Evidence for an Intrathecal Immunoglobulin Synthesis by Kappa Free Light Chains in Neurological Patients with an Isolated Band in Isoelectric Focusing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Laboratory Assay
2.2. Patients
2.3. Statistical Analysis
2.4. Ethical Standards
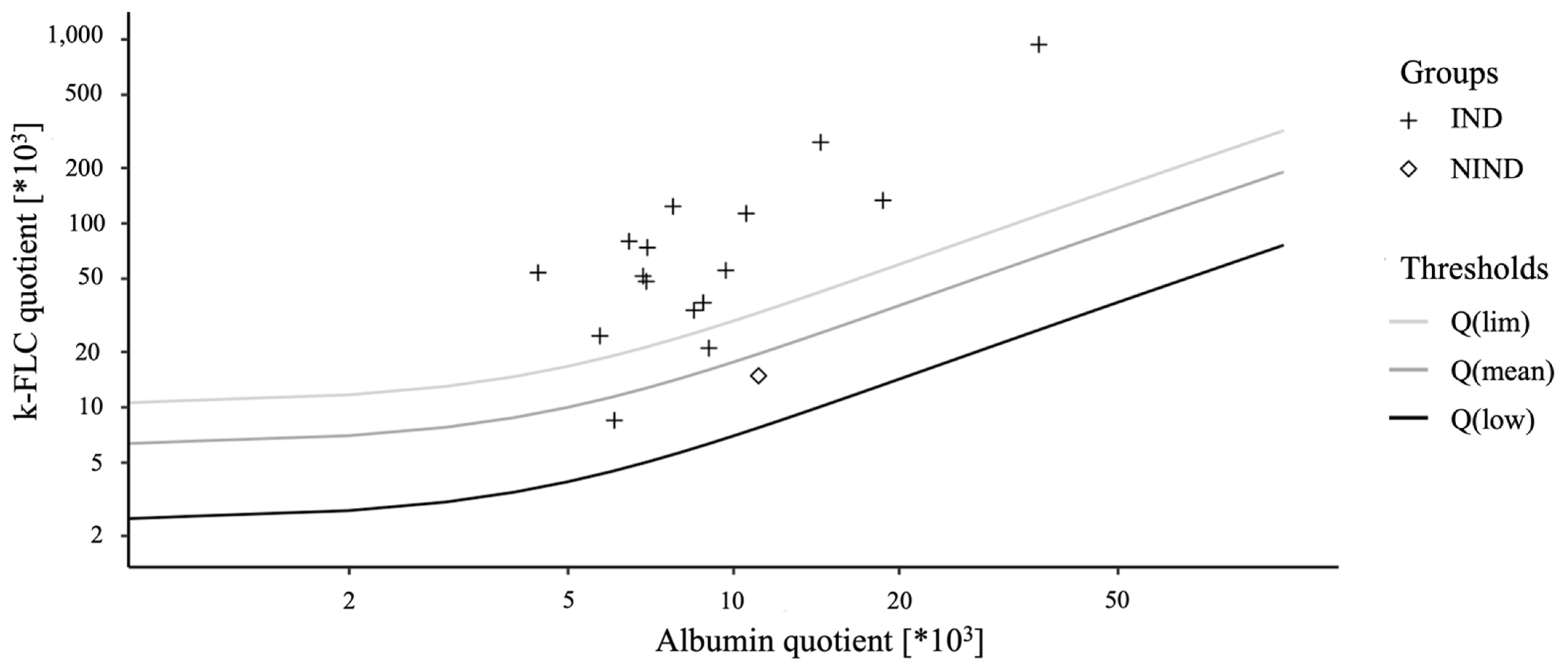
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Freedman, M.S.; Thompson, E.J.; Deisenhammer, F.; Giovannoni, G.; Grimsley, G.; Keir, G.; Ohman, S.; Racke, M.K.; Sharief, M.; Sindic, C.J.; et al. Recommended standard of cerebrospinal fluid analysis in the diagnosis of multiple sclerosis: A consensus statement. Arch Neurol. 2005, 62, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Stangel, M.; Fredrikson, S.; Meinl, E.; Petzold, A.; Stüve, O.; Tumani, H. The utility of cerebrospinal fluid analysis in patients with multiple sclerosis. Nat. Rev. Neurol. 2013, 9, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Alvarez-Cermeño, J.; Bernardi, G.; Cogato, I.; Fredman, P.; Frederiksen, J.; Fredrikson, S.; Gallo, P.; Grimaldi, L.M.; Grønning, M.; et al. Cerebrospinal fluid in the diagnosis of multiple sclerosis: A consensus report. J. Neurol. Neurosurg. Psychiatry 1994, 57, 897–902. [Google Scholar] [CrossRef]
- Hegen, H.; Zinganell, A.; Auer, M.; Deisenhammer, F. The clinical significance of single or double bands in cerebrospinal fluid isoelectric focusing. A retrospective study and systematic review. PLoS ONE 2019, 14, e0215410. [Google Scholar] [CrossRef]
- Ferraro, D.; Franciotta, D.; Bedin, R.; Solaro, C.; Cocco, E.; Santangelo, M.; Immovilli, P.; Gajofatto, A.; Calabrese, M.; Di Filippo, M.; et al. A multicenter study on the diagnostic significance of a single cerebrospinal fluid IgG band. J. Neurol. 2017, 264, 973–978. [Google Scholar] [CrossRef]
- Davies, G.; Keir, G.; Thompson, E.J.; Giovannoni, G. The clinical significance of an intrathecal monoclonal immunoglobulin band: A follow-up study. Neurology 2003, 60, 1163–1166. [Google Scholar] [CrossRef]
- Agnello, L.; Sasso, B.L.; Salemi, G.; Altavilla, P.; Pappalardo, E.M.; Caldarella, R.; Meli, F.; Scazzone, C.; Bivona, G.; Ciaccio, M. Clinical use of κ free light chains index as a screeningtest for multiple sclerosis. Lab. Med. 2020, 51, 402–407. [Google Scholar] [CrossRef]
- Konen, F.F.; Schwenkenbecher, P.; Jendretzky, K.F.; Gingele, S.; Sühs, K.W.; Tumani, H.; Süße, M.; Skripuletz, T. The Increasing Role of Kappa Free Light Chains in the Diagnosis of Multiple Sclerosis. Cells 2021, 10, 3056. [Google Scholar] [CrossRef]
- Leurs, C.E.; Twaalfhoven, H.; Lissenberg-Witte, B.I.; van Pesch, V.; Dujmovic, I.; Drulovic, J.; Castellazzi, M.; Bellini, T.; Pugliatti, M.; Kuhle, J.; et al. Kappa free light chains is a valid tool in the diagnostics of MS: A large multicenter study. Mult. Scler. 2020, 26, 912–923. [Google Scholar] [CrossRef] [Green Version]
- Voortman, M.M.; Stojakovic, T.; Pirpamer, L.; Jehna, M.; Langkammer, C.; Scharnagl, H.; Reindl, M.; Ropele, S.; Seifert-Held, T.; Archelos, J.-J.; et al. Prognostic value of free light chains lambda and kappa in early multiple sclerosis. Mult. Scler. 2017, 23, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Presslauer, S.; Milosavljevic, D.; Huebl, W.; Aboulenein-Djamshidian, F.; Krugluger, W.; Deisenhammer, F.; Senel, M.; Tumani, H.; Hegen, H. Validation of kappa free light chains as a diagnostic biomarker in multiple sclerosis and clinically isolated syndrome: A multicenter study. Mult. Scler. J. 2016, 22, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Matsui, M.; Inoue, I.; Awata, T.; Katayama, S.; Murakoshi, T. Invited critical review Free immunoglobulin light chain: Its biology and implications in diseases. Clin. Chim. Acta 2011, 412, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Süße, M.; Feistner, F.; Holbe, C.; Grothe, M.; Nauck, M.; Dressel, A.; Hannich, M.J. Diagnostic value of kappa free light chains in patients with one isolated band in isoelectric focusing. Clin. Chim. Acta 2020, 507, 205–209. [Google Scholar] [CrossRef]
- Teunissen, C.E.; Petzold, A.; Bennett, J.L.; Berven, F.S.; Brundin, L.; Comabella, M.; Franciotta, D.; Frederiksen, J.L.; Fleming, J.O.; Furlan, R.; et al. A consensus protocol for the standardization of cerebrospinal fluid collection and biobanking. Neurology 2009, 73, 1914–1922. [Google Scholar] [CrossRef]
- Teunissen, C.; Menge, T.; Altintas, A.; Álvarez-Cermeño, J.C.; Bertolotto, A.; Berven, F.S.; Brundin, L.; Comabella, M.; Degn, M.; Deisenhammer, F.; et al. Consensus definitions and application guidelines for control groups in cerebrospinal fluid biomarker studies in multiple sclerosis. Mult. Scler. J. 2013, 19, 1802–1809. [Google Scholar] [CrossRef]
- Reiber, H.; Zeman, D.; Kušnierová, P.; Mundwiler, E.; Bernasconi, L. Diagnostic relevance of free light chains in cerebrospinal fluid—The hyperbolic reference range for reliable data interpretation in quotient diagrams. Clin. Chim. Acta 2019, 497, 153–162. [Google Scholar] [CrossRef]
- Hannich, M.J.; Dressel, A.; Budde, K.; Petersmann, A.; Nauck, M.; Süße, M. Kappa Free Light Chains in the Context of Blood Contamination, and Other IgA- and IgM-Related Cerebrospinal Fluid Disease Pattern. Cells 2021, 10, 616. [Google Scholar] [CrossRef]
- Konen, F.F.; Schwenkenbecher, P.; Jendretzky, K.F.; Gingele, S.; Witte, T.; Sühs, K.-W.; Grothe, M.; Hannich, M.J.; Süße, M.; Skripuletz, T. Kappa Free Light Chains in Cerebrospinal Fluid in Inflammatory and Non-Inflammatory Neurological Diseases. Brain Sci. 2022, 12, 475. [Google Scholar] [CrossRef]
- Tjernberg, I.; Johansson, M.; Henningsson, A.J. Diagnostic performance of cerebrospinal fluid free light chains in Lyme neuroborreliosis—A pilot study. Clin. Chem. Lab. Med. 2019, 57, 2008–2018. [Google Scholar] [CrossRef]
- Hegen, H.; Milosavljevic, D.; Schnabl, C.; Manowiecka, A.; Walde, J.; Deisenhammer, F.; Presslauer, S. Cerebrospinal fluid free light chains as diagnostic biomarker in neuroborreliosis. Clin. Chem. Lab. Med. 2018, 56, 1383–1391. [Google Scholar] [CrossRef]
- Gudowska-Sawczuk, M.; Czupryna, P.; Moniuszko-Malinowska, A.; Pancewicz, S.; Mroczko, B. Free Immunoglobulin Light Chains in Patients with Tick-Borne Encephalitis: Before and after Treatment. J. Clin. Med. 2021, 10, 2922. [Google Scholar] [CrossRef]
- Berek, K.; Bsteh, G.; Auer, M.; Di Pauli, F.; Grams, A.; Milosavljevic, D.; Poskaite, P.; Schnabl, C.; Wurth, S.; Zinganell, A.; et al. Kappa-Free Light Chains in CSF Predict Early Multiple Sclerosis Disease Activity. Neurol.-Neuroimmunol. Neuroinflamm. 2021, 8, e1005. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.M.; Espiño, M.; Costa-Frossard, L.; Muriel, A.; Jiménez, J.; Alvarez-Cermeño, J.C. High levels of cerebrospinal fluid free kappa chains predict conversion to multiple sclerosis. Clin. Chim. Acta 2012, 413, 1813–1816. [Google Scholar] [CrossRef] [PubMed]
- Crespi, I.; Sulas, M.G.; Mora, R.; Naldi, P.; Vecchio, D.; Comi, C.; Cantello, R.; Bellomo, G. Combined use of Kappa Free Light Chain Index and Isoelectrofocusing of Cerebro-Spinal Fluid in Diagnosing Multiple Sclerosis: Performances and Costs. Clin. Lab. 2017, 63, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Süße, M.; Feistner, F.; Grothe, M.; Nauck, M.; Dressel, A.; Hannich, M.J. Free light chains kappa can differentiate between myelitis and noninflammatory myelopathy. Neurol. (R) Neuroimmunol. Neuroinflamm. 2020, 7, e892. [Google Scholar] [CrossRef]
- Schwenkenbecher, P.; Konen, F.F.; Wurster, U.; Witte, T.; Gingele, S.; Sühs, K.W.; Stangel, M.; Skripuletz, T. Reiber’s diagram for kappa free light chains: The new standard for assessing intrathecal synthesis? Diagnostics 2019, 9, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| IND/PIND (n = 29) | NIND/SC (n = 18) | p-Value | |
|---|---|---|---|
| age | 34.0 (26.5–50.5) | 32.0 (24.8–56.0) | 0.776 |
| female | 14 (48.3%) | 10 (55.6%) | 0.627 |
| WBC/µL | 12.0 (3.5–54.5) | 2.0 (1.0–4.0) | 0.001 * |
| Qalb × 10−3 | 7.8 (5.2–11.0) | 4.4 (3.6–6.6) | 0.001 * |
| QIgG × 10−3 | 4.4 (3.2–6.2) | 2.4 (2.1–4.1) | 0.002 * |
| QIgM × 10−3 | 1.0 (0.7–3.0) | 0.6 (0.3–1.3) | 0.013 * |
| QIgA × 10−3 | 0.3 (0.3–0.4) | 0.3 (0.3–0.4) | 0.004 * |
| k-FLC serum (mg/L) | 14.6 (10.9–20.6) | - | |
| k-FLC CSF (mg/L) | 0.8 (0.6–2.2) | - | |
| k-FLC index | 7.3 (4.2–12.3) | - | |
| >Q(lim) | 14 | 0 | |
| <Q(lim) | 2 | 1 | |
| <LML | 13 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, B.; Pichler, A.; Damulina, A.; Buchmann, A.; Hochmeister, S.; Seifert-Held, T.; Enzinger, C.; Archelos, J.-J.; Khalil, M. Evidence for an Intrathecal Immunoglobulin Synthesis by Kappa Free Light Chains in Neurological Patients with an Isolated Band in Isoelectric Focusing. Biomedicines 2022, 10, 2202. https://doi.org/10.3390/biomedicines10092202
Weiss B, Pichler A, Damulina A, Buchmann A, Hochmeister S, Seifert-Held T, Enzinger C, Archelos J-J, Khalil M. Evidence for an Intrathecal Immunoglobulin Synthesis by Kappa Free Light Chains in Neurological Patients with an Isolated Band in Isoelectric Focusing. Biomedicines. 2022; 10(9):2202. https://doi.org/10.3390/biomedicines10092202
Chicago/Turabian StyleWeiss, Bastian, Alexander Pichler, Anna Damulina, Arabella Buchmann, Sonja Hochmeister, Thomas Seifert-Held, Christian Enzinger, Juan-Jose Archelos, and Michael Khalil. 2022. "Evidence for an Intrathecal Immunoglobulin Synthesis by Kappa Free Light Chains in Neurological Patients with an Isolated Band in Isoelectric Focusing" Biomedicines 10, no. 9: 2202. https://doi.org/10.3390/biomedicines10092202
APA StyleWeiss, B., Pichler, A., Damulina, A., Buchmann, A., Hochmeister, S., Seifert-Held, T., Enzinger, C., Archelos, J.-J., & Khalil, M. (2022). Evidence for an Intrathecal Immunoglobulin Synthesis by Kappa Free Light Chains in Neurological Patients with an Isolated Band in Isoelectric Focusing. Biomedicines, 10(9), 2202. https://doi.org/10.3390/biomedicines10092202





