Diagnosis of Malnutrition According to GLIM Criteria Predicts Complications and 6-Month Survival in Cancer Outpatients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Design and Selection Criteria
2.3. Patient Characteristics and Malnutrition Criteria
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics and Malnutrition Prevalence
3.2. Complications Resulting from Malnutrition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schaible, U.E.; Kaufmann, S.H. Malnutrition and infection: Complex mechanisms and global impacts. PLoS Med. 2007, 4, e115. [Google Scholar] [CrossRef] [PubMed]
- Katona, P.; Katona-Apte, J. The interaction between nutrition and infection. Clin. Infect. Dis. 2008, 46, 1582–1588. [Google Scholar] [CrossRef] [PubMed]
- de Las Peñas, R.; Majem, M.; Perez-Altozano, J.; Virizuela, J.A.; Cancer, E.; Diz, P.; Donnay, O.; Hurtado, A.; Jimenez-Fonseca, P.; Ocon, M.J. SEOM clinical guidelines on nutrition in cancer patients (2018). Clin. Transl. Oncol. 2019, 21, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Asakawa, A.; Amitani, H.; Nakamura, N.; Inui, A. Cancer cachexia—Pathophysiology and management. J. Gastroenterol. 2013, 48, 574–594. [Google Scholar] [CrossRef] [PubMed]
- Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Fearon, K.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 2017, 36, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Ryan, A.M.; Power, D.G.; Daly, L.; Cushen, S.J.; Ní Bhuachalla, Ē.; Prado, C.M. Cancer-associated malnutrition, cachexia and sarcopenia: The skeleton in the hospital closet 40 years later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef]
- Haskins, C.P.; Champ, C.E.; Miller, R.; Vyfhuis, M.A.L. Nutrition in Cancer: Evidence and Equality. Adv. Radiat. Oncol. 2020, 5, 817–823. [Google Scholar] [CrossRef]
- Flores-Pérez, J.A.; de la Rosa Oliva, F.; Argenes, Y.; Meneses-Garcia, A. Nutrition, Cancer and Personalized Medicine. Adv. Exp. Med. Biol. 2019, 1168, 157–168. [Google Scholar]
- Ravasco, P. Nutrition in Cancer Patients. J. Clin. Med. 2019, 8, 1211. [Google Scholar] [CrossRef]
- Wiseman, M.J. Nutrition and cancer: Prevention and survival. Br. J. Nutr. 2019, 122, 481–487. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, T.; Pang, L.; Sharma, S.V.; Li, R.; Nyitray, A.G.; Edwards, B.J. Malnutrition and overall survival in older adults with cancer: A systematic review and meta-analysis. J. Geriatr. Oncol. 2019, 10, 874–883. [Google Scholar] [CrossRef]
- Agarwal, E.; Miller, M.; Yaxley, A.; Isenring, E. Malnutrition in the elderly: A narrative review. Maturitas 2013, 76, 296–302. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- Steer, B.; Loeliger, J.; Edbrooke, L.; Deftereos, I.; Laing, E.; Kiss, N. Malnutrition Prevalence according to the GLIM Criteria in Head and Neck Cancer Patients Undergoing Cancer Treatment. Nutrients 2020, 12, 3493. [Google Scholar] [CrossRef]
- Einarsson, S.; Karlsson, H.E.; Björ, O.; Haylock, A.K.; Tiblom Ehrsson, Y. Mapping impact factors leading to the GLIM diagnosis of malnutrition in patients with head and neck cancer. Clin. Nutr. ESPEN 2020, 40, 149–155. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, M.; Zhang, Q.; Zhang, K.P.; Guo, Z.Q.; Xu, H.X.; Yuan, K.-T.; Yu, M.; Braga, M.; Cederholm, T.; et al. The GLIM criteria as an effective tool for nutrition assessment and survival prediction in older adult cancer patients. Clin. Nutr. 2021, 40, 1224–1232. [Google Scholar] [CrossRef]
- Yin, L.; Lin, X.; Li, N.; Zhang, M.; He, X.; Liu, J.; Kang, J.; Chen, X.; Wang, C.; Wang, X.; et al. Evaluation of the Global Leadership Initiative on Malnutrition Criteria Using Different Muscle Mass Indices for Diagnosing Malnutrition and Predicting Survival in Lung Cancer Patients. J. Parenter. Enteral Nutr. 2021, 45, 607–617. [Google Scholar] [CrossRef]
- Gascón-Ruiz, M.; Casas-Deza, D.; Torres-Ramón, I.; Zapata-García, M.; Alonso, N.; Sesma, A.; Lambea, J.; Álvarez-Alejandro, M.; Quílez, E.; Isla, D.; et al. GLIM vs ESPEN criteria for the diagnosis of early malnutrition in oncological outpatients. Clin. Nutr. 2021, 40, 3741–3747. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, K.P.; Zhang, X.; Tang, M.; Song, C.H.; Cong, M.H.; Guo, Z.-Q.; Ding, J.-S.; Braga, M.; Cederholm, T.; et al. Scored-GLIM as an effective tool to assess nutrition status and predict survival in patients with cancer. Clin. Nutr. 2021, 40, 4225–4233. [Google Scholar] [CrossRef]
- Xu, L.B.; Shi, M.M.; Huang, Z.X.; Zhang, W.T.; Zhang, H.H.; Shen, X.; Chen, X. Impact of malnutrition diagnosed using Global Leadership Initiative on Malnutrition criteria on clinical outcomes of patients with gastric cancer. J. Parenter. Enteral Nutr. 2022, 46, 385–394. [Google Scholar] [CrossRef]
- Kakavas, S.; Karayiannis, D.; Bouloubasi, Z.; Poulia, K.A.; Kompogiorgas, S.; Konstantinou, D.; Vougas, V. Global Leadership Initiative on Malnutrition Criteria Predict Pulmonary Complications and 90-Day Mortality after Major Abdominal Surgery in Cancer Patients. Nutrients 2020, 12, 3726. [Google Scholar] [CrossRef] [PubMed]
- Okada, G.; Matsumoto, Y.; Habu, D.; Matsuda, Y.; Lee, S.; Osugi, H. Relationship between GLIM criteria and disease-specific symptoms and its impact on 5-year survival of esophageal cancer patients. Clin. Nutr. 2021, 40, 5072–5078. [Google Scholar] [CrossRef] [PubMed]
- Galindo Martín, C.A.; Aportela Vázquez, V.A.; Becerril Hernández, F.; Aguilar Medina, C.R.; Ayala Carrillo, S.L.; Chávez Flores, A.; Almanza, E.G.; Agredano, M.I.G.; Vilchis, J.D.M. The GLIM criteria for adult malnutrition and its relation with adverse outcomes, a prospective observational study. Clin. Nutr. ESPEN 2020, 38, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Folwarski, M.; Ruszkowski, J.; Świerblewski, M.; Makarewicz, W. Influence of malnutrition stage according to GLIM 2019 criteria and SGA on the quality of life of patients with advanced cancer. Nutr. Hosp. 2020, 37, 1179–1185. [Google Scholar] [CrossRef]
- Shimizu, A.; Maeda, K.; Honda, T.; Ishida, Y.; Ueshima, J.; Nagami, S.; Nagano, A.; Inoue, T.; Murotani, K.; Kayashita, J.; et al. Comparison between the Global Leadership Initiative on Malnutrition and the European Society for Clinical Nutrition and Metabolism definitions for the prevalence of malnutrition in geriatric rehabilitation care. Geriatr. Gerontol. Int. 2020, 20, 1221–1227. [Google Scholar] [CrossRef]
- Einarsson, S.; Laurell, G.; Tiblom Ehrsson, Y. Mapping the frequency of malnutrition in patients with head and neck cancer using the GLIM Criteria for the Diagnosis of Malnutrition. Clin. Nutr. ESPEN 2020, 37, 100–106. [Google Scholar] [CrossRef]
- Contreras-Bolívar, V.; Sánchez-Torralvo, F.J.; Ruiz-Vico, M.; González-Almendros, I.; Barrios, M.; Padín, S.; Alba, E.; Olveira, G. GLIM Criteria Using Hand Grip Strength Adequately Predict Six-Month Mortality in Cancer Inpatients. Nutrients 2019, 11, 2043. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa e Silva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef]
- Prado, C.M.; Purcell, S.A.; Laviano, A. Nutrition interventions to treat low muscle mass in cancer. J. Cachexia Sarcopenia Muscle 2020, 11, 366–380. [Google Scholar] [CrossRef]
- Kapała, A.; Lange, E. Possibility of pain reduction by dietary intervention in patients with advanced cancer. Ann. Agric. Environ. Med. 2013, 1, 18–22. [Google Scholar]
- Omlin, A.; Blum, D.; Wierecky, J.; Haile, S.R.; Ottery, F.D.; Strasser, F. Nutrition impact symptoms in advanced cancer patients: Frequency and specific interventions, a case-control study. J. Cachexia Sarcopenia Muscle 2013, 4, 55–61. [Google Scholar] [CrossRef]
- Bozzetti, F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef]
- Klute, K.A.; Brouwer, J.; Jhawer, M.; Sachs, H.; Gangadin, A.; Ocean, A.; Popa, E.; Dai, T.; Wu, G.; Christos, P.; et al. Chemotherapy dose intensity predicted by baseline nutrition assessment in gastrointestinal malignancies: A multicentre analysis. Eur. J. Cancer 2016, 63, 189–200. [Google Scholar] [CrossRef]
- Kono, T.; Sakamoto, K.; Shinden, S.; Ogawa, K. Pre-therapeutic nutritional assessment for predicting severe adverse events in patients with head and neck cancer treated by radiotherapy. Clin. Nutr. 2017, 36, 1681–1685. [Google Scholar] [CrossRef]

| Variables | Patients N = 165 |
|---|---|
| Sex N (%) | |
| Male | 107 (64.8%) |
| Female | 58 (35.2%) |
| Age (years) | 67.0 [60.0; 74.0] |
| Localization N (%) | |
| Head and neck | 19 (11.5%) |
| Colorectal | 82 (49.7%) |
| Upper GI | 64 (38.8%) |
| Stage N (%) | |
| Localized | 51 (30.9%) |
| Metastatic | 114 (69.1%) |
| Treatment N (%) | |
| Chemotherapy | 137 (83.0%) |
| Chemotherapy + RT | 20 (12.1%) |
| Immunotherapy | 4 (2.42%) |
| Targeted therapy | 4 (2.42%) |
| Duration of treatment (months) | 3.00 [1.00; 7.00] |
| Current state N (%) | |
| Response | 51 (30.9%) |
| Stable | 68 (41.2%) |
| Progressing | 46 (27.9%) |
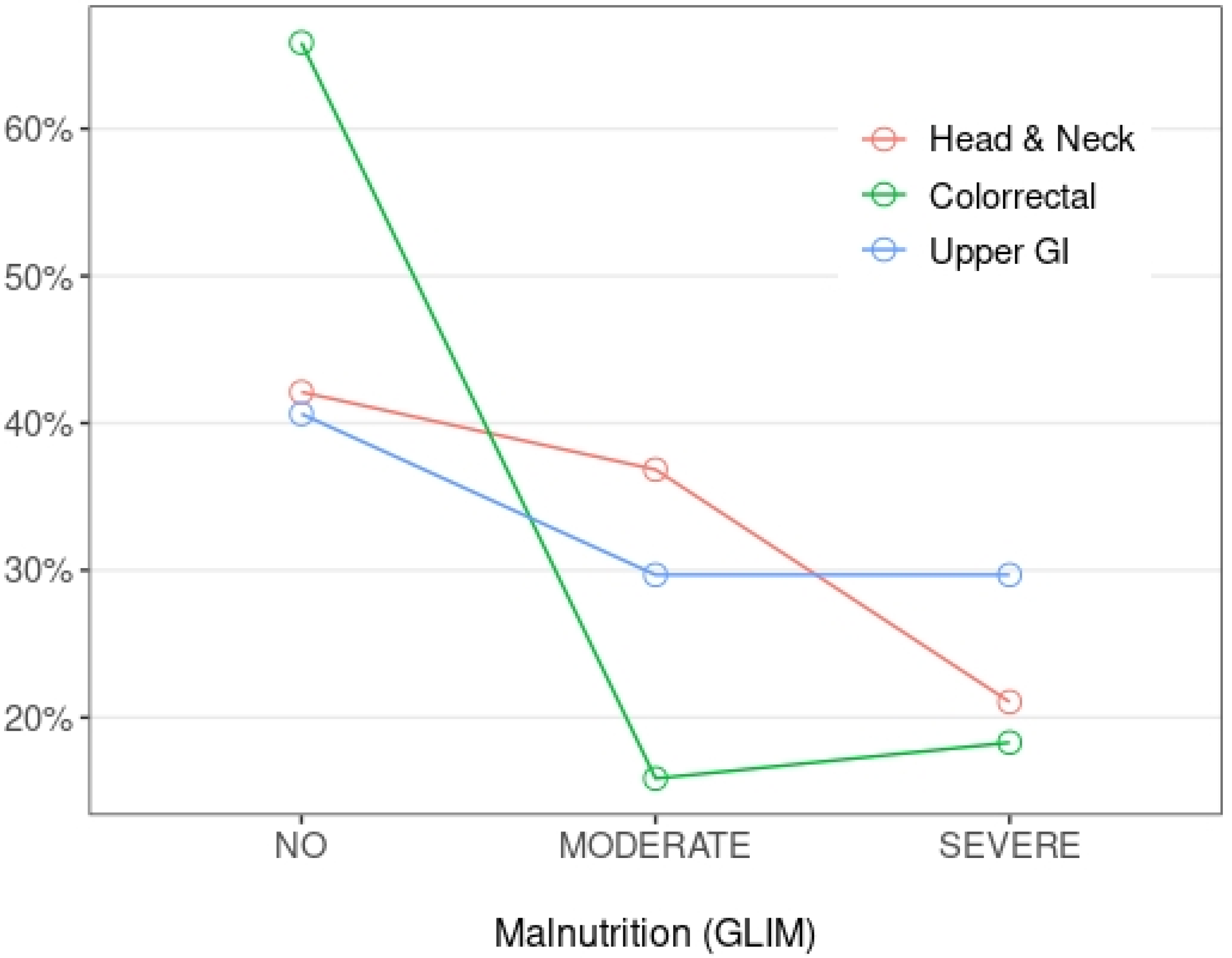
| GLIM Criteria | Head and Neck | Colorectal | Upper GI | p |
|---|---|---|---|---|
| N = 19 | N = 82 | N = 64 | ||
| Phenotypic criteria | ||||
| Non-volitional weight loss | 0.018 | |||
| No | 13 (68.4%) | 53 (64.6%) | 25 (39.1%) | |
| Moderate | 4 (21.1%) | 15 (18.3%) | 18 (28.1%) | |
| Severe | 2 (10.5%) | 14 (17.1%) | 21 (32.8%) | |
| Low BMI | 0.179 | |||
| No | 15 (78.9%) | 77 (93.9%) | 56 (87.5%) | |
| Moderate | 2 (10.5%) | 3 (3.66%) | 3 (4.69%) | |
| Severe | 2 (10.5%) | 2 (2.44%) | 5 (7.81%) | |
| Reduced muscle mass | 0.538 | |||
| No | 11 (57.9%) | 62 (75.6%) | 44 (68.8%) | |
| Moderate | 5 (26.3%) | 12 (14.6%) | 11 (17.2%) | |
| Severe | 3 (15.8%) | 8 (9.76%) | 9 (14.1%) | |
| Etiologic criteria | ||||
| Reduced food intake or assimilation | 11 (57.9%) | 34 (41.5%) | 34 (53.1%) | 0.244 |
| Inflammatory condition | 2 (10.5) | 21 (25.6) | 23 (35.9) | 0.077 |
| GI chronic condition | 5 (26.3%) | 3 (3.66%) | 20 (31.2%) | <0.001 |
| No Malnutrition | Malnutrition | OR | p | |
|---|---|---|---|---|
| N = 95 | N = 70 | |||
| Emergency Room admission | 42 (44.2%) | 41 (58.6%) | 1.78 [0.95; 3.35] | 0.071 |
| Hospitalization | 19 (20.0%) | 35 (50.0%) | 3.95 [2.00; 8.01] | <0.001 |
| Severe infection | 12 (12.6%) | 23 (32.9%) | 3.34 [1.54; 7.57] | 0.002 |
| Mild infection | 26 (27.4%) | 23 (32.9%) | 1.30 [0.66; 2.55] | 0.452 |
| Poor pain control | 26 (27.4%) | 40 (57.1%) | 3.50 [1.83; 6.83] | <0.001 |
| Increase in opioid dosage | 19 (20.0%) | 37 (52.9%) | 4.42 [2.24; 8.99] | <0.001 |
| Toxicity | 76 (80.0%) | 64 (91.4%) | 2.61 [1.03; 7.65] | 0.044 |
| Gastrointestinal toxicity | 49 (51.6%) | 44 (62.9%) | 1.58 [0.84; 3.00] | 0.153 |
| Hematological toxicity | 19 (20.0%) | 25 (35.7%) | 2.21 [1.10; 4.52] | 0.027 |
| Neurological toxicity | 37 (38.9%) | 26 (37.1%) | 0.93 [0.49; 1.76] | 0.817 |
| Decrease or discontinuation of treatment | 60 (63.2%) | 57 (81.4%) | 2.53 [1.23; 5.44] | 0.011 |
| Tumor Progression | 29 (30.5%) | 32 (45.7%) | 1.91 [1.00; 3.65] | 0.049 |
| 6-month survival | 83 (87.4%) | 50 (71.4%) | 0.37 [0.16; 0.81] | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gascón-Ruiz, M.; Casas-Deza, D.; Marti-Pi, M.; Torres-Ramón, I.; Zapata-García, M.; Sesma, A.; Lambea, J.; Álvarez-Alejandro, M.; Quilez, E.; Isla, D.; et al. Diagnosis of Malnutrition According to GLIM Criteria Predicts Complications and 6-Month Survival in Cancer Outpatients. Biomedicines 2022, 10, 2201. https://doi.org/10.3390/biomedicines10092201
Gascón-Ruiz M, Casas-Deza D, Marti-Pi M, Torres-Ramón I, Zapata-García M, Sesma A, Lambea J, Álvarez-Alejandro M, Quilez E, Isla D, et al. Diagnosis of Malnutrition According to GLIM Criteria Predicts Complications and 6-Month Survival in Cancer Outpatients. Biomedicines. 2022; 10(9):2201. https://doi.org/10.3390/biomedicines10092201
Chicago/Turabian StyleGascón-Ruiz, Marta, Diego Casas-Deza, Maria Marti-Pi, Irene Torres-Ramón, María Zapata-García, Andrea Sesma, Julio Lambea, María Álvarez-Alejandro, Elisa Quilez, Dolores Isla, and et al. 2022. "Diagnosis of Malnutrition According to GLIM Criteria Predicts Complications and 6-Month Survival in Cancer Outpatients" Biomedicines 10, no. 9: 2201. https://doi.org/10.3390/biomedicines10092201
APA StyleGascón-Ruiz, M., Casas-Deza, D., Marti-Pi, M., Torres-Ramón, I., Zapata-García, M., Sesma, A., Lambea, J., Álvarez-Alejandro, M., Quilez, E., Isla, D., & Arbonés-Mainar, J. M. (2022). Diagnosis of Malnutrition According to GLIM Criteria Predicts Complications and 6-Month Survival in Cancer Outpatients. Biomedicines, 10(9), 2201. https://doi.org/10.3390/biomedicines10092201








