Beneficial Effects of Table Grape Use on Serum Levels of Omega-3 Index and Liver Function: A Randomized Controlled Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Blood Samples
2.3. Serum Fatty Acids Extraction
2.4. Serum Fatty Acids Analysis and Quantification
2.5. Adiponectin and Fibroblast Growth Factor 21 (FGF21) Assay
3. Results
3.1. Effect of Table Grape on Serum Biochemical Parameters
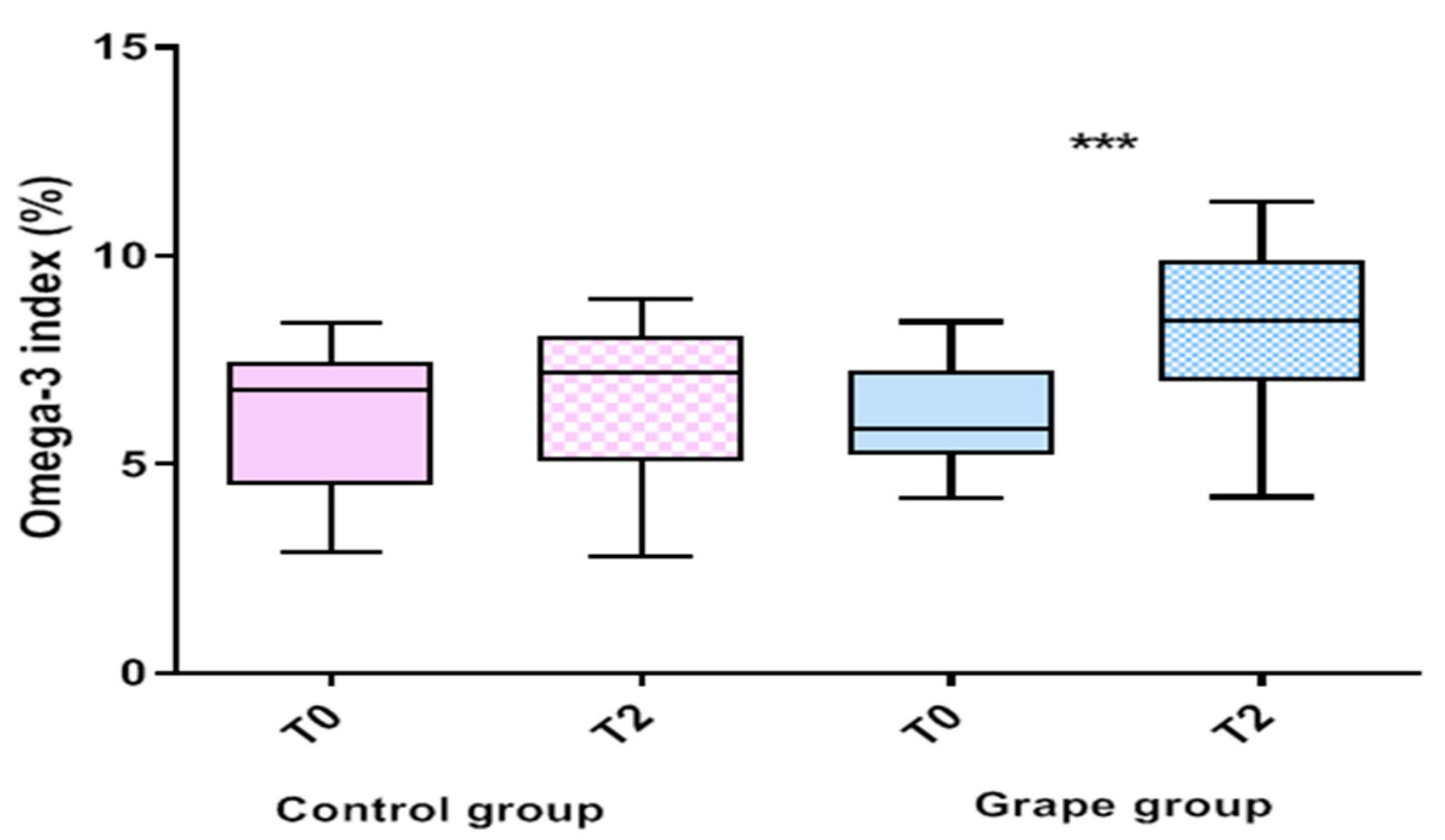
3.2. Effect of Table Grape on Serum Fatty Acids Profile
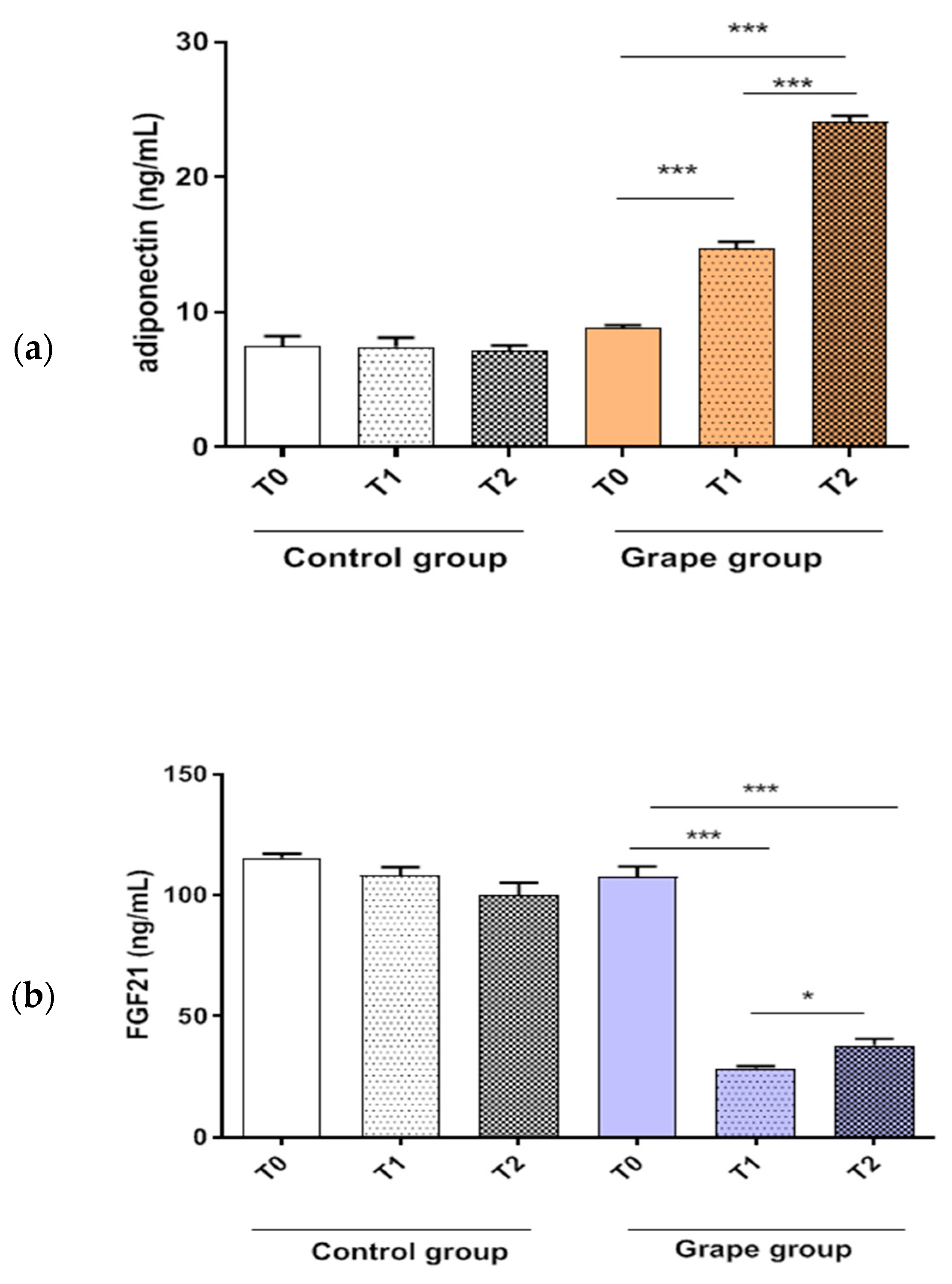
3.3. Impact of Table Grape on Serum Adiponectin and FGF21 Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, J.M.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Tech. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Park, E.; Edirisinghe, I.; Choy, Y.Y.; Waterhouse, A.; Burton-Freeman, B. Effects of grape seed extract beverage on blood pressure and metabolic indices in individuals with pre-hypertension: A randomised, double-blinded, two-arm, parallel, placebo-controlled trial. Br. J. Nutr. 2016, 115, 226–238. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomas-Barberan, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Davinelli, S.; Scapagnini, G. Polyphenols: A Promising Nutritional Approach to Prevent or Reduce the Progression of Prehypertension. High Blood Press. Cardiovasc. Prev. 2016, 23, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrncic, M.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Avtanski, D.; Poretsky, L. Phyto-polyphenols as potential inhibitors of breast cancer metastasis. Mol. Med. 2018, 24, 29. [Google Scholar] [CrossRef]
- Davinelli, S.; Corbi, G.; Zarrelli, A.; Arisi, M.; Calzavara-Pinton, P.; Grassi, D.; De Vivo, I.; Scapagnini, G. Short-term supplementation with flavanol-rich cocoa improves lipid profile, antioxidant status and positively influences the AA/EPA ratio in healthy subjects. J. Nutr. Biochem. 2018, 61, 33–39. [Google Scholar] [CrossRef]
- Tutino, V.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Cisternino, A.M.; Gigante, I.; Lanzilotta, E.; Iacovazzi, P.A.; Lippolis, A.; Lippolis, T.; et al. Impact of Fresh Table Grape Intake on Circulating microRNAs Levels in Healthy Subjects: A Significant Modulation of Gastrointestinal Cancer-Related Pathways. Mol. Nutr. Food Res. 2021, 65, e2100428. [Google Scholar] [CrossRef]
- Dinicola, S.; Cucina, A.; Pasqualato, A.; D’Anselmi, F.; Proietti, S.; Lisi, E.; Pasqua, G.; Antonacci, D.; Bizzarri, M. Antiproliferative and apoptotic effects triggered by Grape Seed Extract (GSE) versus epigallocatechin and procyanidins on colon cancer cell lines. Int. J. Mol. Sci. 2012, 13, 651–664. [Google Scholar] [CrossRef]
- Toufektsian, M.C.; Salen, P.; Laporte, F.; Tonelli, C.; de Lorgeril, M. Dietary flavonoids increase plasma very long-chain (n-3) fatty acids in rats. J. Nutr. 2011, 141, 37–41. [Google Scholar] [CrossRef]
- Tutino, V.; Gigante, I.; Milella, R.A.; De Nunzio, V.; Flamini, R.; De Rosso, M.; Scavo, M.P.; Depalo, N.; Fanizza, E.; Caruso, M.G.; et al. Flavonoid and Non-Flavonoid Compounds of Autumn Royal and Egnatia Grape Skin Extracts Affect Membrane PUFA’s Profile and Cell Morphology in Human Colon Cancer Cell Lines. Molecules 2020, 25, 3352. [Google Scholar] [CrossRef] [PubMed]
- Tutino, V.; Gigante, I.; Scavo, M.P.; Refolo, M.G.; Nunzio, V.; Milella, R.A.; Caruso, M.G.; Notarnicola, M. Stearoyl-CoA Desaturase-1 Enzyme Inhibition by Grape Skin Extracts Affects Membrane Fluidity in Human Colon Cancer Cell Lines. Nutrients 2020, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- de Lorgeril, M.; Salen, P.; Martin, J.L.; Boucher, F.; de Leiris, J. Interactions of wine drinking with omega-3 fatty acids in patients with coronary heart disease: A fish-like effect of moderate wine drinking. Am. Heart J. 2008, 155, 175–181. [Google Scholar] [CrossRef] [PubMed]
- di Giuseppe, R.; de Lorgeril, M.; Salen, P.; Laporte, F.; Di Castelnuovo, A.; Krogh, V.; Siani, A.; Arnout, J.; Cappuccio, F.P.; van Dongen, M.; et al. Alcohol consumption and n-3 polyunsaturated fatty acids in healthy men and women from 3 European populations. Am. J. Clin. Nutr. 2009, 89, 354–362. [Google Scholar] [PubMed]
- Schramm, D.D.; Wang, J.F.; Holt, R.R.; Ensunsa, J.L.; Gonsalves, J.L.; Lazarus, S.A.; Schmitz, H.H.; German, J.B.; Keen, C.L. Chocolate procyanidins decrease the leukotriene-prostacyclin ratio in humans and human aortic endothelial cells. Am. J. Clin. Nutr. 2001, 73, 36–40. [Google Scholar] [CrossRef]
- Davinelli, S.; Corbi, G.; Righetti, S.; Sears, B.; Olarte, H.H.; Grassi, D.; Scapagnini, G. Cardioprotection by Cocoa Polyphenols and omega-3 Fatty Acids: A Disease-Prevention Perspective on Aging-Associated Cardiovascular Risk. J. Med. Food 2018, 21, 1060–1069. [Google Scholar] [CrossRef]
- Chacinska, M.; Zabielski, P.; Ksiazek, M.; Szalaj, P.; Jarzabek, K.; Kojta, I.; Chabowski, A.; Blachnio-Zabielska, A.U. The Impact of OMEGA-3 Fatty Acids Supplementation on Insulin Resistance and Content of Adipocytokines and Biologically Active Lipids in Adipose Tissue of High-Fat Diet Fed Rats. Nutrients 2019, 11, 835. [Google Scholar] [CrossRef]
- Diez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef]
- Fasshauer, M.; Bluher, M. Adipokines in health and disease. Trends Pharmacol. Sci. 2015, 36, 461–470. [Google Scholar] [CrossRef]
- Zebrowska, A.; Hall, B.; Stolecka-Warzecha, A.; Stanula, A.; Sadowska-Krepa, E. The Effect of Omega-3 Fatty Acid Supplementation on Serum Adipocytokines, Lipid Profile and Biochemical Markers of Inflammation in Recreational Runners. Nutrients 2021, 13, 456. [Google Scholar] [CrossRef]
- Domouzoglou, E.M.; Vlahos, A.P.; Cholevas, V.K.; Papafaklis, M.I.; Chaliasos, N.; Siomou, E.; Michalis, L.K.; Tsatsoulis, A.; Naka, K.K. Association of fibroblast growth factor 21 with metabolic syndrome and endothelial function in children: A prospective cross-sectional study on novel biomarkers. Ann. Pediatr. Endocrinol. Metab. 2021, 26, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Tezze, C.; Romanello, V.; Sandri, M. FGF21 as Modulator of Metabolism in Health and Disease. Front. Physiol. 2019, 10, 419. [Google Scholar] [CrossRef] [PubMed]
- Rebollo-Hernanz, M.; Aguilera, Y.; Martin-Cabrejas, M.A.; Gonzalez de Mejia, E. Phytochemicals from the Cocoa Shell Modulate Mitochondrial Function, Lipid and Glucose Metabolism in Hepatocytes via Activation of FGF21/ERK, AKT, and mTOR Pathways. Antioxidants 2022, 11, 136. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Yamaguchi, K.; Seko, Y.; Okishio, S.; Ishiba, H.; Tochiki, N.; Takahashi, A.; Kataoka, S.; Okuda, K.; Liu, Y.; et al. Hepatocyte-specific fibroblast growth factor 21 overexpression ameliorates high-fat diet-induced obesity and liver steatosis in mice. Lab. Investig. 2022, 102, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Luo, Y.; Fang, A.P.; Wusiman, M.; He, T.T.; Liu, X.Z.; Yishake, D.; Chen, S.; Lu, X.T.; Zhang, Y.J.; et al. High serum fibroblast growth factor 21 is associated with inferior hepatocellular carcinoma survival: A prospective cohort study. Liver Int. 2022, 42, 663–673. [Google Scholar] [CrossRef]
- Walker, R.E.; Jackson, K.H.; Tintle, N.L.; Shearer, G.C.; Bernasconi, A.; Masson, S.; Latini, R.; Heydari, B.; Kwong, R.Y.; Flock, M.; et al. Predicting the effects of supplemental EPA and DHA on the omega-3 index. Am. J. Clin. Nutr. 2019, 110, 1034–1040. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Neubronner, J.; Block, R.C.; von Schacky, C.; Hahn, A. Associations between Omega-3 Index increase and triacylglyceride decrease in subjects with hypertriglyceridemia in response to six month of EPA and DHA supplementation. Prostaglandins Leukot. Essent. Fatty Acids 2014, 91, 129–134. [Google Scholar] [CrossRef]
- Richardson, C.E.; Krishnan, S.; Gray, I.J.; Keim, N.L.; Newman, J.W. The Omega-3 Index Response to an 8 Week Randomized Intervention Containing Three Fatty Fish Meals Per Week Is Influenced by Adiposity in Overweight to Obese Women. Front. Nutr. 2022, 9, 810003. [Google Scholar] [CrossRef]
- Li, J.; Zheng, Z.; Liu, M.; Ren, Y.; Ruan, Y.; Li, D. Relationship between the n-3 index, serum metabolites and breast cancer risk. Food Funct. 2021, 12, 7741–7748. [Google Scholar] [CrossRef]
- Harris, W.S.; Von Schacky, C. The Omega-3 Index: A new risk factor for death from coronary heart disease? Prev. Med. 2004, 39, 212–220. [Google Scholar] [CrossRef]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean diet on metabolic syndrome, cancer and longevity. Oncotarget 2017, 8, 8947–8979. [Google Scholar] [CrossRef] [PubMed]
- Milella, R.A.; Gasparro, M.; Alagna, F.; Cardone, M.F.; Rotunno, S.; Ammollo, C.T.; Semeraro, F.; Tullo, A.; Marzano, F.; Catalano, D.; et al. Microarray data and pathway analyses of peripheral blood mononuclear cells from healthy subjects after a three weeks grape-rich diet. Data Brief. 2020, 29, 105278. [Google Scholar] [CrossRef]
- Athinarayanan, S.; Fan, Y.Y.; Wang, X.; Callaway, E.; Cai, D.; Chalasani, N.; Chapkin, R.S.; Liu, W. Fatty Acid Desaturase 1 Influences Hepatic Lipid Homeostasis by Modulating the PPARalpha-FGF21 Axis. Hepatol. Commun. 2021, 5, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Takahashi, S.; Higashiura, Y.; Sakai, A.; Koyama, M.; Saitoh, S.; Shimamoto, K.; Ohnishi, H.; Furuhashi, M. Circulating level of fatty acid-binding protein 4 is an independent predictor of metabolic dysfunction-associated fatty liver disease in middle-aged and elderly individuals. J. Diabetes Investig. 2022, 13, 878–888. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. [Google Scholar] [CrossRef]
- Ammollo, C.T.; Semeraro, F.; Milella, R.A.; Antonacci, D.; Semeraro, N.; Colucci, M. Grape intake reduces thrombin generation and enhances plasma fibrinolysis. Potential role of circulating procoagulant microparticles. J. Nutr. Biochem. 2017, 50, 66–73. [Google Scholar] [CrossRef]
- Dushay, J.; Chui, P.C.; Gopalakrishnan, G.S.; Varela-Rey, M.; Crawley, M.; Fisher, F.M.; Badman, M.K.; Martinez-Chantar, M.L.; Maratos-Flier, E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology 2010, 139, 456–463. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Lin, X.; Liu, Y.B.; Hu, H. Metabolic role of fibroblast growth factor 21 in liver, adipose and nervous system tissues. Biomed. Rep. 2017, 6, 495–502. [Google Scholar] [CrossRef]
- Jimenez, V.; Jambrina, C.; Casana, E.; Sacristan, V.; Munoz, S.; Darriba, S.; Rodo, J.; Mallol, C.; Garcia, M.; Leon, X.; et al. FGF21 gene therapy as treatment for obesity and insulin resistance. EMBO Mol. Med. 2018, 10, e8791. [Google Scholar] [CrossRef] [PubMed]
- Spann, R.A.; Morrison, C.D.; den Hartigh, L.J. The Nuanced Metabolic Functions of Endogenous FGF21 Depend on the Nature of the Stimulus, Tissue Source, and Experimental Model. Front. Endocrinol. 2021, 12, 802541. [Google Scholar] [CrossRef] [PubMed]

| Control Group (n = 19) | Grape Group (n = 21) | |
|---|---|---|
| Sex | ||
| Male | 4 | 9 |
| Female | 15 | 12 |
| Age | 44.1 ± 10.14 | 47.4 ± 9.5 |
| Glucose (mg/dL) | ||
| T0 | 82.53 ± 6.05 | 79.33 ± 6.08 |
| T1 | 84.05 ± 7.7 | 79.76 ± 5.43 |
| T2 | 80.00 ± 9.81 | 77.41 ± 10.67 |
| AST (U/L) | ||
| T0 | 18.89 ± 4.34 | 18.67 ± 3.54 |
| T1 | 19.9 ± 7.34 | 18.48 ± 3.65 |
| T2 | 19.07 ± 4.33 | 19.71 ± 5.64 |
| ALT (U/L) | ||
| T0 | 22.47 ± 10.6 | 18.62 ± 7.26 |
| T1 | 23.58 ± 12.0 | 19.9 ± 6.24 |
| T2 | 19.93 ± 7.96 | 18.53 ± 7.19 |
| γ-GT (U/L) | ||
| T0 | 19.32 ± 8.39 | 17.20 ± 9.30 |
| T1 | 15.80 ± 7.40 | 16.20 ± 6.50 |
| T2 | 15.47 ± 7.68 | 14.65 ± 6.65 |
| Cholesterol (mg/dL) | ||
| T0 | 196.1 ± 30.0 | 194.3 ± 37.5 |
| T1 | 180.5 ± 20.32 * | 181.4 ± 21.9 * |
| T2 | 188.9 ± 33.96 | 198.8 ± 41.85 |
| HDL-C (mg/dL) | ||
| T0 | 51.20 ± 12.80 | 54.62 ± 7.99 |
| T1 | 49.70 ± 10.60 | 51.77 ± 4.24 |
| T2 | 51.33 ± 11.60 | 54.88 ± 13.13 |
| LDL-C (mg/dL) | ||
| T0 | 120.1 ± 21.6 | 116.1 ± 23.24 |
| T1 | 118.3 ± 22.5 | 118.5 ± 21.5 |
| T2 | 123.1 ± 27.33 | 120.7 ± 25.24 |
| Triglycerides (mg/dL) | ||
| T0 | 87.50 ± 41.53 | 69.33 ± 37.34 |
| T1 | 76.60 ± 29.03 | 67.35 ± 25.49 |
| T2 | 72.53 ± 45.29 | 71.06 ± 39.53 |
| Protein C reactive (mg/dL) | ||
| T0 | 0.14 ± 0.26 | 0.14 ± 0.26 |
| T1 | 0.13 ± 0.36 | 0.18 ± 0.16 |
| T2 | 0.24 ± 0.67 | 0.10 ± 0.26 |
| Fatty Acid (%) | Control Group | Grape Group | ||
|---|---|---|---|---|
| T0 | T2 | T0 | T2 | |
| Palmitic acid | 18.30 ± 2.80 | 19.98 ± 3.92 | 18.44 ± 1.91 | 18.27 ± 2.99 |
| Stearic acid | 14.33 ± 1.98 | 15.51 ± 2.42 | 14.27 ± 1.83 | 14.29 ± 2.12 |
| Oleic acid | 13.04 ± 1.87 | 14.38 ± 1.48 | 12.85 ± 1.60 | 14.15 ± 1.86 * |
| Vaccenic acid | 0.89 ± 0.23 | 0.99 ± 0.21 | 0.83 ± 0.24 | 0.90 ± 0.35 |
| Linoleic acid | 10.61 ± 1.39 | 10.93 ± 1.21 | 11.00 ± 1.58 | 11.45 ± 1.66 |
| γ-linoleic acid (GLA) | 0.69 ± 1.74 | 0.59 ± 1.29 | 0.34 ± 0.85 | 0.37 ± 1.45 |
| α-linolenic acid (ALA) | 0.11 ± 0.07 | 0.13 ± 0.14 | 0.12 ± 0.11 | 0.16 ± 0.30 |
| Arachidonic acid (AA) | 18.25 ± 3.96 | 19.19 ± 3.43 | 17.72 ± 1.98 | 18.84 ± 3.43 |
| Eicosapentaenoic acid (EPA) | 1.65 ± 1.09 | 2.07 ± 1.15 | 2.02 ± 0.98 | 2.94 ± 1.18 * |
| Docosahexaenoic acid (DHA) | 4.37 ± 1.53 | 4.60 ± 2.01 | 4.07 ± 1.19 | 5.29 ± 1.45 * |
| Saturated fatty acids | 40.62 ± 3.91 | 41.67 ± 4.27 | 41.83 ± 2.82 | 41.65 ± 5.22 |
| Monounsaturated fatty acids | 17.65 ± 1.70 | 18.01 ± 1.41 | 17.26 ± 1.29 | 17.53 ± 1.64 |
| Polyunsaturated fatty acids | 38.67 ± 4.88 | 37.75 ± 4.35 | 37.76 ± 3.05 | 38.81 ± 5.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Notarnicola, M.; De Nunzio, V.; Lippolis, T.; Tutino, V.; Cisternino, A.M.; Iacovazzi, P.A.; Milella, R.A.; Gasparro, M.; Negro, R.; Polignano, M.; et al. Beneficial Effects of Table Grape Use on Serum Levels of Omega-3 Index and Liver Function: A Randomized Controlled Clinical Trial. Biomedicines 2022, 10, 2310. https://doi.org/10.3390/biomedicines10092310
Notarnicola M, De Nunzio V, Lippolis T, Tutino V, Cisternino AM, Iacovazzi PA, Milella RA, Gasparro M, Negro R, Polignano M, et al. Beneficial Effects of Table Grape Use on Serum Levels of Omega-3 Index and Liver Function: A Randomized Controlled Clinical Trial. Biomedicines. 2022; 10(9):2310. https://doi.org/10.3390/biomedicines10092310
Chicago/Turabian StyleNotarnicola, Maria, Valentina De Nunzio, Tamara Lippolis, Valeria Tutino, Anna Maria Cisternino, Palma Aurelia Iacovazzi, Rosa Anna Milella, Marica Gasparro, Roberto Negro, Maurizio Polignano, and et al. 2022. "Beneficial Effects of Table Grape Use on Serum Levels of Omega-3 Index and Liver Function: A Randomized Controlled Clinical Trial" Biomedicines 10, no. 9: 2310. https://doi.org/10.3390/biomedicines10092310
APA StyleNotarnicola, M., De Nunzio, V., Lippolis, T., Tutino, V., Cisternino, A. M., Iacovazzi, P. A., Milella, R. A., Gasparro, M., Negro, R., Polignano, M., & Caruso, M. G. (2022). Beneficial Effects of Table Grape Use on Serum Levels of Omega-3 Index and Liver Function: A Randomized Controlled Clinical Trial. Biomedicines, 10(9), 2310. https://doi.org/10.3390/biomedicines10092310







