Google Scholar] [CrossRef]
- Krueger, F. Trim Galore. Available online: https://www.bioinformatics.babraham.ac.uk/projects/trim_galore/ (accessed on 24 October 2022).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon provides fast and bias-aware quantification of transcript expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Cho, J.W.; Lee, S.; Yun, A.; Kim, H.; Bae, D.; Yang, S.; Kim, C.Y.; Lee, M.; Kim, E.; et al. TRRUST v2: An expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2018, 46, D380–D386. [Google Scholar] [CrossRef]
- Han, H.; Shim, H.; Shin, D.; Shim, J.E.; Ko, Y.; Shin, J.; Kim, H.; Cho, A.; Kim, E.; Lee, T.; et al. TRRUST: A reference database of human transcriptional regulatory interactions. Sci. Rep. 2015, 5, 11432. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef]
- Sodhi, A.; Montaner, S.; Miyazaki, H.; Gutkind, J.S. MAPK and Akt act cooperatively but independently on hypoxia inducible factor-1alpha in rasV12 upregulation of VEGF. Biochem. Biophys. Res. Commun. 2001, 287, 292–300. [Google Scholar] [CrossRef]
- Li, Z.; Gou, J.; Jia, J.; Zhao, X. MicroRNA-429 functions as a regulator of epithelial-mesenchymal transition by targeting Pcdh8 during murine embryo implantation. Hum. Reprod. 2015, 30, 507–518. [Google Scholar] [CrossRef]
- Gassmann, M.; Fandrey, J.; Bichet, S.; Wartenberg, M.; Marti, H.H.; Bauer, C.; Wenger, R.H.; Acker, H. Oxygen supply and oxygen-dependent gene expression in differentiating embryonic stem cells. Proc. Natl. Acad. Sci. USA 1996, 93, 2867–2872. [Google Scholar] [CrossRef]
- Rodesch, F.; Simon, P.; Donner, C.; Jauniaux, E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet. Gynecol. 1992, 80, 283–285. [Google Scholar]
- Matsumoto, L.; Hirota, Y.; Saito-Fujita, T.; Takeda, N.; Tanaka, T.; Hiraoka, T.; Akaeda, S.; Fujita, H.; Shimizu-Hirota, R.; Igaue, S.; et al. HIF2α in the uterine stroma permits embryo invasion and luminal epithelium detachment. J. Clin. Investig. 2018, 128, 3186–3197. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Jiang, B.H.; Rue, E.A.; Semenza, G.L. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 1995, 92, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, R.; Kelly, B.; Semenza, G.L. Vascular endothelial growth factor gene expression in colon cancer cells exposed to prostaglandin E2 is mediated by hypoxia-inducible factor 1. Cancer Res. 2003, 63, 2330–2334. [Google Scholar] [PubMed]
- Hirota, K.; Fukuda, R.; Takabuchi, S.; Kizaka-Kondoh, S.; Adachi, T.; Fukuda, K.; Semenza, G.L. Induction of hypoxia-inducible factor 1 activity by muscarinic acetylcholine receptor signaling. J. Biol. Chem. 2004, 279, 41521–41528. [Google Scholar] [CrossRef] [PubMed]
- Van Uden, P.; Kenneth, N.S.; Rocha, S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem. J. 2008, 412, 477–484. [Google Scholar] [CrossRef]
- Görlach, A.; Bonello, S. The cross-talk between NF-kappaB and HIF-1: Further evidence for a significant liaison. Biochem. J. 2008, 412, e17–e19. [Google Scholar] [CrossRef]
- Frede, S.; Stockmann, C.; Freitag, P.; Fandrey, J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem. J. 2006, 396, 517–527. [Google Scholar] [CrossRef]
- Di Conza, G.; Trusso Cafarello, S.; Loroch, S.; Mennerich, D.; Deschoemaeker, S.; Di Matteo, M.; Ehling, M.; Gevaert, K.; Prenen, H.; Zahedi, R.P.; et al. The mTOR and PP2A Pathways Regulate PHD2 Phosphorylation to Fine-Tune HIF1α Levels and Colorectal Cancer Cell Survival under Hypoxia. Cell Rep. 2017, 18, 1699–1712. [Google Scholar] [CrossRef]
- Zhong, H.; Chiles, K.; Feldser, D.; Laughner, E.; Hanrahan, C.; Georgescu, M.M.; Simons, J.W.; Semenza, G.L. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: Implications for tumor angiogenesis and therapeutics. Cancer Res. 2000, 60, 1541–1545. [Google Scholar]
| Target | Gene Symbol | Forward Primer 5′–3′ | Reverse Primer 5′–3′ |
|---|---|---|---|
| HIF-1a HIF-2a HIF-1b GLUT1 VEGF SNAI2 ZEB1 ZEB2 MMP1 MMP3 MMP9 Vimentin EF1α | HIF-1A EPAS1 ARNT SLC2A1 VEGFA SNAI2 ZEB1 ZEB2 MMP1 MMP3 MMP9 VIM EEF1A1 | ACACACAGAAATGGCCTTGTGA ATGGGACTTACACAGGTGGAG TGTTGGCTACCAGCCACAGGAACT TCCACGAGCATCTTCGAGA CGAAACCATGAACTTTCTGC TGTGACAAGGAATATGTGAGCC TTACACCTTTGCATACAGAACCC CAAGAGGCGCAAACAAGCC ACAAACCCCAAAAGCGTGTG TTCGTTTTCTCCTGCCTGTG ATGCCTGCAACGTGAACATC GACGCCATCAACACCGAGTT TCTGGTTGGAATGGTGACAACATGC | CCTGTGCAGTGCAATACCTTC GCTCTGTGGACATGTCTTTGC ACCGGAACCGGAACATGACAGA ATACTGGAAGCACATGCCC CCTCAGTGGGCACACACTCC TGAGCCCTCAGATTTGACCTG TTTACGATTACACCCAGACTGC GGTTGGCAATACCGTCATCC AGAAGGGATTTGTGCGCATG AGCAGCAGCCCATTTGAATG AGAATCGCCAGTACTTCCCATC CTTTGTCGTTGGTTAGCTGGT AGAGCTTCACTCAAAGCTTCATGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashimoto, Y.; Tsuzuki-Nakao, T.; Kida, N.; Matsuo, Y.; Maruyama, T.; Okada, H.; Hirota, K. Inflammatory Cytokine-Induced HIF-1 Activation Promotes Epithelial–Mesenchymal Transition in Endometrial Epithelial Cells. Biomedicines 2023, 11, 210. https://doi.org/10.3390/biomedicines11010210
Hashimoto Y, Tsuzuki-Nakao T, Kida N, Matsuo Y, Maruyama T, Okada H, Hirota K. Inflammatory Cytokine-Induced HIF-1 Activation Promotes Epithelial–Mesenchymal Transition in Endometrial Epithelial Cells. Biomedicines. 2023; 11(1):210. https://doi.org/10.3390/biomedicines11010210
Chicago/Turabian StyleHashimoto, Yoshiko, Tomoko Tsuzuki-Nakao, Naoko Kida, Yoshiyuki Matsuo, Tetsuo Maruyama, Hidetaka Okada, and Kiichi Hirota. 2023. "Inflammatory Cytokine-Induced HIF-1 Activation Promotes Epithelial–Mesenchymal Transition in Endometrial Epithelial Cells" Biomedicines 11, no. 1: 210. https://doi.org/10.3390/biomedicines11010210
APA StyleHashimoto, Y., Tsuzuki-Nakao, T., Kida, N., Matsuo, Y., Maruyama, T., Okada, H., & Hirota, K. (2023). Inflammatory Cytokine-Induced HIF-1 Activation Promotes Epithelial–Mesenchymal Transition in Endometrial Epithelial Cells. Biomedicines, 11(1), 210. https://doi.org/10.3390/biomedicines11010210








